Abstract
Dynamic nucleocytoplasmic shuttling of class IIa histone deacetylases (HDACs) is a fundamental mechanism regulating gene transcription. Recent studies have identified several protein kinases that phosphorylate HDAC5, leading to its exportation from the nucleus. However, the negative regulatory mechanisms for HDAC5 nuclear exclusion remain largely unknown. Here we show that cAMP-activated protein kinase A (PKA) specifically phosphorylates HDAC5 and prevents its export from the nucleus, leading to suppression of gene transcription. PKA interacts directly with HDAC5 and phosphorylates HDAC5 at serine 280, an evolutionarily conserved site. Phosphorylation of HDAC5 by PKA interrupts the association of HDAC5 with protein chaperone 14-3-3 and hence inhibits stress signal-induced nuclear export of HDAC5. An HDAC5 mutant that mimics PKA-dependent phosphorylation localizes in the nucleus and acts as a dominant inhibitor for myocyte enhancer factor 2 transcriptional activity. Molecular manipulations of HDAC5 show that PKA-phosphorylated HDAC5 inhibits cardiac fetal gene expression and cardiomyocyte hypertrophy. Our findings identify HDAC5 as a substrate of PKA and reveal a cAMP/PKA-dependent pathway that controls HDAC5 nucleocytoplasmic shuttling and represses gene transcription. This pathway may represent a mechanism by which cAMP/PKA signaling modulates a wide range of biological functions and human diseases such as cardiomyopathy.
Keywords: nucleocytoplasmic shuttling, phosphorylation
Gene transcription is governed in part by the acetylation and deacetylation of histones, the latter of which is mediated by histone deacetylases (HDACs) (1–4). In particular, class IIa HDACs, such as HDAC5, acting as transcriptional repressors, have been implicated in cardiac hypertrophy, skeletal muscle differentiation, and angiogenesis (5–10). Dynamic nucleocytoplasmic shuttling has been proposed as a fundamental mechanism regulating the function of class IIa HDACs (1, 11–13). Recent studies have identified several protein kinases, including calmodulin-dependent protein kinases (CaMKs), protein kinase D (PKD) and salt-inducible kinase, that phosphorylate HDAC5, leading to its export from the nucleus (1, 9, 14). However, much less is understood about the negative regulatory mechanisms for the nuclear exclusion of HDAC5 (15). To date, specific protein kinases that may inhibit export of HDAC5 from the nucleus have not been identified.
The cAMP/protein kinase A (PKA) signaling pathway regulates a variety of cellular functions and numerous important biological processes (16, 17). Many of the effects of cAMP/PKA are mediated via changes in gene transcription. A large body of research has defined the cAMP-response element binding (CREB) proteins as PKA substrates that mediate an increase in gene expression in response to cAMP (18–20). However, whether and how the cAMP/PKA pathway inhibits gene expression remains unclear. In this study, we found that cAMP/PKA signaling represses gene transcription and cardiomyocyte hypertrophy by phosphorylating HDAC5 and preventing its export from the nucleus.
Results and Discussion
PKA Prevents Export of HDAC5 from the Nucleus.
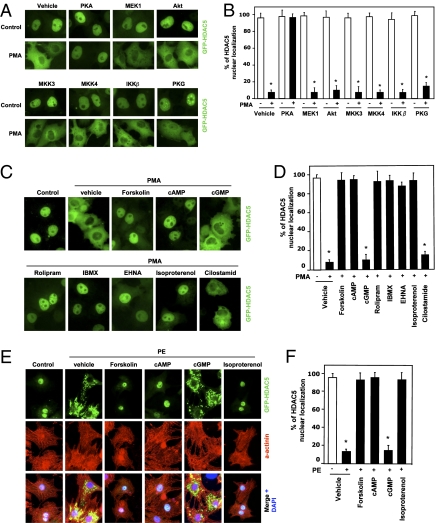
To search for possible protein kinases inhibiting the export of HDA5 from the nucleus, we examined the effects of various protein kinases on the nucleocytoplasmic shuttling of HDAC5. We cotransfected Cos7 cells with GFP-tagged HDAC5 and various constitutively active kinases, followed by treatment with phorbol 12-myristate 13-acetate (PMA), a well-documented stimulus for the nuclear export of class IIa HDACs (7, 21). Interestingly, we found that the PKA catalytic (PKA-CA) subunit (22) blocked PMA-induced nuclear export of HDAC5 (Fig. 1 A and B). Other kinases tested in our studies did not have an inhibitory effect on the nucleocytoplasmic shuttling of HDAC5. Furthermore, cotransfection experiments showed that PKA-CA also inhibited PKD- and CaMK-induced nuclear export of HDAC5 in Cos7 cells (SI Appendix, Fig. S1). Consistent with the PKA effect, the PKA activators forskolin and cAMP totally blocked nuclear export of HDAC5 (Fig. 1 C and D). The effects of forskolin and cAMP are PKA dependent because a specific PKA inhibitor, PKI-(14–22)-amide, abolished the inhibition of HDAC5 nuclear export by forskolin and cAMP (SI Appendix, Fig. S2). Treatment with the phosphodiesterase (PDE) inhibitors rolipram (a cAMP-specific PDE IV inhibitor), 3-isobutyl-1-methylxanthine (IBMX), erythro-9-(2-hydroxy-3-nonyl)-adenine (EHNA, a PDE II inhibitor), and the β-adrenergic receptor (β-AR) agonist isoproterenol, which increase the intracellular cAMP level, also inhibited the nuclear export of HDAC5 (Fig. 1 C and D). However, cGMP and the selective cGMP-specific PDE inhibitor cilostamide did not block export of HDAC5 from the nucleus, indicating the specificity of the cAMP/PKA pathway in regulating the nuclear export of HDAC5.
