Abstract
Background
HU a small, basic, histone like protein is a major component of the bacterial nucleoid. E. coli has two subunits of HU coded by hupA and hupB genes whereas Mycobacterium tuberculosis (Mtb) has only one subunit of HU coded by ORF Rv2986c (hupB gene). One noticeable feature regarding Mtb HupB, based on sequence alignment of HU orthologs from different bacteria, was that HupBMtb bears at its C-terminal end, a highly basic extension and this prompted an examination of its role in Mtb HupB function.
Methodology/Principal Findings
With this objective two clones of Mtb HupB were generated; one expressing full length HupB protein (HupBMtb) and another which expresses only the N terminal region (first 95 amino acid) of hupB (HupBMtbN). Gel retardation assays revealed that HupBMtbN is almost like E. coli HU (heat stable nucleoid protein) in terms of its DNA binding, with a binding constant (Kd) for linear dsDNA greater than 1000 nM, a value comparable to that obtained for the HUαα and HUαβ forms. However CTR (C-terminal Region) of HupBMtb imparts greater specificity in DNA binding. HupBMtb protein binds more strongly to supercoiled plasmid DNA than to linear DNA, also this binding is very stable as it provides DNase I protection even up to 5 minutes. Similar results were obtained when the abilities of both proteins to mediate protection against DNA strand cleavage by hydroxyl radicals generated by the Fenton's reaction, were compared. It was also observed that both the proteins have DNA binding preference for A:T rich DNA which may occur at the regulatory regions of ORFs and the oriC region of Mtb.
Conclusions/Significance
These data thus point that HupBMtb may participate in chromosome organization in-vivo, it may also play a passive, possibly an architectural role.
Introduction
The Escherichia coli (E. coli) protein HU (heat stable nucleoid protein) is an abundant DNA binding protein, which is a major component of the bacterial nucleoid [1]. HU is a small, basic, histone like protein, initially called factor U, isolated for the first time from E. coli strain U93 [1], [2]. In E. coli HU activity is comprised of a hetero-dimer of HupA (Hu α) and HupB (Hu β) coded by hupA and hupB genes, respectively. Besides this, homo-dimeric forms (Hu α2 and Hu β2) are also observed with different forms (mainly Hu α2 and Hu αβ) predominating in distinct phases of growth [3]. HU is highly conserved [4] and unlike most DNA binding proteins, binds to both single stranded (ss) and double stranded (ds) DNA in a sequence independent manner [5], [6]. The other major nucleoid associated proteins are FIS (factor for inversion stimulation), H-NS (histone like nucleoid protein), IHF (integration host factor) and a stationary phase specific DNA binding protein, DPS (DNA binding protein from starved cells [2], [7], [8]. HU, which resembles eukaryotic proteins of the high mobility group (HMG) class in terms of its DNA binding properties e.g. it binds dsDNA with low affinity and negligible sequence specificity; also displays high affinity for some altered DNA structures such as junctions, nicks, gaps, forks, and overhangs even under stringent salt conditions [9], [10]. In E. coli HU action is fairly pleiotropic in the sense that HU plays a role in many cellular processes such as site specific recombination [11], [12], initiation of DNA replication [13], [14], phage Mu transposition [15] and introducing negative supercoiling into relaxed DNA molecule in presence of Topoisomerase I [2]. Furthermore, cells deficient for HU are known to be highly sensitive to gamma and UV irradiation and it is thought that HU might assist in the processes of recombinational repair [11], [12].
HU also mediates ring closure of linear DNA enhancing DNA cyclization rates [16]. Crystal structure of Anabaena HU bound to DNA revealed that binding of dimers of HU to linear DNA with cohesive ends produces an overall bend of ∼105–140° thus stimulating rate of ring formation [17]. Once cohesive DNA ends are brought together they can be sealed by T4 DNA ligase [16], [18], [19]. E. coli HU binds to linear DNA fragments in a weakly cooperative fashion with one dimer occupancy per 9 bp and this binding is observed only under low salt conditions [20]. The binding of HU to a DNA four way junction is several orders of magnitude higher in comparison to binding to linear DNA even under high salt conditions [21] and is not inhibited by a large (100 fold) excess of competitor linear DNA. This binding preference is thought to be commensurate with the role of HU in DNA inversion [22].
