Abstract
Toll-like receptors (TLRs) are innate immune receptors that have recently emerged as regulators of neuronal survival and developmental neuroplasticity. Adult TLR3-deficient mice exhibited enhanced hippocampus-dependent working memory in the Morris water maze, novel object recognition, and contextual fear-conditioning tasks. In contrast, TLR3-deficient mice demonstrated impaired amygdala-related behavior and anxiety in the cued fear-conditioning, open field, and elevated plus maze tasks. Further, TLR3-deficient mice exhibited increased hippocampal CA1 and dentate gyrus volumes, increased hippocampal neurogenesis, and elevated levels of the AMPA receptor subunit GluR1 in the CA1 region of the hippocampus. In addition, levels of activated forms of the kinase ERK and the transcription factor CREB were elevated in the hippocampus of TLR3-deficient mice, suggesting that constitutive TLR3 signaling negatively regulates pathways known to play important roles in hippocampal plasticity. Direct activation of TLR3 by intracerebroventricular infusion of a TLR3 ligand impaired working memory, but not reference memory. Our findings reveal previously undescribed roles for TLR3 as a suppressor of hippocampal cellular plasticity and memory retention.
Keywords: anxiety, cognition, GluR1, CREB
Toll-like receptors (TLRs) are innate immunity-related receptors that sense pathogen-associated molecular patterns (1) as well as tissue damage-associated molecular patterns (2). Although TLRs are abundant in the immune system, some TLRs are also expressed in central nervous system (CNS) cells where they mediate infection and injury responses (3). For example, activation of TLR4 by bacterial lipopolysaccharide up-regulates proinflammatory cytokine and chemokine production in microglia, and this TLR4 action on microglia is critical for the recruitment of circulating leukocytes into the brain (4). Neurons also express TLRs, and it was recently reported that activation of TLR2 and TLR4 in cortical neurons increases their vulnerability to ischemic injury (5). TLRs mediate innate immune responses, in part, through nuclear factor-κB (NF-κB) and IFN regulatory factor (IRF)-dependent pathways (3). Recent findings suggest that some TLRs modulate neural plasticity. For example, TLR3 inhibits neural progenitor cell (NPC) proliferation in the embryonic mouse telencephalon (6) and inhibits axonal growth in DRG neurons (7). More recently, Peltier et al. (8) found that human neurons are capable of a functional TLR3 signaling mechanism. Here we provide evidence that TLR3 signaling negatively regulates hippocampus-dependent learning and memory and suppresses hippocampal neurogenesis and AMPA receptor expression in CA1 neurons. We further show that constitutive physiological TLR3 signaling suppresses ERK and CREB activities, suggesting a mechanism whereby TLR3 regulates hippocampal neurogenesis and behavioral plasticity.
Results
TLR3 Deficiency Enhances Hippocampus-Dependent, but Impairs Amygdala-Dependent, Learning and Memory.
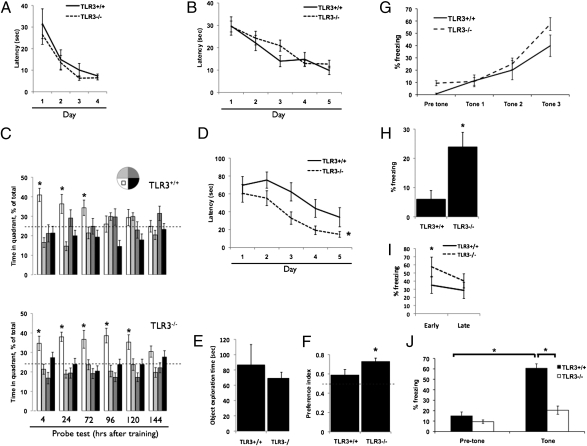
To determine whether TLR3 affects cognitive function, we compared spatial memory acquisition and retention abilities in TLR3−/− and TLR3+/+ mice. We first assessed the performance of mice in a nonspatial visible platform variant of the Morris water maze (MWM) to test for baseline differences in vision and motivation. TLR3−/− and TLR3+/+ mice exhibited similar escape latencies to the visible platform, suggesting no baseline differences in vision or motivation between the two strains (Fig. 1A, P > 0.05). We then tested the mice in a spatial, hippocampus-dependent MWM. TLR3−/− and TLR3+/+ mice exhibited similar escape latencies to the hidden platform, suggesting that the learning kinetics of reference memory were similar between these groups (Fig. 1B, P > 0.05). There were no differences in swim speed (P > 0.05), mean distance from the platform (P > 0.05), or distance traveled (P > 0.05) (Fig. S1 A–C). In probe trials to test memory retention conducted 4–72 h following acquisition training, TLR3−/− and TLR3+/+ mice spent a similar amount of time searching in the target quadrant (Fig. 1C, P > 0.05). However, at 120 h TLR3−/− mice spent a significantly greater percentage of time searching in the target quadrant (35.3 ± 3.7% in the target quadrant compared with 23.7 ± 3%, 17.2 ± 2.2%, and 23.7 ± 2.8% in the left, opposite, and right quadrants, respectively; Fig. 1C, P < 0.01), whereas TLR3+/+ mice showed no significant preference for the target quadrant (29.3 ± 4.1% in the target quadrant compared with 29.8 ± 1.9%, 22.9 ± 4.2%, and 17.8 ± 3.1% in the left, opposite, and right quadrants, respectively; Fig. 1C, P > 0.05). Memory of the platform location was completely extinct at 144 h post training in TLR3−/− mice (Fig. 1C).