Fig. 1.
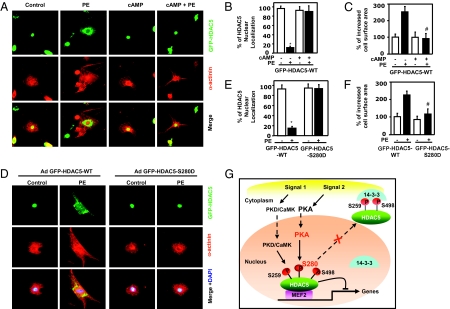
PKA inhibits stress signal-regulated HDAC5 nuclear export. (A and B) Cos7 cells were cotransfected with expression vectors encoding GFP-tagged HDAC5 (GFP-HDAC5) and constitutively active protein kinases as indicated and then were exposed to 500 μM PMA for 3 h. (C and D) Cos7 cells were cotransfected with GFP-HDAC5 and then were pretreated with the vehicle (DMSO) as control, forskolin (10 μM), cAMP (500 μM), cGMP (500 μM), rolipram (10 μM), IBMX (500 μM), EHNA (30 μM), isoproterenol (1 μM), and cilostamide (5 μM), followed by exposure to PMA for 3 h. (E and F) NRVMs were infected with adenoviral expression vector encoding GFP-HDAC5 and then were pretreated with the vehicle (DMSO) as control, forskolin (10 μM), cAMP (500 μM), cGMP (500 μM), and isoproterenol (1 μM) for 30 min, followed by exposure to the α-adrenergic agonist PE (10 μM) for 3 h. In A–F, cells were fixed, and the subcellular localization of GFP-HDAC5 was visualized by fluorescence microscopy. Values represent the percentage of expressing cells in which HDAC5 exhibited nuclear staining. Cardiomyocyte protein marker α-actinin immunofluorescence staining (red) and nuclei stained with DAPI (blue) are shown. *P < 0.05 versus without PMA or PE; n = 4.
To investigate whether the export of HDAC5 from the nucleus is regulated by the cAMP/PKA pathway in other types of cells, we infected neonatal rat ventricular myocytes (NRVMs) with adenovirus expressing GFP-tagged HDAC5 and then treated the NRVMs with forskolin, cAMP, or the β-AR agonist isoproterenol for 30 min, followed by treatment with the α-adrenergic receptor agonist phenylephrine (PE, 10 μM). Forskolin, cAMP, and isoproterenol markedly inhibited the PE-stimulated export of HDAC5 from the nucleus (Fig. 1 E and F, high resolution images for Fig. 1E in SI Appendix, Fig. S3). The positive staining of the myocyte marker sarcomeric α-actinin was confirmed. Similar results were observed when adult rat ventricular myocytes were used (SI Appendix, Fig. S4). In agreement with the results observed in Cos7 cells, the inhibition of PKA by PKI and siRNA in NRVMs abolished the inhibitory effects of cAMP on the PE-induced HDAC5nuclear export (SI Appendix, Figs. S5 and S6). Because PKA-CA has long been shown to enter and exit the nucleus of cells when intracellular cAMP is raised and lowered, respectively (23), we asked whether nuclear PKA could affect HDAC5 localization. The transfection of the nuclear-localized HcRed1-tagged PKA-CA-nuclear localization sequence (NLS) inhibited HDAC5 nuclear export (SI Appendix, Fig. S7), suggesting that the inhibitory effect of PKA on HDAC5 nuclear export could occur in the nucleus. We also observed that cAMP had the same inhibitory effect on PE-induced endogenous HDAC5 nuclear export in NRVMs (SI Appendix, Fig. S8). Collectively, our findings show that cAMP/PKA signaling specifically and negatively regulates stress signal-dependent HDAC5nuclear export.
To investigate whether the cAMP/PKA pathway regulates other members of class IIa HDACs, we examined the effects of forskolin/cAMP on PMA-induced nuclear export of YFP-tagged HDAC7 in Cos7 cells. Interestingly, cAMP/PKA had no inhibitory effect on HDAC7 nuclear export (Fig. 2 B and C). Taken together, these results indicate that the cAMP/PKA pathway selectively controls nucleocytoplasmic shuttling of HDAC5 but not of HDAC7.
Fig. 2.