Although in E. coli the heterodimeric state of HU is quite preponderant, in many bacterial species HU exists as a homodimer like in Bacillus subtilis [23], [24], Mycobacterial family etc. The annotated genome of Mycobacterium tuberculosis strain H37Rv, (Mtb) bears the potential to encode only one subunit of HU - the product of ORF Rv2986c (hupBMtb). The product of ORF Rv2986c is also referred to as mycobacterial DNA binding protein (MDP-1) or histone like protein (HLPMt) [25], [26]. HupBMtb is 214 amino acids long, has a high content of alanine (23.78%) and lysine (18.93%) and has a calculated pI of 12.4 with the ratio of basic to acidic amino acid residues being 12. This ratio in HupA and HupB of E. coli is 1.4, while for histone H1 or H5 this ratio is approximately 7 [25]. The N-terminal portion of HupBMtb exhibits significant homology to histone like proteins of E. coli while the C-terminal part displays homology to eukaryotic H1 histone [25]. Sequence alignment of HU homolog's from different members of the Mtb complex shows that N terminal end of HupB is conserved, but the C-terminal end is variable (Supplementary Figure S2) and this feature has therefore been used as a diagnostic marker for differentiating members of Mtb complex [27]. HupBMtb, like other bacterial HU proteins, lacks tryptophan, cysteine and tyrosine residues. These properties and an overall homology to bacterial HU suggest that HupBMtb could be involved in the packaging of mycobacterial DNA and functions as a nucleoid associated protein. Interestingly HupBMtb interacts with the immune system, an interaction that may occur due to release of protein during natural cell lysis or release resulting from interaction with the host immune system [25], [28]. Recently HupBMtb ortholog in M. smegmatis has been shown to possess DNA end joining/ring closure protein activity [19].
In this report we describe biochemical studies on recombinant, purified HupBMtb and show that it displays properties of a nucleoid associated protein in terms of non-specific DNA binding activity. By undertaking comparative studies on HupBMtb and a derivative that lacks 119 amino acids located at the C-terminal end, we provide evidence to show that this C-terminal region (CTR) of HupBMtb is required for providing increased specificity of DNA binding and for specific recognition of altered nucleic acid structures under stringent (high salt) conditions. DNA binding by HupBMtb is stable and, in contrast to its derivative lacking the CTR, protects DNA from attack by reactive oxygen species produced by the Fenton's reagent and also affords substantial protection against cleavage by DNaseI. HupBMtb displayed substantial preference in binding to AT rich DNA. These observations suggest that HupBMtb action is different from enterobacterial HU and the CTR of HupBMtb may act like a DNA clasp. Given its increased preference to bind to AT rich DNA it is speculated that the site(s) of HupBMtb action on the Mtb genome could be AT rich regions that are found in the replication origin and regulatory regions of genes.
Results
Presence of a basic CTR in HupB orthologs from the mycobacterial clade
As noted previously a very visible feature of the polypeptide sequence of HupB orthologs belonging to the mycobacterial clade is the presence of a basic CTR [25], [27]. Member proteins of this clade are roughly 214 amino acids long whereas E. coli HupA and HupB are 90 amino acids long. The N terminal region of HupBMtb shows significant homology to individual subunits of enterobacterial HU namely HupA, HupB from E. coli, HupB from S. enterica and HU from B. subtilis (Supplementary Figure S1A), whereas the CTR despite retaining its overall basic character displays some variation in amino acid composition within members of the mycobacterial clade (Supplementary Figure S2). Amino acid sequence of the HupBMtb CTR shows that it is rich in lysine and alanine. It has six PAKK and one KAAK repeats (Supplementary Figure S1A and Supplementary S1B,) which are also present in histone H1, and are known to facilitate DNA binding [29]. The CTR of HupBMtb also shows significant homology to sea urchin histone H1 (Supplementary Figure S1B) primarily in terms of the presence of these tetrapeptide repeats. In order to determine the specific function related to this CTR, two hupBMtb ORFs were generated by PCR; one bearing the full length hupBMtb DNA sequence (encoding HupBMtb) and another encoding a hupBMtb variant bearing only the N-terminal 95 amino acids (HupBMtbN).