Fig. 1.
Hippocampus-dependent working memory is enhanced and amygdala-dependent memory is impaired in mice lacking TLR3. (A) TLR3+/+ (n = 16) and TLR3−/− (n = 16) mice show similar latencies to reach a visible platform in a nonspatial variant of the MWM. (B) TLR3+/+ and TLR3−/− mice exhibit similar learning rates, as measured by their escape latencies. (C) Whereas TLR3+/+ mice retain memory of the platform up to 72 h; TLR3−/− mice retain memory of the platform location up to 120 h posttraining. (D) TLR3−/− mice exhibit enhanced working memory acquisition compared with TLR3+/+ mice. As early as day 2 of the test, TLR3−/− show shorter latencies to find the platform in its new location. (E) No significant difference in total exploration during object familiarization between TLR3+/+ and TLR3−/− mice. (F) TLR3−/− mice exhibit significantly higher preference for a novel object than TLR3+/+ mice in the novel object recognition test. (G) TLR3+/+ and TLR3−/− mice show similar association curves in the fear-conditioning paradigm. (H) TLR3−/− mice exhibit significantly greater hippocampus-dependent contextual fear than TLR3+/+ mice, as measured by time freezing. (I) TLR3−/− mice exhibit slower contextual memory extinction than TLR3+/+ mice. (J) TLR3−/− mice exhibit impaired cued fear and freeze less than TLR3+/+ mice in the presence of tone.
Categorical pattern recognition analysis of behavioral strategies used in the MWM are valuable in distinguishing between cognitive as well as noncognitive effects of a particular genetic or environmental factor (9). The platform searching strategies of TLR3−/− mice and TLR3+/+ mice were different: 85% of TLR3−/− mice exhibited a focused searching strategy compared with only 20% of TLR3+/+ mice (Fig. S1D). Although reference memory relates to long-term memory, working memory is a system for temporarily storing and managing the information required to carry out complex cognitive tasks. To study whether TLR3 alters working memory, we performed a variation of the MWM in which the platform location is moved to a new location every day. In this test, TLR3−/− mice showed a marked improvement compared with TLR3+/+ mice in finding the changed location of the platform as early as training day 2, strongly suggesting enhanced working memory efficiency in TLR3−/− mice (TLR3+/+ mice: 70 ± 9.7 s, 76 ± 9 s, 62 ± 10.1 s, 44 ± 10 s, and 33.9 ± 10.8 s; TLR3−/− mice: 61 ± 9.9 s, 55 ± 8.3 s, 33 ± 6.9 s, 19 ± 4.8 s, and 14 ±3.2 s for days 1, 2, 3, 4, and 5, respectively; P < 0.05, Fig. 1D). The superior performance of TLR3−/− mice was not due to differences in body weight (32.9 ± 3.5 g and 29.69 ± 2.7 g in TLR3+/+ and TLR3−/− mice, respectively; Fig. S2A, P > 0.05). Performance in the MWM was not attributable to alterations in motor function because TLR3+/+ mice performed better than TLR3−/− mice on the Rotarod task, falling at a higher rpm (TLR3+/+: 30.98 ± 1.7; TLR3−/−: 25 ± 1.46; Fig. S2 B and D, P < 0.05), falling after a longer time period (TLR3+/+: 224.9 ± 14.7 s; TLR3−/−: 180.4 ± 12.05 s; Fig. S2 C and F, P < 0.05), and falling fewer times (TLR3+/+: 2.7 ± 0.8; TLR3−/−: 5.5 ± 0.5; Fig. S2 D and G, P < 0.05). Furthermore, TLR3+/+ mice showed superior motor learning, which was defined by improved performance between trials, compared with TLR3−/− mice (Fig. S2G, P < 0.05). We next determined whether direct activation of TLR3 in the CNS could have a behavioral effect opposite to that observed in TLR3-deficient mice. Mice were implanted with an intracerebral cannula and either artificial cerebral spinal fluid (aCSF) or Polyinosinic:polycytidylic acid [(Poly(I:C)] was infused into the lateral ventricle at a rate of 0.1 μL/h. Following 1 week of recovery from the surgery (Fig. S3A), mice were tested in the MWM. Both aCSF- and Poly(I:C)-treated mice exhibited similar escape latencies to a visible platform in the nonspatial MWM, suggesting that there are no baseline differences in vision or motivation between the two experimental groups (Fig S3B, Left, P > 0.05). We then tested the mice in a spatial, hippocampus-dependent MWM. aCSF- and Poly(I:C)-treated mice exhibited similar escape latencies to the hidden platform, suggesting that the learning kinetics of reference memory were similar between these groups (Fig. S3B, Right, P > 0.05). In probe trials to test memory retention conducted 24–72 h following acquisition training, control and Poly(I:C)-treated mice spent a similar amount of time searching in the target quadrant (Fig. S3C, P > 0.05), suggesting that TLR3 activation had no effect on extinction of reference memory. We next tested whether CNS infusion of Poly(I:C) affected working memory in the MWM. In this test, Poly(I:C)-treated mice showed impaired performance compared with aCSF-treated mice in finding the changed location of the platform as early as training day 1, suggesting that central activation of TLR3 impairs working memory (aCSF: 26.4 ± 3.5 s, 25.5 ± 3.6 s, 18.1 ± 2.1 s, and 20.6 ± 3.3 s; Poly(I:C): 32.5 ± 4.3 s, 31.1 ± 4.02 s, 25.1 ± 4.03 s, and 23.3 ± 2.99 s for days 1, 2, 3, and 4, respectively; P < 0.05, Fig. S3D).