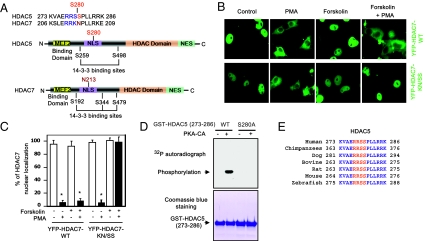
HDAC5 is a substrate for PKA. (A) (Upper) Comparison of amino acids surrounding the regulatory serine (arrowhead) between HDAC5 and HDAC7. (Lower) Schematic diagram of the HDAC5 and HDAC7 functional domains. (B and C) Nuclear export of YFP-HDAC7-WT is resistant to forskolin treatment, but nuclear export of YFP-HDAC7-K196S/N197S (mouse sequence) mutant was inhibited by forskolin in Cos7. *, P < 0.05 versus without PMA; n = 5. (D) (Upper) 32P autoradiograph image from in vitro kinase assays performed with recombinant PKA-CA and GST-HDAC5-WT (273–286) or GST-HDAC5-S280A (273–286) peptides. (Lower) The equal loading of GST proteins was shown by Coomassie blue staining. (E) Cos7 cells were transfected with Flag-tagged HDAC5-WT or Flag-tagged HDAC5-S280A and then were treated with forskolin (10 μM) at different times. Phosphorylation of HDAC5 in cell lysates was detected by immunoblotting with PKA phospho-substrate antibodies after immunoprecipitation with anti-Flag antibodies. (E) Alignment of amino acid sequences surrounding HDAC5 S280 in various species.
HDAC5 Is a Substrate for PKA.
To begin to address the mechanisms by which PKA regulates HDAC5 subcellular localization, we compared the amino acid sequences of human HDAC5 and human HDAC7. We noticed that a potential PKA-targeting motif “RRSS” in HDAC5 is replaced by “RRKN” in HDAC7 (Fig. 2A). Both HDAC5 and HDAC7 have an N-terminal myocyte enhancer factor-2 (MEF2) binding domain, an NLS, and a C-terminal HDAC domain and nuclear export sequence (4). Several conserved phosphorylation sites near the NLS are scaffolding protein 14-3-3 binding sites (1, 24). The potential PKA phospho-site serine (S280) in human HDAC5 is replaced with asparagine (N213) in human HDAC7. To determine whether the “RRSS” motif is responsible for PKA-dependent inhibition of HDAC5 nuclear export, we performed site-directed mutagenesis to replace “KN” in HDAC7 with “SS.” Although PKA did not inhibit export of YFP-HDAC7-WT from the nucleus, treatment with the PKA activator forskolin blocked nuclear export of the YFP-HDAC7-KN/SS mutant (Fig. 2 B and C). Because there is only 50% identity of amino acids between HDAC5 and HDAC7 (SI Appendix, Fig. S9), these results indicate that the “RRSS” motif is responsible for the inhibitory effect of PKA on HDAC5 nuclear export.
Because PKA prefers to phosphorylate the serine in the “RRXS” motif (18, 19), we hypothesized that phosphorylation of Ser280 by PKA in HDAC5 inhibits HDAC5 nuclear export. To determine whether PKA is able to phosphorylate HDAC5 at the Ser280 site, we performed an in vitro kinase assay using GST-tagged peptides containing residues 273–286 of HDAC5-WT and the S280A mutant in which Ser280 was replaced by alanine. We observed that recombinant PKA-CA phosphorylated the GST-tagged peptide of HDAC5-WT but not the GST-tagged peptide of the HDAC5-S280A mutant (Fig. 2D). Using a larger, more natural fragment of HDAC5 (amino acids 1-360, covering Ser280 and containing the entire NLS) (1), we detected similar phosphorylation by PKA-CA in an in vitro kinase assay (SI Appendix, Fig. S10). In addition, using a phospho-(Ser/Thr) PKA substrate antibody, we observed PKA-dependent phosphorylation of full-length HDAC5-WT, but not HDAC5-S280A mutant, in Cos7 cells (SI Appendix, Fig. S11). Notably, the PKA phosphorylation site in HDAC5 is evolutionally conserved from zebrafish to human (Fig. 2E). Moreover, we also detected the association between PKA and HDAC5 in Cos7 cells by coimmunoprecipitation (SI Appendix, Fig. S12). Taken together, our results demonstrate that HDAC5 is a substrate of PKA.
Phosphorylation of HDAC5 on Ser280 Mediates the Inhibition of HDAC5 Nuclear Export by the cAMP/PKA Pathway.
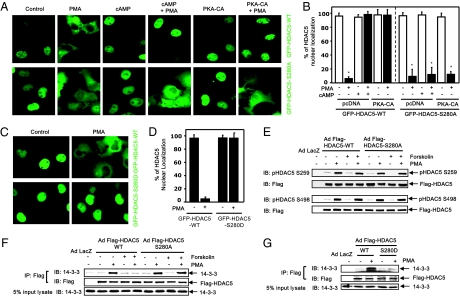
To investigate whether PKA-dependent phosphorylation of HDAC5 mediates the inhibitory effect of Ser280 on the nuclear export of HDAC5, we studied the effect of PKA on the subcellular localization of the HDAC5-S280A mutant in Cos7 cells. In contrast to the inhibition by PKA of GFP-HDAC5-WT nuclear export, PKA had no inhibitory effect on PMA-induced nuclear export of GFP-HDAC5-S280A (Fig. 3 A and B). Similar results were observed in cardiomyocytes infected with adenoviral GFP-HDAC5-S280A (SI Appendix, Fig. S13). These results indicate that Ser280 is necessary for PKA-mediated inhibition of HDAC5 nuclear export. To investigate whether the phosphorylation of Ser280 is sufficient to mediate PKA inhibition of HDAC5 nuclear export, we generated a GFP-tagged HDAC5-S280D mutant in which Ser280 was replaced with aspartic acid to mimic PKA-dependent phosphorylation. When this protein was expressed in Cos7 cells, we found that the GFP-HDAC5-S280D mutant is resistant to nuclear exclusion in response to PMA (Fig. 3 C and D). Similar results were observed when cardiomyocytes were infected with adenoviral GFP-HDAC5-S280D (SI Appendix, Fig. S14). Collectively, our results show that PKA-dependent phosphorylation of Ser280 mediates nuclear retention of HDAC5.