The CTR of HupBMtb imparts increased specificity in DNA binding
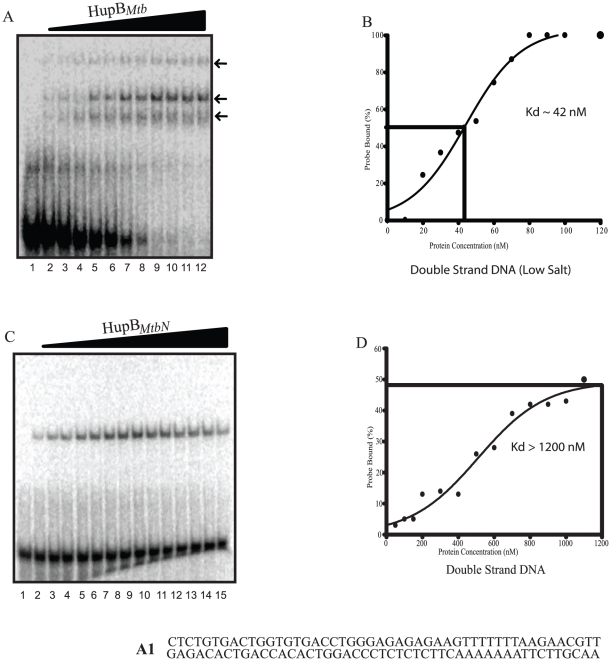
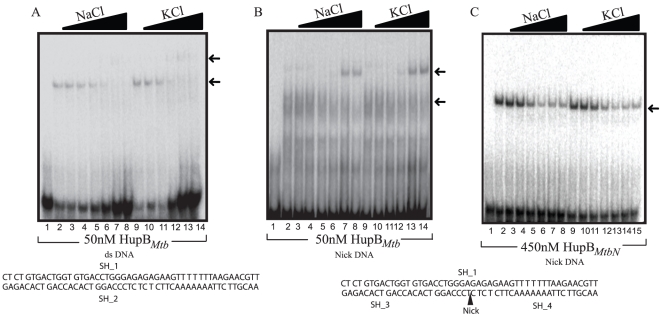
Under low salt conditions (10 mM NaCl or KCl) HupBMtb bound to linear ds DNA (ds oligonucleotide A1) in a weakly cooperative manner with a Kd of 42 nM whereas even at 1200 nM HupBMtbN barely displayed 50% binding to A1. (Figure 1A and 1B). For HupBMtb linear ds DNA interaction three retarded complexes (Figure 1A shown by arrowheads) were apparent with complex 2 being the major species. However with HupBMtbN only a single band could be observed (Figure 1C) which shows that the binding is non-cooperative. Based upon the length of ds DNA used herein (that is 48 bp) and assuming that retardation is provoked by dimeric HupBMtb, one can roughly estimate that HupBMtb binds to linear DNA with one dimer occupying DNA ranging from 16 to 24 bp. Binding of HupBMtb (at 50 nM) to A1 under increasing salt concentrations revealed that HupBMtb binding was greatly impaired at salt concentrations above 150 mM (NaCl or KCl; Figure 2A). E. coli HU is known to bind with duplex DNA containing a nick or a gap of one or two nucleotides with high affinity [30]. A specific DNA protein complex (Kd 65 nM), under high salt conditions was detected when the interaction of HupBMtb with linear DNA bearing a nick (ds oligonucleotide A2) was studied (Figure 2B). On the other hand, binding of higher concentrations of HupBMtbN (employed at 450 nM) promoted binding to A2 under low salt conditions, which was impeded under high salt conditions (Figure 2C). Under low salt HupBMtbN bound to nicked DNA with a Kd of 1000 nM (Table 1). HU is known to bind specifically to DNA containing either nick or a gap or DNA junction (cruciform DNA) without sequence preference [9], [10], [30]. These structures are associated with DNA damage and repair.
Figure 1. HupBMtb shows higher binding affinity to double stranded DNA.
Comparative gel retardation analysis showing the binding of increasing amounts of (A) HupBMtb (0–120 nM, lanes 1–12) and (C) HupBMtbN (0–1200 nM, lanes 1–15) to radioactively labeled ds DNA under low salt conditions (10 mM KCl) (sequences shown in Table 2). Dissociation constants (Kd) were also calculated (B) for HupBMtb and (D) for HupBMtbN binding to ds DNA. The Kd values are indicated as inset in the respective panels. Sequence of the oligonucleotide A1 used here is provided at the bottom of the figure.
Figure 2. HupBMtb binds specifically to nick DNA under high salt conditions.
Comparative gel retardation analysis showing the binding of (A) 50 nM of HupBMtb under increasing salt concentration (0, 10, 50, 100, 150, 200 and 250 mM) of either NaCl (lanes 1–7) or KCl (lanes 8–14) as indicated in figure, to DNA (Table 2) ds or (B) nick DNA. (C) 450 nM of HupBMtbN binding to nick DNA under increasing salt concentration (0, 10, 50, 100, 150, 200 and 250 mM) of either NaCl or KCl as indicated in figure. Position of the nick in the DNA is marked by an arrowhead.
Table 1. Binding constants (Kd) of HupBMtb and HupBMtbN to different structures of nucleic acid.
| DNA Structure | HupBMtb | HupBMtbN | |
| Kd (200 mM KCl) | Kd (10 mM KCl) | Kd (10 mM KCl) | |
| ds DNA | - | 42 nM | >1200 nM |
| Nick DNA | 65 nM | - | 1000 nM |
| 1 nucleotide gap DNA | - | 55 nM | 725 nM |
| 2 nucleotide gap DNA | 88 nM | 60 nM | 536 nM |
| Cruciform DNA | 66 nM | 60 nM | 160 nM |
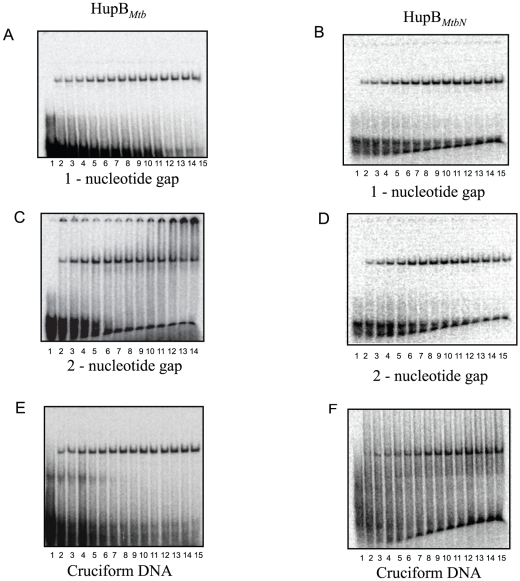
In order to address the question whether HupBMtb and HupBMtbN displayed differential binding we studied their interaction with linear ds DNA bearing one or two nucleotide gap and cruciform DNA, represented by ds oligonucleotides A3, A4 and A5 respectively, in the presence of low salt (10 mM KCl) (Supplementary Figure S3 and Supplementary Figure S4; for details refer Supplementary Methods S1). It is apparent (Figure 3; Table 1) that the CTR of HupBMtb is required for specific recognition and high affinity recognition of a duplex DNA containing a nick or a gap of one or two nucleotides and cruciform DNA.