We next used the object preference test, which assesses nonspatial short term memory of a novel object in a familiar setting. Exploration time during familiarization of the mice to the objects did not differ significantly between TLR3+/+ and TLR3−/− mice (Fig. 1E). Upon introduction of a novel object, TLR3−/− mice exhibited stronger preference for the novel object than TLR3+/+ mice (0.72 ± 0.03 s compared with 0.58 ± 0.05 s; Fig. 1F, P < 0.05).
We further examined learning and memory using the fear-conditioning paradigm. During fear conditioning, TLR3+/+ and TLR3−/− mice demonstrated similar learning curves for the association between the tone and the shock (Fig. 1G, P > 0.05). However, in the hippocampus-dependent contextual fear paradigm, whereas both TLR3+/+ and TLR3−/− froze more than naive animals, TLR3−/− mice exhibited significantly increased freezing (23.8 ± 5%) compared with TLR3+/+ mice (6.03 ± 2.9%; Fig. 1H, P < 0.05). Analysis of contextual memory extinction shows that, in TLR3−/− mice, extinction of the memory occurs more slowly than in TLR3+/+ mice (TLR3−/−: 57.57 ± 10.08 early phase and 40.24 ± 9.95 in late phase; TLR3+/+: 35 ± 11.8 in early phase and 28.61 ± 8.7 in late phase, Fig. 1I, P < 0.05). In contrast, in the cued test, in which freezing is measured in the presence of the tone, albeit in a different context, TLR3−/− mice showed significantly less freezing than TLR3+/+ mice (20.4 ± 3.9% and 60.6 ± 4.2%, respectively; Fig. 1J, P < 0.0001). Together with the results of the MWM and novel object recognition, these data indicate that TLR3 is a negative regulator of hippocampus-dependent learning and memory.
TLR3 Deficiency Reduces Anxiety-Related Behaviors.
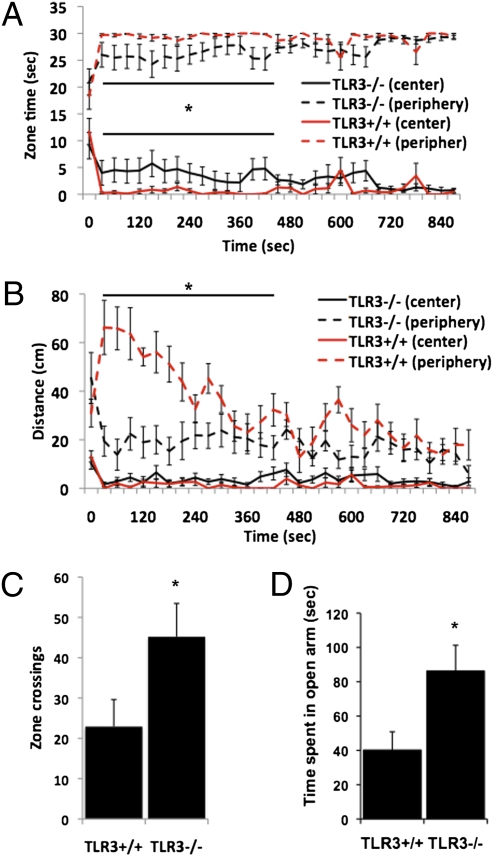
To further evaluate the effects of TLR3 deficiency on behavior, we used the open field exploration test, in which anxious mice spend more time in the periphery than in the center of the arena. TLR3−/− mice display reduced anxiety, indicated by greater time spent in the center of the field than at its periphery (Fig. 2A, P < 0.05). TLR3−/− mice were significantly less active in the periphery than TLR3+/+ mice (Fig. 2B, P < 0.01) and show significantly more zone crosses between the center and the periphery of the open field (22.9 ± 6.71 and 45.2 ± 8.2 for TLR3+/+ and TLR3−/− mice, respectively; Fig. 2C, P < 0.01). Anxiety was further evaluated by using the elevated plus maze (10). In this test, animals that exhibit higher anxiety levels spend less time in the open arms and more time in the closed arms of the maze. TLR3−/− mice displayed significantly reduced anxiety levels, spending 86.25 ± 15.02 s compared with 40.18 ± 10.66 s for TLR3+/+ mice in the open arms of the maze (Fig. 2D, P < 0.01). In humans, although depression is not always associated with anxiety, elevated anxiety often precedes depression (11). We therefore tested whether the altered anxiety levels of TLR3−/− mice affects their performance in the Tail Suspension Test (TST) and the Forced Swim Test (FST), two widely used models for depression in rodents. No significant difference was observed in freezing behavior between TLR3−/− and TLR3+/+ mice in either the FST (Fig. S4A) or the TST (Fig. S4B), suggesting that the altered anxiety response in TLR3−/− mice is not associated with reduced depressive behavior.