Fig. 3.
Phosphorylation of HDAC5 on Ser280 mediates the inhibition of HDAC5 nuclear export by the cAMP/PKA pathway. (A and B) Cos7 cells were transfected with GFP-HDAC5-WT or GFP-HDAC5-S280A, with or without HA-PKA-CA, and then were pretreated with cAMP followed by exposure to PMA for 3 h. cAMP and PKA-CA inhibited nuclear export of GFP-HDAC5-WT but not GFP-HDAC5-S280A by PMA. (C and D) Cos7 cells were transfected with GFP-HDAC5-WT or GFP-HDAC5-S280D and then were exposed to PMA for 3 h. GFP-HDAC5-S280D, but not GFP-HDAC5-WT, was resistant to PMA-induced nuclear export. *P < 0.05 versus without PMA; n = 4. (E) Cos7 cells were transfected with Flag-HDAC5-WT or Flag-HDAC5-S280A mutant and then were pretreated with forskolin followed by exposure to PMA. Phosphorylation of HDAC5 in cell lysates was analyzed by immunoblotting with phospho-specific HDAC5 antibodies that recognize the HDAC5 phosphorylated at Ser259 (E) and Ser498 (F). (F and G) Cos7 cells were transfected Flag-HDAC5-WT, Flag-HDAC5-S280A, or Flag-HDAC5-S280D and then were pretreated with forskolin, followed by exposure to PMA. Coimmunoprecipitation of Flag-HDAC5 in cell lysates and then immunoblotting with anti-14-3-3 and anti-Flag antibodies in immunoprecipitates were performed. The presence of14-3-3 protein in total cell lysates (5% of the input) was determined by immunoblotting with anti-14-3-3 antibodies. Representative blots are shown; n = 3.
PKA-Dependent Phosphorylation of HDAC5 Impairs the Association of HDAC5 and 14-3-3 Proteins.
To determine the potential mechanisms by which PKA-dependent phosphorylation of HDAC5 controls its subcellular localization, we examined whether PKA affected HDAC5 phosphorylation on Ser259 and Ser498 residues that are responsible for the recruitment of 14–3-3 proteins and subsequent nuclear export of HDAC5 (1, 24). Western blot analysis showed that PKA activators did not affect HDAC5 phosphorylation at Ser259 and Ser498 sites in response to PMA (Fig. 3 E and F). We observed similar results when Cos7 cells were cotransfected with the constitutively active PKD1-S738E/S742E mutant (10) and Flag-HDAC5-WT (SI Appendix, Fig. S15). These results indicate that there is no cross talk between these two functionally distinct phosphorylation events. Next, we examined whether PKA affected the recruitment of 14–3-3 proteins by HDAC5. Coimmunoprecipitation experiments showed that PKA stimulation markedly attenuated the PMA-induced association of HDAC5 and 14-3-3 proteins (Fig. 3F). However, PKA had no inhibitory effect on the association of HDAC5-S280A mutant and 14-3-3 proteins (Fig. 3F). Furthermore, HDAC5-S280D mutant prevented interaction with 14-3-3 proteins (Fig. 3G). These results demonstrate that PKA-dependent HDAC5 phosphorylation at Ser280 interferes with the interaction of HDAC5 and 14-3-3 proteins, resulting in the inhibition of the nuclear export of HDAC5. Because the Ser280 residue lies within the region of the NLS and between two 14-3-3 binding sites, Ser259 and Ser498 (Fig. 2A), we speculate that PKA-dependent Ser280 phosphorylation may change the conformation of HDAC5 and hence block 14-3-3 binding, resulting in the retention of HDAC5 in the nucleus (25).
PKA-Dependent Phosphorylation and Nuclear Retention of HDAC5 Repress MEF2-Dependent Gene Transcription and Cardiac Fetal Gene Expression.