Figure 3. HupBMtb binds with high affinity to 1-nucleotide gap, 2-nucleotide gap and cruciform DNA under low salt (10 mM KCl) in comparison to HupBMtbN.
Gel retardation analysis showing the binding of increasing amounts of (A) HupBMtb (0–210 nM) and (B) HupBMtbN (0–1200 nM) to 1-nucleotide gap DNA (C) HupBMtb (0–210 nM) and (D) HupBMtbN (0–1200 nM) to 2-nucleotide gap DNA (E) HupBMtb (0–210 nM) and (F) HupBMtbN (0–1200 nM) to cruciform DNA. The gel retardation conditions are described in Materials and Methods.
Interaction of HupBMtb with supercoiled plasmid DNA
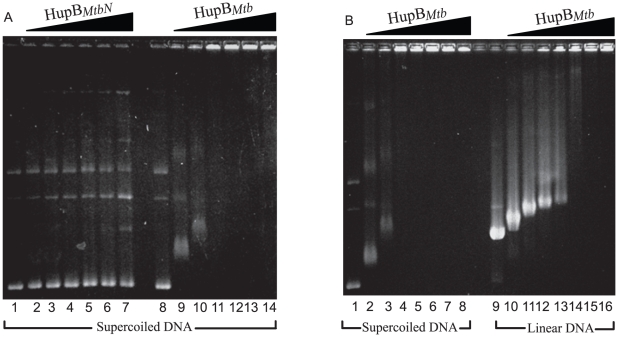
Despite a lack of sequence specificity in DNA binding, HU is known to bind preferentially to negatively supercoiled DNA [17], [31]. The interaction of HupBMtb and HupBMtbN with supercoiled pBluescriptSK (pBSK) plasmid DNA was studied in buffer conditions such that the molar ratio of protein to DNA ranged from 15∶1 to 180∶1. It could be seen that HupBMtbN displayed a markedly reduced interaction with supercoiled plasmid DNA (Figure 4A, lanes 1–7). Even at the highest protein to DNA molar ratio sufficient amount of unbound pBSK plasmid was visible (Figure 4A, lane 7). HupBMtb on the other hand bound very proficiently with supercoiled DNA, in the sense that at intermediate protein to DNA molar ratio the intensity of DNA protein complex was large and the DNA remained lodged in the wells Figure 4A, lanes 11 to 14). This property is reminiscent of proteins that can induce DNA compaction [32]. In order to test the preference of HupBMtb towards supercoiled/linear DNA, pBluescript SK (pBSK) plasmid was linearized using EcoR1. EMSA was carried out with supercoiled pBSK (Figure 4B, lanes 1–8) and with linear pBSK (lanes 9–16). It was observed that interaction of HupBMtb with supercoiled pBSK at a molar ratio (protein: DNA) of 75∶1 the intensity of DNA protein complex was large and the DNA remained lodged in the well (lane 4). However, the interaction of HupBMtb with linear pBSK was observed at a molar ratio of 112.5∶1 (lane 14). It therefore appears that HupBMtb exhibits ∼1.5-fold higher affinity towards supercoiled plasmid DNA. Furthermore, the interaction of HupBMtb with supercoiled DNA was more avid than with linear DNA of the same size (Figure 4B lanes 9–16).
Figure 4. HupBMtb binds strongly to supercoiled and linear plasmid DNA in comparison to HupBMtbN as evident from agarose gel electrophoresis of pBluescript SK (linear and supercoiled).
A) 500 ng of supercoiled pBluescript SK plasmid was incubated with increasing amounts (0, 1.2, 0.5, 1.0, 1.5, 2.0 and 2.5 µM) of either HupBMtbN (lanes 1–7) or HupBMtb (lanes 8–14) in low salt conditions (10 mM KCl). The DNA-protein interaction products were resolved by electrophoresis on a 1% agarose gel buffered with 0.5× TBE at 35 V, 16–20 hrs. B) 500 ng of either supercoiled (lanes 1–8) or linear (lanes 9–16) pBluescript SK DNA was incubated with increasing amount of HupBMtb. The DNA-protein interaction products were resolved as described above.