Fig. 2.
TLR3−/− mice exhibit reduced anxiety-like behaviors. (A) TLR3−/− (n = 16) mice spend more time in the center of the arena compared with TLR3+/+ (n = 16) mice. (B) TLR3−/− mice cover less distance in the periphery of the arena compared with TLR3+/+ mice. (C) TLR3−/− mice cross more between the center and peripheral zones in an open field. (D) TLR3−/− mice spend more time than TLR3+/+ mice in the open arm in an elevated plus maze.
These data suggest that hippocampus-dependent memory is significantly improved in TLR3−/− mice, and anxiety-like behavior and cued fear conditioning are reduced.
TLR3 Is a Negative Regulator of Hippocampal Cellular Plasticity.
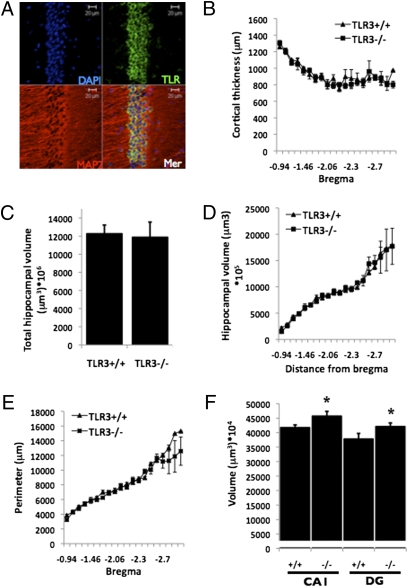
We next evaluated whether the improved behavioral performance in memory retention tests in TLR3−/− mice was associated with changes in cortical or hippocampal structure. TLR3 is expressed in the CA1 subregion of the hippocampus (Fig. 3A) as well as in the dentate gyrus (DG), Hillus, and the CA3 subregions of the hippocampus (Fig. S5). No differences were observed between TLR3−/− and TLR3+/+ mice in cortical thickness (Fig. 3B), nor in total hippocampal volume (Fig. 3 C and D) or perimeter length (Fig. 3E). However, the volumes of the DG and CA1 in TLR3−/− mice were significantly larger than in TLR3+/+ mice (DG: 42,106 ± 1,275 μm3 × 104 and 37,808 ± 1,931 μm3 × 104 in TLR3−/− and TLR3+/+ mice, respectively; P < 0.05; CA1: 45,760 ± 1,682 μm3 × 104 and 41,722 ± 962 μm3 × 104 in TLR3−/− and TLR3+/+ mice, respectively; P < 0.05, Fig. 3F).
Fig. 3.
Loss of TLR3 alters hippocampal structure. (A) TLR3 is expressed in the CA1 in adult TLR3+/+ mice. (B–E) Cortical thickness (B), hippocampal volume (C and D), and hippocampal perimeter (E) were similar in TLR3+/+ (n = 6) and TLR3−/− (n = 6) mice. (F) TLR3−/− mice exhibit increased DG and CA1 volumes.
Because the DG volume of TLR3-deficient mice was greater than wild-type mice, we next assessed whether TLR3−/− mice have increased hippocampal neurogenesis. Total cell genesis within the DG estimated by unbiased stereological counting of BrdU-labeled cells was not significantly elevated (TLR3+/+: 41 ± 4.1; TLR3−/−: 51 ± 5.4; P > 0.05, Fig. S6A). In TLR3−/− mice, however, we observed a significant increase in BrdU-labeled cells coexpressing the mature neuronal marker NeuN, indicative of increased neuronal differentiation and/or cell survival (TLR3+/+: 21 ± 4; TLR3−/−: 38 ± 6.1; P < 0.05, Fig. S6B). In agreement with our previous findings (6), nestin-positive NPCs in the DG did not express TLR3 (Fig. S7), further confirming that the effect of TLR3 on neurogenesis in the adult is via differentiation/cell survival rather than adult NPC proliferation.
Because the AMPA receptor subunit GluR1 contributes to hippocampus-dependent spatial working memory (12), we also examined GluR1 protein levels in the hippocampus of TLR3−/− mice by immunofluorescence and immunoblotting. GluR1 was significantly increased in hippocampal extracts from TLR3−/− mice compared with TLR3+/+ mice (0.97 ± 0.14 and 0.22 ± 0.083, respectively; P < 0.001; Fig. S6C). GluR1 is predominantly expressed in CA1 neurons (Fig. S6D) where it was significantly elevated in hippocampal slices from TLR3−/− mice (45,700 ± 1,682) compared with TLR3+/+ mice (41,722 ± 962; P < 0.05). In contrast, GluR1 protein levels were not significantly affected by TLR3 deficiency in the DG (37,808 ± 1,931 and 42,106 ± 1,275 in TLR3+/+ and TLR3−/− mice, respectively; P > 0.05, Fig. S6D).