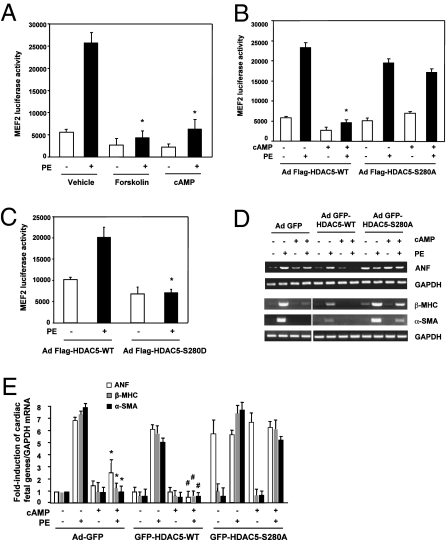
HDAC5 is highly expressed in the heart, skeletal muscle, vasculature, and brain (5, 10, 26). HDAC5 binds and represses MEF2 transcriptional factor to silence MFE2-dependent gene transcription programs that control cell differentiation and cell growth (6, 27, 28). To address the biological role of PKA-dependent HDAC5 phosphorylation, we examined the effects of the PKA activators and the HDAC5 mutants on MEF2 transcriptional activity. We used a luciferase reporter containing 3xMEF2 sites to assess MEF2 transcriptional activity. In NRVMs infected with adenovirus expressing GFP alone and in GFP-tagged HDAC5-WT, cAMP significantly inhibited PE-stimulated MEF2 transcriptional activity (Fig. 4A), a result that is consistent with previous reports showing that cAMP inhibits MEF2 activity in skeletal myocytes and neuronal cells (29, 30). Interestingly, coinfection by the adenoviral MEF2 luciferase construct and adenoviral GFP-HADC5-S280A abolished the cAMP-induced decrease in MEF2 transcriptional activation (Fig. 4B). Of note, overexpression of HDAC5-WT or mutant dose-dependently decreased endogenous HDAC5 expression (SI Appendix, Fig. S16), suggesting a dominant effect of infected HDAC5 on exogenous HDAC5. In contrast to infection with the HDAC5-S280A mutant, infection with adenoviruses expressing GFP-HADC5-S280D blocked PE-stimulated MEF2 transcriptional activity (Fig. 4C). These results indicate that cAMP/PKA-dependent phosphorylation and nuclear accumulation of HDAC5 negatively regulate MEF2 transcriptional activity.
Fig. 4.
PKA-dependent HDAC5 phosphorylation and nuclear retention repress MEF2-dependent gene transcription and cardiac fetal gene expression. (A–C) Luciferase reporter assays for MEF2 transcriptional activity. NRVMs were infected with adenoviruses expressing 3xMEF2-luciferase reporter gene (A) along with adenoviruses expressing Flag-HDAC5-WT or Flag-HDAC5-S280A (B) or Flag-HDAC5-S280D (C) and then were pretreated with forskolin or cAMP for 30 min, followed by stimulation with PE for 24 h. *P < 0.05 compared with PE + vehicle; n = 4. (D and E) RT-PCR analysis of cardiomyocyte fetal gene expression. NRVMs were infected with adenoviruses expressing GFP alone (control), GFP-HDAC5-WT, or GFP-HDAC5-S280A for 24 h and then were pretreated with cAMP, followed by stimulation with PE for 24 h. The mRNA was extracted from the cell lysates; then RT-PCR with the primers for ANF, β-MHC, α-SMA, and GADPH (internal control) was performed. *P < 0.05 versus PE + Ad-GFP; #P < 0.05 versus PE + GFP-HDAC5-WT; n = 4.
To determine the potential influence of PKA-induced HDAC5 phosphorylation on gene expression in NRVMs, we used RT-PCR and real-time PCR to study the expression of several cardiac fetal genes (hypertrophic marker genes) including atrial natriuretic factor (ANF), β-myosin heavy chain (β-MHC), and α-skeletal muscle actin (α-SMA) (5). Treatment with PE significantly increased expression of ANF, β-MHC, and α-SMA in cells infected with adenoviruses expressing GFP-tagged HDAC5-WT; cAMP treatment blocked this elevated gene expression (Fig. 4 D and E and SI Appendix, Fig. S17). In contrast, when cells were infected with adenoviruses expressing the HDAC5-S280A mutant, the inhibitory effect of PKA was markedly attenuated (Fig. 4 D and E). These results indicate that cAMP/PKA-dependent phosphorylation and nuclear accumulation of HDAC5 negatively regulate cardiac fetal gene expression.
PKA-Dependent Phosphorylation and Nuclear Retention of HDAC5 Inhibit Cardiomyocyte Hypertrophy.
Cardiac fetal gene expression contributes to cardiac growth and hypertrophy (4, 31–33). A major feature of the hypertrophic response of cardiomyocytes is a pronounced sarcomeric rearrangement and enlargement of cell size that can be detected by immunostaining with α-actinin antibody. Thus, we studied the effect of the PKA/HDAC5 pathway on cardiomyocyte hypertrophy. NRVMs were infected with adenoviruses expressing with GFP-tagged HDAC5-WT for 24 h and then were treated with cAMP for 30 min, followed by treatment with PE for 24 h. We found that PE treatment leads to increased cell size and nuclear export of HDAC5 (Fig. 5 A–C). However, the addition of cAMP decreased the size of cardiomyocytes, and HDAC5 was prevented from translocating from the nucleus to the cytoplasm (Fig. 5 A–C). Unlike the cells infected with GFP-tagged HDAC5-WT, the cells infected with adenoviruses expressing the GFP-tagged HDAC5-S280D mutant showed the nuclear accumulation of HDAC5-S280D and reduced cell size after PE treatment (Fig. 5 D–F and SI Appendix, Fig. S18). Furthermore, infection of GFP-tagged HDAC5-S280A attenuated the inhibitory effect of PKA on the PE-stimulated increase in cardiomyocyte size (SI Appendix, Fig. S19). Similar effects were observed when NRVMs were treated with angiotensin II instead of PE (SI Appendix, Fig. S20). These results show that PKA-dependent phosphorylation and nuclear retention of HDAC5 inhibit cardiomyocyte hypertrophy.