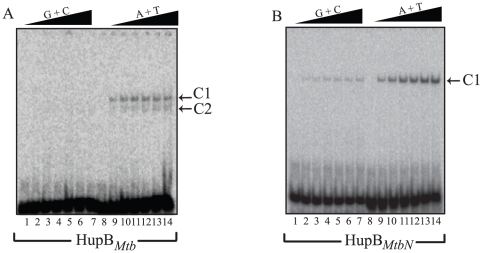
HupBMtb displays greater affinity in binding to AT rich DNA
Since the average GC content of the Mtb H37Rv genome works out to around 65%, we wondered whether the CTR of HupBMtb would endow it with the ability to interact more proficiently with GC rather than AT rich DNA. To assess whether HupBMtb bore this attribute, interaction of this protein was studied with ds DNA oligonucleotides A6, (GC content 84%) and A7 (AT content 64%). Surprisingly, HupBMtb interacted proficiently with AT rich DNA forming two gel-retarded complexes (C1, C2; Figure 5A marked by arrowheads) where as its interaction with GC rich DNA was negligible. On the other hand HupBMtbN while retaining its ability to interact more proficiently with AT rich DNA, forming a single gel retarded complex (Figure 5B, lanes 9–14), displayed weak interaction with GC rich DNA (Figure 5B, lanes 2–7). We then determined by circular dichroism spectrometry the secondary structure parameters of the two proteins, which revealed that the two proteins exhibited some differences in the content of alpha helix and beta sheet structures (Supplementary Figure S5; for details refer Supplementary Methods S1). Perhaps these differences may account for the ability of HupBMtbN to display binding to GC rich DNA.
Figure 5. HupBMtb displays higher affinity for AT rich DNA.
Electrophoretic mobility shift assay was carried out with GC rich ds DNA (A6) or AT rich ds DNA (A7) with increasing amount of either (A) HupBMtb (0–300 nM) or (B) HupBMtbN (0–300 nM). Lanes 1–7 of both the panels have GC rich oligo with protein concentrations ranging from 0, 50, 100, 150, 200, 250 and 300 nM. Similarly, lanes 8–14 of both the panels have AT rich oligo with protein concentrations ranging from 0, 50, 100, 150, 200, 250 and 300 nM, respectively.
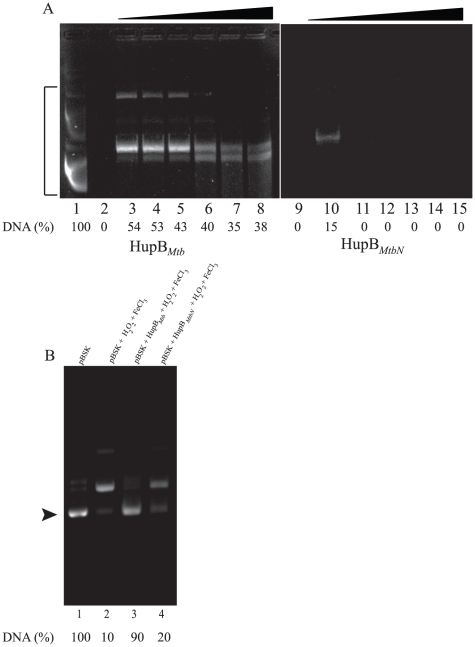
Enhanced DNA protection mediated by the CTR of HupBMtb
Given that the CTR of HupBMtb imparts upon it increased avidity with respect to DNA binding we tested whether the said region could protect DNA from enzymatic or non-enzymatic DNA strand breakage. In the first instance we tested the abilities of HupBMtb and HupBMtbN to mediate protection of DNA from cleavage by DNaseI. Supercoiled pBSK plasmid was exposed to 1 unit of DNase I for 30 seconds at 25°C in the presence of varying concentrations of HupBMtb and HupBMtbN (0.2–2.5 µM). Furthermore, timed digestions with DNase I were carried out with 2.5 µM of HupBMtb and HupBMtbN (Figure 6A and 6B). While both proteins at 2.5 µM gave substantial protection to DNA from DNase I cleavage (data not shown), analysis of timed digestion with DNaseI revealed that the binding of HupBMtb was stable enough to provide protection from DNase I even up to 5 min (Figure 6A, lane 8). However HupBMtbN was significantly impaired in mediating protection from DNase I, and could not provide protection beyond 30 sec (Figure 6A, lanes 11–15), as could be seen from the absence of DNA band (lanes 11–15). Similar results were obtained when the abilities of both proteins to mediate protection against DNA strand cleavage by hydroxyl radicals generated by the Fenton's reaction, were compared. HupBMtb was significantly more proficient than HupBMtbN in preventing DNA strand cleavage upon exposure to the Fenton system (Figure 6B). These studies thus suggest that the CTR of HupBMtb promotes associations with DNA that are stable enough to promote substantial DNA protection.
Figure 6. CTR of HupBMtb provides protection from DNaseI.