We next studied whether cellular pathways that are central to TLR3 signaling could explain the enhanced plasticity changes in TLR3−/− mice. In canonical TLR3 signaling, Akt phosphorylation is required to obtain the full phosphorylation and activation of IRF3 in the nucleus. However, there were no significant differences in the phosphorylation levels of Akt and IRF3 in TLR3−/− compared with TLR3+/+ hippocampi (Fig. S6E). Furthermore, quantitative RT–PCR analysis of the mRNAs for IFN-β (an IRF3-dependent transcript) and Tnfaip3 (TNF-α–induced protein 3, a NF-κB–dependent transcript) showed no differences between TLR3−/− and TLR3+/+ hippocampi (Fig. S6F). Since the activation of the canonical TLR pathway is not altered in TLR3−/− mice compared with TLR3+/+ hippocampi, it is plausible that the canonical activation-mediated TLR3 signaling is not functional under physiological conditions in the absence of external stimuli.
Interestingly, we observed increased phosphorylation of the extracellular signal-regulated kinases ERK1 and ERK2 but not total ERK protein levels (Fig. S6E), as well as increased levels of total and phosphorylated cAMP response element-binding protein (pCREB) in the hippocampi of TLR3−/− compared with TLR3+/+ mice (Fig. S6E).
Although both CREB and GluR1 protein levels were up-regulated, only mRNA levels of CREB were up-regulated (Fig. S6F), suggesting both transcriptional and posttranscriptional (phosphorylation) up-regulation of CREB and posttranslational up-regulation of GluR1 in the hippocampus of TLR3−/− mice. The increased expression and phosphorylation of ERK and CREB was specific to the hippocampus, as analysis of the same signaling mediators in the cortex revealed no difference between TLR3+/+ and TLR3−/− mice (Fig. S8). These data showing that ERK and CREB are more active in hippocampi lacking TLR3 compared with those containing TLR3 suggest a unique role for TLR3 in inhibiting these plasticity-related signaling molecules.
Discussion
Our findings suggest that TLR3 plays complex roles in modulating CNS plasticity. Comparisons of the performance of TLR3+/+ and TLR3−/− mice in the MWM and novel object recognition and contextual fear-conditioning tests, as well as the effects of direct TLR3 activation on performance in the MWM, suggest that TLR3 signaling inhibits working memory acquisition and long-term memory retention without affecting reference memory acquisition. The fact that, in the probe trials following the reference memory test, TLR3−/− mice have slower memory extinction is not at odds with their enhanced working memory performance. Other studies have shown that different mechanisms underlie encoding and extinction of reference memory and working memory (13). In the case of TLR3−/− mice, their slower memory extinction in the MWM is in agreement with the slower extinction of fear memory in these mice (Fig. 1I). In contrast, TLR3-deficient mice performed poorly in the cued test component of the fear-conditioning tests and showed abnormal performance in the elevated plus maze and open field arena, suggesting that TLR3 signaling plays a key role in amygdala-dependent memory retention. Interestingly, TLR3−/− mice performed worse in the Rotarod tests of motor function and motor learning, suggesting a role for TLR3 signaling in the motor system.
TLR3 Modulates Hippocampal Plasticity.
Our findings suggest two mechanisms whereby TLR3 signaling may negatively regulate hippocampus-dependent memory retention. Previous studies have demonstrated strong associations between increased dentate neurogenesis and environmental and pharmacological manipulations that enhance learning and memory including exercise, environmental enrichment, and antidepressants (14, 15). Consistent with an inhibitory role for TLR3 in hippocampal memory, we found that neurogenesis was increased in TLR3-deficient mice. The increased hippocampal neurogenesis in TLR3-deficient mice was correlated with an increased volume of the DG of the hippocampus. Therefore, increased neurogenesis may contribute to increases in DG volume. Alternatively, increased DG volume may result from increased subventricular zone NPC proliferation during embryonic developmental stages (6) or from the cumulative result of both processes. However, in the adult subependymal zone (SEZ), NPCs contribute to olfactory rather than hippocampal neurogenesis (16), which excludes this route from contributing to the DG neurogenesis effect that we observed. Additionally, in agreement with our previous findings in which adult-derived NPCs express little TLR3 compared with embryonic-derived NPCs, nestin-expressing NPCs in the DG do not express TLR3. It remains to be determined whether TLR3 plays a role in adult SEZ NPC proliferation. Another possible mechanism yet to be tested is that the increase in DG volume in TLR3-deficient mice is due to altered regulation of dendritic arbors resulting in increased granule cell neuropil in the DG.
Recent findings point to a role for hippocampal neurogenesis in maintaining flexibility for retention of new information, a prime requirement for working memory (17). Indeed, in TLR3−/− mice, which exhibit greater neurogenesis, working memory is enhanced compared with TLR3+/+ mice.