Fig. 5.
PKA-dependent HDAC5 phosphorylation and nuclear retention inhibit cardiomyocyte hypertrophy. (A–H) Cardiomyocyte size detected by immunostaining with anti-α-actinin antibody. NRVMs were pretreated with cAMP for 30 min and then were treated with PE for 24 h (A–C). NRVMs were infected by adenoviruses expressing GFP-HDAC5-WT or GFP-HDAC5-S280D (D–F) and then were pretreated with the vehicle (DMSO) as control, with cAMP for 30 min, followed by exposure to PE for 24 h. The cells were fixed and analyzed for GFP-HDAC5 localization, α-actinin staining (red), and nuclear DAPI staining (blue). *P < 0.05 versus without PE; #P < 0.05 versus with PE; n = 4. (G) Schematic of PKA-dependent regulation of HDAC5 subcellular localization and gene transcription.
The regulation by PKA of HDAC5 accumulation in the nucleus provides a mechanism for cell type-specific responses to extracellular stimulation. In contrast to the positive regulation of CREB-dependent gene transcription by the cAMP/PKA pathway (18–20), we show in this study that PKA phosphorylates HDAC5 and blocks its export from the cell nucleus, thereby negatively regulating MEF2-dependent gene expression and cardiomyocyte hypertrophy in response to stress signals (Fig. 5G and SI Appendix, Fig. S21). Interestingly, these two pathways are distinctly regulated by PKA, because HDAC5 has no inhibitory effect on CREB transcriptional activity (SI Appendix, Fig. S22). Given the important regulatory functions of cAMP/PKA and HDAC5/MEF2 signaling in cell differentiation, proliferation, morphogenesis, survival, and apoptosis in various tissues and systems (27), the identification of a molecular link between the two pathways may have broad implications for the regulation of a wide range of biological functions and human diseases such as cardiomyopathy, neural diseases, and metabolic disorders (34, 35).
In the heart, cAMP/PKA signaling that is activated via stimulation of β-ARs plays a key role in cardiac contractility through target proteins downstream of PKA (36, 37). In this study, we found that the cAMP/PKA pathway inhibited cardiac fetal gene expression and cardiomyocyte hypertrophy by affecting the subcellular localization of HDAC5. Consistent with our results, it has been shown that HDAC5-deficient mice developed cardiac hypertrophy under stress (26). It has been documented that sustained β-AR stimulation induces cardiomyocyte apoptosis and heart failure through cAMP/PKA-dependent and independent pathways (36–38). Antos et al. (39) reported that overexpression of the constitutively active PKA catalytic subunit in mouse heart led to dilated cardiomyopathy and cardiomyocyte hypertrophy, although there was no significant change in the heart-to-body weight ratio in PKA transgenic mice. Besides HDAC5, PKA has many other substrates including ryanodine receptor and phospholamban, L-type calcium channels, and cardiac troponin I (36). It is possible that pathways independent of HDAC5 may be involved in the cardiomyocyte hypertrophy induced in mice by sustained PKA activation (39, 40). In addition, we observed that the β-AR agonist isoproterenol inhibited the nuclear export of HDAC5 in cultured cardiomyocytes. However, long-term treatment with isoproterenol typically induced cardiac hypertrophy (36, 41). This discrepancy could result from the signaling complexity triggered by isoproterenol (37). Isoproterenol can bind to all three β-AR isoforms expressed in the heart, namely β1-AR, β2-AR, and β3-AR (36, 37, 42). Although β1-AR is coupled to Gsα that mediates classic cAMP/PKA signaling, β2-AR is coupled both to Gsα and to Giα, which mediates MAPKs and PI3K/Akt pathways. Selective β1-AR stimulation caused hypertrophy growth of ventricular cardiomyocytes by a mechanism that is independent of cAMP but dependent on a tyrosine kinase and CaMKII (38, 43). The MAPK pathway has been implicated in cardiac hypertrophy induced by β2-AR stimulation (44, 45). PI3Kγ, which is activated through Gi-associated Gβγ, plays an essential role in isoproterenol-induced cardiac hypertrophy and heart failure (46, 47). Although the net effect of isoproterenol through multiple signaling pathways is enhanced cardiac hypertrophy, our studies suggest a negative pathway for cardiomyocyte hypertrophy in isoproterenol signaling that may provide a means for manipulating isoproterenol/β-AR responses in heart. Notably, down-regulation and desensitization of β-AR are hallmarks of the failing heart (36, 48). Clinically, the use of β-AR blockers has become the standard treatment for heart failure, but whether they act by blocking or resensitizing the β-AR system is still debated (36, 49). Whether there is any link between β-AR–blocker therapy and the PKA/HDAC5 pathway remains to be investigated. Collectively, our findings suggesting that the PKA/HDAC5 pathway is involved in regulating cardiac fetal gene expression and cardiomyocyte hypertrophy warrant further investigation and may lead to the design of therapeutic strategies for the prevention or treatment of cardiac hypertrophy and heart failure.
Materials and Methods
Detailed methods appear in the SI Appendix, SI Materials and Methods. These methods include materials, cell culture, plasmid transfection and adenoviral infection, fluorescence images, in vitro kinase assay, Western blot and immunoprecipitation, luciferase assay, RT-PCR, and statistical analysis.