(A) Timed digestion of supercoiled pBSK DNA with DNase I was carried out with 2.5 µM of HupBMtb (A, lanes 1–8) or HupBMtbN (lanes 9–15). Lanes 1, 2 and 9 have no protein while lanes 3–8 and lanes 10–15 have 2.5 µM of HupBMtb and HupBMtbN, respectively. The reaction was initiated by the addition of one unit of DNaseI to all the lanes except lane 1. Incubation time with DNaseI in lanes 2–3 and 9–10 was 30 sec; lanes 4 and 11 for 1 min; lanes 5 and 12 for 2 min; lanes 6 and 13 for 3 min, lanes 7 and 14 for 4 min and lanes 8 and 15 for 5 min respectively. Area quantitated in this figure is marked by bracket and the amount of DNA (%) in each lane is indicated at the bottom of figure. (B) Protection of DNA from H2O2 mediated damage. Lane 1, pBSK DNA alone; lane 2, pBSK treated with FeCl3 and H2O2; lane 3, first incubated with HupBMtb and then treated with FeCl3 and H2O2 and lane 4, first incubated with HupBMtbN and then treated with FeCl3 and H2O2 as indicated in material and methods. The lower band in Figure 6B represents supercoiled DNA while the upper band represents relaxed DNA. The band marked by arrowhead was quantitated and the amount of DNA (%) in each lane is indicated at the bottom of figure.
Discussion
In bacteria the chromosomal DNA is present in a highly compacted form, called the bacterial nucleoid, in which DNA exists in association with basic low molecular weight proteins. Unlike that seen for eukaryotic chromatin, bacterial chromosome lacks perceptible DNA protein organization. In the well-studied case of E. coli, low molecular weight proteins such as H-NS, HU, IHF and FIS are thought to be the key protein constituents of the bacterial nucleoid [7], [8], [33], [34]. While both HU and H-NS are very abundant, HU has been considered to be a prokaryotic histone, primarily based upon its resemblance to eukaryotic histones in terms of its non-specific DNA binding capacity and amino acid sequence composition [2], [8]. HU is highly conserved and in enterobacteriacae HU exists as a heterodimer comprising of two homologous protein subunits, Huα and Huβ. The heterodimeric state of HU appears to be restricted to enterobacteriacae, whereas in many other bacteria HU exists as a homodimer.
In this study we have examined the biochemical properties of the HU ortholog from M. tuberculosis (Mtb) strain H37Rv, the product of ORF Rv2986c, designated as HupBMtb. One noticeable feature regarding HupBMtb, based upon sequence alignment of HU orthologs from different bacteria, was the presence at its C-terminal end a highly basic extension (Supplementary Figure S1), designated as the C-terminal region (CTR), which prompted us to examine its role. Using purified recombinant HupBMtb and HupBMtbN, a variant of the former that lacks the CTR, comparative biochemical studies were undertaken. HupBMtb and HupBMtbN exhibited HU-like DNA binding properties, in that both displayed non-specific DNA binding with the difference that HupBMtb was more proficient in binding to DNA than HupBMtbN in several DNA binding assays employed. These results therefore suggest that the CTR of HupBMtb imparts upon it greater specificity in DNA binding (Figure 1 and Figure 2). Recently, HU ortholog from M.smegmatis has been shown to possess similar properties [19]. It is worth noting that HupBMtbN is almost E. coli HU- like in terms of its DNA binding, its DNA binding constant (Kd) with linear dsDNA is greater than 1000 nM, a value comparable to that obtained for the HUαα and HUαβ forms [10]. Furthermore, HupBMtbN like E. coli HU displayed comparatively greater affinity towards DNA bearing a nick or gap and cruciform DNA (Supplementary Figure S4, Table 1). In all these instances DNA binding affinities of HupBMtb were comparatively greater. These observations suggest that the basic or skeletal DNA binding property of HupBMtb resides in its first 90 amino acid residues and that the basic CTR acts to enhance DNA binding.
Recent crystal structure studies of Anabaena HU (homodimeric protein of size similar to and homologous to E. coli HU) have shown that in the HU dimer, two monomers associate to form an α-helical base extending outwards from each monomer and contribution from its C-terminal region is a largely β-sheeted structure (extends as two β-ribbon arms that contact the DNA within its minor groove) [17], [35]. The N-terminal half of each monomer contributes the α-helical base. The presence of a highly conserved proline residue at position 63 (present in HupBMtb and HupBMtbN, Supplementary Figure S1) at the tip of each arm is thought to be critical for DNA binding. Given these observations, it is possible to place the presence of the basic DNA binding property of HupBMtb in its N-terminal 90 amino acids. HupBMtb displayed more stable DNA associations than HupNMtbN, HupBMtb bound more proficiently to supercoiled plasmid DNA and, commensurate with this property, mediated enhanced protection of DNA from enzymatic or non-enzymatic DNA strand breakage (Figure 4). From these studies it appears that the CTR of HupBMtb may act like a DNA clasp promoting more stable DNA interactions. It is apparent that though presence of CTR lowers the Kd of dsDNA binding by at least one order of magnitude in comparison to HupBMtbN, (and E. coli HU), the Kd (42 nM for HupBMtb) is not lowered to the extent expected for a site specific DNA binding protein. Thus, HupBMtb bears the propensity to function as a nucleoid-associated protein in its natural setting.