In addition to increased neurogenesis, our data indicate that levels of GluR1, the essential subunit of the AMPA subtype of the glutamate receptor, are increased in the CA1 region of TLR3-deficient mice. AMPA receptors play a critical role in synaptic plasticity and spatial learning and memory (18). Mice lacking GluR1 exhibit normal acquisition of hippocampus-dependent reference memory but are impaired in working memory, which is dependent on information in short-term memory (18). Increased GluR1 expression may therefore contribute to the enhanced hippocampus-dependent working memory retention in mice lacking TLR3. The observation that central TLR3 activation leads to impaired working memory, the opposite of the enhanced working memory in TLR3-deficient mice, provides a strong case for the involvement of TLR3 signaling in establishing working memory. However, the possibility that noncanonical TLR3 activation contributes to working memory processes is yet to be determined; this could be tested by over-expressing TLR3 in the hippocampus and assessing its effects on working memory. To further understand the molecular mechanisms by which TLR3 inhibits hippocampus-dependent memory processes, we examined the levels and activation states of the kinase ERK and the transcription factor CREB, both of which are believed to play critical roles in hippocampal synaptic plasticity (19) and neurogenesis (20). We found that levels of phosphorylated ERK and phosphorylated CREB were elevated in hippocampi of TLR3−/− mice compared with TLR3+/+ mice. These data show that TLR3 negatively regulates the ERK-CREB pathway. The mechanism(s) that couples TLR3 to CREB activation, neurogenesis, and GluR1 expression remains to be determined.
TLR3 Deficiency Impairs Amygdala-Dependent Processes.
In addition to roles in hippocampal cellular plasticity and learning and memory, our findings suggest a role for TLR3 signaling in amygdala-dependent memory and anxiety behaviors. TLR3 deficiency resulted in reduced amygdala-dependent memory and reduced anxiety-related behaviors, suggesting that TLR3 signaling is normally involved in amygdala plasticity. The mechanism(s) by which TLR3 interacts with amygdala-dependent processes is unknown and warrants further investigation. Despite the fact that TLR3−/− mice show impaired anxiety in fear conditioning and in elevated plus maze and open field paradigms, interpretation of tasks such as the novel object recognition were not hindered as TLR3−/− mice showed increased preference to the novel object without increased overall exploration during object familiarization. Moreover, altered anxiety levels in TLR3−/− mice appear not to be associated with a depression-related phenotype because TLR3-deficient mice showed no abnormalities in two tests of depression-like behavior (TST and FST). Slower memory extinction in TLR3−/− mice, as observed in the fear-conditioning paradigm, is in line with the performance of these mice in the MWM, as memory of the platform location was retained longer by TLR3−/− mice in the probe trials compared with TLR3+/+ mice. Because both contextual fear-conditioning and the MWM assess hippocampal-dependent cognitive performance, one could expect that TLR3−/− mice would perform better in the reference memory variant of the MWM, as they do in the contextual fear task. The difference probably stems from the fact that context (as assessed using the fear-conditioning task) and spatial processing (as assessed using the MWM task) are different in nature and could involve different neuronal populations. Moreover, the fact that TLR3−/− mice express higher levels of GluR1 compared with TLR3+/+ mice is in line with findings that show that GluR1−/− mice have impaired contextual fear conditioning (21). In contrast, GluR1−/− mice also exhibit impaired cued fear conditioning, whereas TLR3−/− mice show impaired cued fear conditioning despite increased GluR1 protein levels. However, the effects of GluR1 over-expression on contextual and cued fear conditioning are unknown.
Physiological TLR3 Signaling.
The functions of TLR3 under physiological conditions, in the absence of infection or injury, are unknown (22). The canonical TLR3 signaling pathway activated in response to pathogenic molecules involves Akt phosphorylation and translocation of the transcription factors IRF3 and NF-κB into the nucleus. Because we did not observe functional activation of IRF3 or Akt and because mRNA levels for IRF3– and NF-κB–dependent genes were not changed in the hippocampi of TLR3+/+ compared with TLR3−/− mice, it is plausible that canonical TLR3 signaling does not occur under physiological conditions. Instead, our data suggest that the presence of TLR3 suppresses ERK and CREB signaling pathways.
Our findings on the involvement of TLR3 in adult CNS plasticity and behavior add to a growing body of evidence positioning innate immune receptors in the arena of CNS plasticity. The linked fate of this class of ancient receptors and CNS physiology as well as plasticity is both surprising and intriguing. A better understanding of the involvement of TLRs in neurophysiology as well neuropathology can provide opportunities for therapeutic intervention in neurological disorders as suggested by recent findings that have revealed roles for TLRs in experimental models of ischemic stroke (5) and Alzheimer's disease (23).
Glial cells such as astrocytes are emerging as important regulators of synaptic plasticity and cognition and may play an important role in hippocampus-dependent learning and memory (24). It is yet to be determined whether the effects of TLR3 signaling on working memory are mediated solely by changes in neurons or whether there is a also a role for glial TLR3 signaling. This could be determined in future studies by manipulating TLR3 signaling in a cell type-specific manner.
Studies of experimental models have implicated TLR4 in the pathogenesis of cognitive impairment that occurs in systemic inflammation/bacterial sepsis (25) and in Alzheimer's disease (23, 26). TLR2 and TLR4 signaling were also associated with worsened functional outcome in a mouse model of focal ischemic stroke (5). In addition, systemic administration of the double-stranded RNA TLR3 ligand poly I:C resulted in proinflammatory changes in the CNS and an associated sickness behavior (27). Our findings suggest that TLR3 negatively regulates hippocampal synaptic plasticity and neurogenesis by a mechanism involving suppression of ERK and CREB signaling, suggesting a mechanism whereby TLR3 activation might contribute to cognitive impairment in several disorders. In addition, the reduced anxiety-like behaviors in TLR3-deficient mice suggest potential roles for TLR3 signaling in anxiety disorders.