Supplementary Material
Acknowledgments
We thank E. Olson (University of Texas Southwestern Medical Center, Dallas) and T. McKinsey (University of Colorado Cardiovascular Institute, Denver) for HDAC5 plasmids and pHDAC5 antibody, H. Kao (Case Western Reserve University, Cleveland) for HDAC7 plasmids, C. Yan (University of Rochester Medical Center, Rochester, NY) for PDE inhibitors, and J. O-Uchi (University of Rochester Medical Center) for helping isolate rat adult ventricular myocytes. We also thank J. Sottile (University of Rochester Medical Center) for a critical reading of the manuscript. This work was supported by National Institutes of Health Grant HL80611 (to Z.G.J.), Thomas R. Lee Award 1-06-CD-13 from the American Diabetes Association (to Z.G.J.), Grant-in-Aid Award 0755916T from the American Heart Association (to Z.G.J.), an American Heart Association Postdoctoral Fellowship (to C.H.H.), and an American Heart Association Predoctoral Fellowship (to W.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1000462107/-/DCSupplemental.
References
- 1.McKinsey TA, Zhang CL, Lu J, Olson EN. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature. 2000;408:106–111. doi: 10.1038/35040593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–352. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- 3.Yang XJ, Grégoire S. Class II histone deacetylases: From sequence to function, regulation, and clinical implication. Mol Cell Biol. 2005;25:2873–2884. doi: 10.1128/MCB.25.8.2873-2884.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: Implications for disease and therapy. Nat Rev. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang CL, et al. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell. 2002;110:479–488. doi: 10.1016/s0092-8674(02)00861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu J, McKinsey TA, Zhang CL, Olson EN. Regulation of skeletal myogenesis by association of the MEF2 transcription factor with class II histone deacetylases. Mol Cell. 2000;6:233–244. doi: 10.1016/s1097-2765(00)00025-3. [DOI] [PubMed] [Google Scholar]
- 7.Vega RB, et al. Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell. 2004;119:555–566. doi: 10.1016/j.cell.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 8.Chang S, et al. Histone deacetylase 7 maintains vascular integrity by repressing matrix metalloproteinase 10. Cell. 2006;126:321–334. doi: 10.1016/j.cell.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 9.Berdeaux R, et al. SIK1 is a class II HDAC kinase that promotes survival of skeletal myocytes. Nat Med. 2007;13:597–603. doi: 10.1038/nm1573. [DOI] [PubMed] [Google Scholar]
- 10.Ha CH, et al. Protein kinase D-dependent phosphorylation and nuclear export of histone deacetylase 5 mediates vascular endothelial growth factor-induced gene expression and angiogenesis. J Biol Chem. 2008;283:14590–14599. doi: 10.1074/jbc.M800264200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kao HY, et al. Mechanism for nucleocytoplasmic shuttling of histone deacetylase 7. J Biol Chem. 2001;276:47496–47507. doi: 10.1074/jbc.M107631200. [DOI] [PubMed] [Google Scholar]
- 12.Ago T, et al. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell. 2008;133:978–993. doi: 10.1016/j.cell.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 13.Carnegie GK, et al. AKAP-Lbc mobilizes a cardiac hypertrophy signaling pathway. Mol Cell. 2008;32:169–179. doi: 10.1016/j.molcel.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vega RB, et al. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol Cell Biol. 2004;24:8374–8385. doi: 10.1128/MCB.24.19.8374-8385.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parra M, Mahmoudi T, Verdin E. Myosin phosphatase dephosphorylates HDAC7, controls its nucleocytoplasmic shuttling, and inhibits apoptosis in thymocytes. Genes Dev. 2007;21:638–643. doi: 10.1101/gad.1513107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beavo JA, Brunton LL. Cyclic nucleotide research—still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3:710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- 17.Rehmann H, Wittinghofer A, Bos JL. Capturing cyclic nucleotides in action: Snapshots from crystallographic studies. Nat Rev Mol Cell Biol. 2007;8:63–73. doi: 10.1038/nrm2082. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez GA, et al. A cluster of phosphorylation sites on the cyclic AMP-regulated nuclear factor CREB predicted by its sequence. Nature. 1989;337:749–752. doi: 10.1038/337749a0. [DOI] [PubMed] [Google Scholar]
- 20.Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- 21.Dequiedt F, et al. Phosphorylation of histone deacetylase 7 by protein kinase D mediates T cell receptor-induced Nur77 expression and apoptosis. J Exp Med. 2005;201:793–804. doi: 10.1084/jem.20042034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim C, Xuong NH, Taylor SS. Crystal structure of a complex between the catalytic and regulatory (RIalpha) subunits of PKA. Science. 2005;307:690–696. doi: 10.1126/science.1104607. [DOI] [PubMed] [Google Scholar]
- 23.Kurosawa A, Guidotti A, Costa E. Induction of tyrosine 3-monooxygenase in adrenal medulla: Role of protein kinase activation and translocation. Science. 1976;193:691–693. doi: 10.1126/science.7836. [DOI] [PubMed] [Google Scholar]