An interesting finding was that HupBMtb interacted much more proficiently with substantially A:T rich than with G:C rich DNA. The G:C content of the Mtb genome is around 65% and is fairly uniform. Admittedly the G:C content of the dsDNA oligonucleotide A13 used in these studies is high (84%), nevertheless it is present in the Mtb genome located upstream of the ORF Rv494. On the other hand HupBMtb bound proficiently with dsDNA oligonucleotide A1 that constitutes a DNA sequence located within the origin of chromosomal replication on the Mtb genome, present between the dnaA and the dnaN genes. Recently we showed that Mtb DnaA in-vitro can mediate DNA duplex unwinding in the vicinity of this sequence [36]. At the E. coli origin of replication oriC, strand opening by DnaA is known to require the presence of either HU or IHF [14]. It is thus possible that the site(s) of biological HupBMtb activity in Mtb may be restricted to A:T rich regions, at least one that is present in the origin of replication where HupBMtb could promote efficient DnaA mediated helix unwinding and/or to other regions of limited A:T richness that may occur at the regulatory regions of ORFs. It is interesting to note that HupBMtb is considered to be essential for mycobacterial growth, an observation that is apparently compatible with its suspected role in DNA replication [37]. Future studies in this regard, directed towards exploring the possible requirement of HupBMtb in the initiation of chromosomal DNA replication are underway.
Materials and Methods
Cloning and purification of recombinant His tagged HupBMtb and HupBMtbN protein
The M. tuberculosis ORF Rv2986c coding for HupBMtb and HupBMtbN protein was PCR amplified using genomic DNA from H37Rv as a template and primers HupBMtb _F, HupBMtb _R and HupBMtbN_R, carrying specific restriction enzyme sites (Table 2), by Accutaq DNA polymerase (Sigma). The amplicons thus generated were digested with Nde1/HindIII restriction enzymes and cloned into the corresponding sites of pET28a expression vector. The resultant plasmids were labeled as pETHupBMtb and pETHupBMtbN. Restriction analysis and DNA sequencing confirmed the authenticity of all constructs. Recombinant HupBMtb and HupBMtbN, coded by M.tb ORF Rv2986c, were purified from the soluble fraction of BL21 (DE3) cells transformed with pETHupBMtb and pETHupBMtbN. The transformants were grown overnight at 18°C and induced with 0.15 mM IPTG at an OD600 of 0.2 for the expression of recombinant protein as described earlier [38], [39]. The recombinant protein was purified in buffer having 30 mM Tris (pH 8.5), 5 mM MgCl2, 250 mM NaCl, 100 mM potassium glutamate and 7% glycerol. SDS PAGE confirmed the purity of the protein. The concentration of the protein was estimated by BCA (Bichinconic acid) and protein was stored at −20°C until further use.
Table 2. Sequence of oligonucleotides used for Electrophoretic Mobility Shift Assays.
| Oligonucleotide name | Oligonucleotide sequence | Oligonucleotide length |
| HupBMtb _F | GGAATTCATATGAACAAAGCAGAGCTCATTGACG | 34 |
| HupBMtb _R | GCAAGCTTCTATTTGCGACCCCGCCGA | 27 |
| HupBMtbN_R | GCAAGCTTCTAGAGACGCTGCGCGCCAGAC | 31 |
| SH_1 | CTCTGTGACTGGTGTGACCTGGGAGAGAGAAGTTTTTTTAAGAACGTT | 48 |
| SH_2 | AACGTTCTTAAAAAAACTTCTCTCTCCCAGGTCACACCAGTCACAGAG | 48 |
| SH_3 | GAGACACTGACCACACTGGACCCT | 24 |
| SH_4 | CTCTCTTCAAAAAAATTCTTGCAA | 24 |
| SH_5 | TCTCTTCAAAAAAATTCTTGCAA | 23 |
| SH_6 | CTCTTCAAAAAAATTCTTGCAA | 22 |
| SH_7 | GAACTGACCGGACTGGACGAGCGCGAGAGAAGTTTTTTTAAGAACGTT | 48 |
| SH_8 | AGGACGGCAATTACTCGCCGCAGCGCGCTCGTCCAGTCCGGTCAGTTC | 48 |
| SH_9 | CTCTGTGACTGGTGTGACCTGGGAGCTGCGGCGAGTAATTGCCGTCCT | 48 |
| SH_10 | GCCCGGCTGCACCGCGCCACCGCGG | 25 |
| SH_11 | CCGCGGTGGCGCGGTGCAGCCGGGC | 25 |
| SH_12 | TCAAATCTAATCGGAGTCGTTTTGA | 25 |
| SH_13 | TCAAAACGACTCCGATTAGATTTGA | 25 |
Electrophoretic mobility shift assays
The interaction of increasing amounts of protein (0.2–2.5 µM) (HupBMtb and HupBMtbN) with 480 ng of supercoiled or linearized plasmid DNA (pBSK linearized with EcoR1) was carried out in buffer A (20 mM HEPES-KOH pH 7.5, 5 mM magnesium acetate, 1 mM EDTA, 0.05 mM bovine serum albumin and 7% glycerol). The reactions were incubated at 25°C for 30 min and the products resolved by electrophoresis on a 1% agarose gel in 0.5× TBE at 35 V for 16–20 hrs. The gels were visualized by staining with ethidium bromide.