Materials and Methods
Mice.
TLR3-deficient male mice (TLR3−/−, n = 16) and wild-type littermates (TLR3+/+, n = 16) (breeding pairs were initially purchased from Jackson Laboratories) were maintained on a 12-h light/12-h dark cycle with food and water provided ad libitum. For testing of the effect of intracerebroventricular administration of the TLR3 ligand Poly(I:C) on spatial learning and memory, wild-type littermates were infused with either aCSF (Harvard Apparatus) (n = 10) or Poly(I:C) (Invitrogen), (n = 10). For detailed methods, see SI Materials and Methods. All experiments were performed using 6- to 7-mo-old mice. All procedures followed National Institutes of Health guidelines and were approved by the National Institute on Aging Animal Care and Use Committee.
Behavioral Testing.
The methods for testing mice in the open field—Rotarod, MWM, novel object recognition, fear conditioning, elevated plus maze, tail suspension test, and forced swim test—have been described previously (28–34) and are detailed in SI Materials and Methods.
Immunofluoresence.
Mice were anesthetized using isofluorane and perfused transcardially with cold 4% paraformaldehyde (PFA) in 0.1 M PBS. Brains were removed and postfixed in 4% PFA overnight and then cryoprotected in 30% sucrose in 0.1 M PBS. Brains were then sectioned 30 μm thick on a freezing microtome in the coronal planes. All immunohistochemistry was completed on tissue mounted on gelatin-coated slides. For BrdU staining, slides were first immersed in a 2N HCl bath for 30 min at 37 °C, followed by 0.1 M borate buffer (pH 8.5) for 10 min at room temperature. Slides were washed six times in Tris-buffered saline (TBS) for a total of 90 min. Nonspecific binding was blocked with 10% normal goat serum and 0.1% Triton X-100 in TBS for 30 min. Primary antibodies used for staining (listed Table S1) were diluted in PBS supplemented with 0.1% Triton-X-100 with 2% goat serum. Following 48 h primary antibody incubation, slides were washed three times in TBS for a total of 15 min. Slices were subsequently incubated with a fluorescent-tagged secondary antibody (Alexa-488 or Alexa-568 1:2,000; Invitrogen), diluted in PBS supplemented with 0.1% Triton X-100 with 2% goat serum for 1 h at room temperature. Slides were counterstained with 4′, 6-diamidino-2-phenylindole, 1:10,000; Invitrogen. The staining with the TLR3 antibody was specific because no immunoreactivity was observed in brain sections from TLR3−\− mice (6), nor in brain sections from wild-type mice when the primary antibody was excluded from the processing procedure.
Western Blotting.
Hippocampi from TLR3+/+ and TLR3−/− mice were dissected and then tissue was lysed and protein concentration was determined using the Bradford reagent (BioRad). Protein samples (30 μg) were separated by electrophoresis in a 4–12% bis-Tris polyacrylamide gel. Proteins were then electrophoretically transferred to a 45-μm nitrocellulose membrane and incubated with primary and peroxidase-conjugated secondary antibodies (Table S1), and immunolabeled proteins were visualized using enhanced chemiluminescence (Thermo Scientific).
BrdU Labeling and Quantification of Labeled Cells.
Mice received a daily i.p. injection of BrdU (dissolved in 0.9% saline and filtered sterile at 0.2 μm, 50 mg/kg body weight at 10 μg/mL; Sigma) for 7 consecutive days to label dividing cells, and mice were euthanized 5 weeks later. BrdU injections were carried out 2 mo after the completion of behavioral testing to avoid any confounding effects of the behavioral tests on cell proliferation and neurogenesis. Brain tissue section preparation and quantification of BrdU-labeled cells were performed as described previously (31), and details are provided in SI Materials and Methods.
Quantification of Brain Structure Sizes.
Methods for quantification of brain structure sizes are detailed in SI Materials and Methods.
Quantitative RT–PCR.
RNA from whole hippocampi of TLR3−/− and TLR3+/+ mice was extracted using TRIzol Reagent (Invitrogen) and was DNaseI digested before cDNA synthesis. cDNA was synthesized using the SuperScript III first-strand kit (Invitrogen). All samples within an experiment were reverse transcribed at the same time, and the resulting cDNA was diluted 1:5 in nuclease-free water and stored in aliquots at –80 °C until used. Real-time PCR with SYBR green detection was performed using a PTC-200 thermocycler (MJ Research) with a Chromo4 fluorescence detector (BioRad). Gene-specific primers are listed in Table S2.
Statistical Analysis.
Analyses of the MWM and fear-conditioning and open field experiments were performed using two-way ANOVA with repeated measures and Fisher posthoc tests. Analyses of the MWM probe tests were performed using one-way repeated measures ANOVA. All other tests were analyzed using t test. Results are expressed as mean ± SEM.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Aging of the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005807107/-/DCSupplemental.