- 24.Grozinger CM, Schreiber SL. Regulation of histone deacetylase 4 and 5 and transcriptional activity by 14-3-3-dependent cellular localization. Proc Natl Acad Sci USA. 2000;97:7835–7840. doi: 10.1073/pnas.140199597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKinsey TA, Zhang CL, Olson EN. Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin-dependent protein kinase-stimulated binding of 14-3-3 to histone deacetylase 5. Proc Natl Acad Sci USA. 2000;97:14400–14405. doi: 10.1073/pnas.260501497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang S, et al. Histone deacetylases 5 and 9 govern responsiveness of the heart to a subset of stress signals and play redundant roles in heart development. Mol Cell Biol. 2004;24:8467–8476. doi: 10.1128/MCB.24.19.8467-8476.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Potthoff MJ, Olson EN. MEF2: A central regulator of diverse developmental programs. Development. 2007;134:4131–4140. doi: 10.1242/dev.008367. [DOI] [PubMed] [Google Scholar]
- 28.Liang FS, Crabtree GR. Developmental biology: The early heart remodelled. Nature. 2009;459:654–655. doi: 10.1038/459654a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du M, et al. Protein kinase A represses skeletal myogenesis by targeting myocyte enhancer factor 2D. Mol Cell Biol. 2008;28:2952–2970. doi: 10.1128/MCB.00248-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belfield JL, Whittaker C, Cader MZ, Chawla S. Differential effects of Ca2+ and cAMP on transcription mediated by MEF2D and cAMP-response element-binding protein in hippocampal neurons. J Biol Chem. 2006;281:27724–27732. doi: 10.1074/jbc.M601485200. [DOI] [PubMed] [Google Scholar]
- 31.Hunter JJ, Chien KR. Signaling pathways for cardiac hypertrophy and failure. N Engl J Med. 1999;341:1276–1283. doi: 10.1056/NEJM199910213411706. [DOI] [PubMed] [Google Scholar]
- 32.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 33.Mudd JO, Kass DA. Tackling heart failure in the twenty-first century. Nature. 2008;451:919–928. doi: 10.1038/nature06798. [DOI] [PubMed] [Google Scholar]
- 34.Vinge LE, Raake PW, Koch WJ. Gene therapy in heart failure. Circ Res. 2008;102:1458–1470. doi: 10.1161/CIRCRESAHA.108.173195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eschenhagen T. Beta-adrenergic signaling in heart failure-adapt or die. Nat Med. 2008;14:485–487. doi: 10.1038/nm0508-485. [DOI] [PubMed] [Google Scholar]
- 36.Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- 37.Xiao RP, et al. Subtype-specific alpha1- and beta-adrenoceptor signaling in the heart. Trends Pharmacol Sci. 2006;27:330–337. doi: 10.1016/j.tips.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Zhu WZ, et al. Linkage of beta1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J Clin Invest. 2003;111:617–625. doi: 10.1172/JCI16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antos CL, et al. Dilated cardiomyopathy and sudden death resulting from constitutive activation of protein kinase A. Circ Res. 2001;89:997–1004. doi: 10.1161/hh2301.100003. [DOI] [PubMed] [Google Scholar]
- 40.Marx SO, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): Defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 41.Antos CL, et al. Activated glycogen synthase-3 beta suppresses cardiac hypertrophy in vivo. Proc Natl Acad Sci USA. 2002;99:907–912. doi: 10.1073/pnas.231619298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiang Y, Kobilka BK. Myocyte adrenoceptor signaling pathways. Science. 2003;300:1530–1532. doi: 10.1126/science.1079206. [DOI] [PubMed] [Google Scholar]
- 43.Schäfer M, Frischkopf K, Taimor G, Piper HM, Schlüter KD. Hypertrophic effect of selective beta(1)-adrenoceptor stimulation on ventricular cardiomyocytes from adult rat. Am J Physiol Cell Physiol. 2000;279:C495–C503. doi: 10.1152/ajpcell.2000.279.2.C495. [DOI] [PubMed] [Google Scholar]
- 44.Ueyama T, et al. Requirement of activation of the extracellular signal-regulated kinase cascade in myocardial cell hypertrophy. J Mol Cell Cardiol. 2000;32:947–960. doi: 10.1006/jmcc.2000.1135. [DOI] [PubMed] [Google Scholar]
- 45.Zou Y, et al. Isoproterenol activates extracellular signal-regulated protein kinases in cardiomyocytes through calcineurin. Circulation. 2001;104:102–108. doi: 10.1161/hc2601.090987. [DOI] [PubMed] [Google Scholar]
- 46.Oudit GY, et al. Phosphoinositide 3-kinase gamma-deficient mice are protected from isoproterenol-induced heart failure. Circulation. 2003;108:2147–2152. doi: 10.1161/01.CIR.0000091403.62293.2B. [DOI] [PubMed] [Google Scholar]
- 47.Nienaber JJ, et al. Inhibition of receptor-localized PI3K preserves cardiac beta-adrenergic receptor function and ameliorates pressure overload heart failure. J Clin Invest. 2003;112:1067–1079. doi: 10.1172/JCI18213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 49.Tevaearai HT, Koch WJ. Molecular restoration of beta-adrenergic receptor signaling improves contractile function of failing hearts. Trends Cardiovasc Med. 2004;14:252–256. doi: 10.1016/j.tcm.2004.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.