References
- 1.Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: Endogenous danger signaling. Mol Med. 2008;14:476–484. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okun E, et al. Toll-like receptors in neurodegeneration. Brain Res Brain Res Rev. 2009;59:278–292. doi: 10.1016/j.brainresrev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou H, Lapointe BM, Clark SR, Zbytnuik L, Kubes P. A requirement for microglial TLR4 in leukocyte recruitment into brain in response to lipopolysaccharide. J Immunol. 2006;177:8103–8110. doi: 10.4049/jimmunol.177.11.8103. [DOI] [PubMed] [Google Scholar]
- 5.Tang SC, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci USA. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lathia JD, et al. Toll-like receptor 3 is a negative regulator of embryonic neural progenitor cell proliferation. J Neurosci. 2008;28:13978–13984. doi: 10.1523/JNEUROSCI.2140-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cameron JS, et al. Toll-like receptor 3 is a potent negative regulator of axonal growth in mammals. J Neurosci. 2007;27:13033–13041. doi: 10.1523/JNEUROSCI.4290-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peltier DC, Simms A, Farmer JR, Miller DJ. Human neuronal cells possess functional cytoplasmic and TLR-mediated innate immune pathways influenced by phosphatidylinositol-3 kinase signaling. J Immunol. 2010;184:7010–7021. doi: 10.4049/jimmunol.0904133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolfer DP, Lipp HP. Dissecting the behaviour of transgenic mice: Is it the mutation, the genetic background, or the environment? Exp Physiol. 2000;85:627–634. [PubMed] [Google Scholar]
- 10.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anisman H, Merali Z. Rodent models of depression: Learned helplessness induced in mice. In: Jacqueline N, Crawley, et al., editors. Current Protocols in Neuroscience. Malden, MA: Wiley InterScience; 2001. Chapter 8, Unit 8.10C.1–8.10C.15. [DOI] [PubMed] [Google Scholar]
- 12.Reisel D, et al. Spatial memory dissociations in mice lacking GluR1. Nat Neurosci. 2002;5:868–873. doi: 10.1038/nn910. [DOI] [PubMed] [Google Scholar]
- 13.Sanderson DJ, et al. Spatial working memory deficits in GluA1 AMPA receptor subunit knockout mice reflect impaired short-term habituation: Evidence for Wagner's dual-process memory model. Neuropsychologia. 2010;48:2303–2315. doi: 10.1016/j.neuropsychologia.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Praag H. Exercise and the brain: Something to chew on. Trends Neurosci. 2009;32:283–290. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 16.Suh H, Deng W, Gage FH. Signaling in adult neurogenesis. Annu Rev Cell Dev Biol. 2009;25:253–275. doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- 17.Waddell J, Shors TJ. Neurogenesis, learning and associative strength. Eur J Neurosci. 2008;27:3020–3028. doi: 10.1111/j.1460-9568.2008.06222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanderson DJ, et al. The role of the GluR-A (GluR1) AMPA receptor subunit in learning and memory. Prog Brain Res. 2008;169:159–178. doi: 10.1016/S0079-6123(07)00009-X. [DOI] [PubMed] [Google Scholar]
- 19.Davis S, Vanhoutte P, Pages C, Caboche J, Laroche S. The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J Neurosci. 2000;20:4563–4572. doi: 10.1523/JNEUROSCI.20-12-04563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakagawa S, et al. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci. 2002;22:3673–3682. doi: 10.1523/JNEUROSCI.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Humeau Y, et al. A pathway-specific function for different AMPA receptor subunits in amygdala long-term potentiation and fear conditioning. J Neurosci. 2007;27:10947–10956. doi: 10.1523/JNEUROSCI.2603-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vercammen E, Staal J, Beyaert R. Sensing of viral infection and activation of innate immunity by toll-like receptor 3. Clin Microbiol Rev. 2008;21:13–25. doi: 10.1128/CMR.00022-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang SC, et al. Toll-like receptor-4 mediates neuronal apoptosis induced by amyloid beta-peptide and the membrane lipid peroxidation product 4-hydroxynonenal. Exp Neurol. 2008;213:114–121. doi: 10.1016/j.expneurol.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pertusa M, et al. Expression of GDNF transgene in astrocytes improves cognitive deficits in aged rats. Neurobiol Aging. 2008;29:1366–1379. doi: 10.1016/j.neurobiolaging.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 25.Cunningham C, et al. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry. 2009;65:304–312. doi: 10.1016/j.biopsych.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tahara K, et al. Role of toll-like receptor signalling in Abeta uptake and clearance. Brain. 2006;129:3006–3019. doi: 10.1093/brain/awl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cunningham C, Campion S, Teeling J, Felton L, Perry VH. The sickness behaviour and CNS inflammatory mediator profile induced by systemic challenge of mice with synthetic double-stranded RNA (poly I:C) Brain Behav Immun. 2007;21:490–502. doi: 10.1016/j.bbi.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Martin B, et al. Exendin-4 improves glycemic control, ameliorates brain and pancreatic pathologies, and extends survival in a mouse model of Huntington's disease. Diabetes. 2009;58:318–328. doi: 10.2337/db08-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porton B, et al. Mice lacking synapsin III show abnormalities in explicit memory and conditioned fear. Genes, Brain, and Behavior. 2009;3:257–268. doi: 10.1111/j.1601-183X.2009.00555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soliman F, et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stranahan AM, et al. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008;11:309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bevins RA, Besheer J. Object recognition in rats and mice: A one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc. 2006;1:1306–1311. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- 33.Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: A review of antidepressant activity. Psychopharmacology (Berl) 2005;177:245–255. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- 34.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.