The interaction of HU (HupBMtb and HupBMtbN) protein with dsDNA oligonucleotide probes was characterized by native PAGE. Increasing amounts of protein was incubated with 5′32P-labelled DNA for 30 min at room temperature in 20 µl of binding buffer A. The DNA protein complex were resolved on a 5% polyacrylamide gel (29∶1) buffered with 0.25× TBE as mentioned in figure legends. The binding of HU protein (HupBMtb and HupBMtbN) to nicked, 1-nt gap, 2-nt gap or cruciform DNA was carried out in buffer A, either in low salt (10 mM KCl) or in high salt (200 mM KCl) conditions. Any variations apart from the above-mentioned conditions are specified in the figure legends.
DNaseI protection Assay
The DNaseI protection assays were carried out in presence of 500 ng of pBluescriptK plasmid either in presence of increasing protein concentrations (HupB full length and mutant protein) (0.2–2.5 µM) or constant protein concentration (2.5 µM). The reactions were performed in buffer B (20 mM HEPES-KOH pH 7.5, 5 mM magnesium chloride, 1 mM EDTA, 0.05 mM bovine serum albumin and 7% glycerol) and were incubated for 30 min at 25°C. The DNaseI digestion was initiated by the addition of 1 unit of DNaseI (PROMEGA, USA) for 30 sec or an increasing time interval ranging from 30 sec to 5 minutes as mentioned in figure legends. The reactions were stopped by the addition of 3 µl of 20% SDS and 2 µl of 0.5 M EDTA. The reaction products were subsequently resolved by electrophoresis on 1% agarose gel in 1× TAE.
DNA protection from metal catalyzed oxidation system
The ability of HupBMtb and HupBMtbN to protect 500 ng of plasmid pBluescript II SK (+) from Metal catalyzed oxidation system (MCO) was tested in presence of either 1 µM of HupBMtb or HupBMtbN in a reaction volume of 15 µl for 30 minutes at room temperature. MCO (0.4 µM FeCl3, 10 mM DTT, 100 mM Ethanol and 2 mM H2O2) was added to the protein DNA complex and the reaction was further continued for 30 min. The reaction was terminated by the addition of 10 mM EDTA, phenol extracted and analyzed on a 1% agarose gel in 1× TAE at 7 V/cm for 30 minutes.
Supporting Information
A detailed description of method employed for multiple sequence alignment, calculation of dissociation constant and estimation of secondary structure of protein.
(0.05 MB DOC)
A) Sequence alignment of HU protein from M. tuberculosis (M._tb_HU), E. coli (E._coli), S. enterica (S._enterica_Hup) and B. subtilis (B._subtilis_HU) using Clustal W programme shows conservation of N terminal region. Completely conserved residues are shaded green, identical residues are shaded yellow, similar residues are shaded cyan and different residues are white. B) Sequence alignment between M. tuberculosis HupB (M._tb_HU) and sea urchin Histone H1 (Sea_Urchin_H1-g) revealed conservation of amino acids at C terminal region. The rectangular shaded boxes represent conserved PAKK and KAAK amino acid residues.
(0.66 MB PDF)
Amino acid sequence alignment of HupBMtb from M. tuberculosis (M.tb) with M. bovis, M. bovis BCG, M. ulcerans, M. marinum, M. leprae, M. kansasii, M. smegmatis, M. avium, M. parascrofula, M. gilvium, M. vanbaalenii and M. abscessus. Completely conserved residues are shaded green, identical residues are shaded yellow, similar residues are shaded cyan and different residues are white.
(1.35 MB PDF)
Plots indicating the half maximal saturation of A) 2- nucleotide gap DNA (high salt), B) 2- nucleotide gap DNA (low salt), C) Cruciform DNA (high salt), D) 1- nucleotide gap DNA (low salt), E) nick DNA (high salt), in presence of indicated HupBMtb. Respective Kd values are shown as inset in each panel.
(1.89 MB EPS)
Plots indicating the half maximal saturation of A) nick DNA B) 1- nucleotide gap DNA, C) 2- nucleotide gap DNA, D) Cruciform DNA, in presence of indicated HupBMtbN in low salt conditions. Respective Kd values are shown as inset in each panel.
(1.54 MB EPS)
Far UV CD spectrum analysis of HupBMtb and HupBMtbN was carried out as described in supplementary methods and shown by arrowheads. HupBMtb has 8.3% as α helix, 50.8% as β sheet, 7.6% as turn and 33.3% as random, however HupBMtbN has 16.9% as α helix, 35.2% as β sheet, 16.9% as turn and 31.1% as random.
(1.36 MB EPS)
Acknowledgments
We wish to thank Dr. Niyaz Ahmed and Dr. M. Krishnaveni for their help during this study.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was partly supported by research grant under the “Centre of Excellence” to SEH from the Department of Biotechnology (DBT), Ministry of Science and Technology, Government of India; SK and DB thank the Council of Scientific and Industrial Research (CSIR), Government of India for Senior Research Fellowships. KM and SEH are JC Bose National Fellows. SEH is a Robert Koch Fellow of the Robert Koch Institute, Berlin. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rouviere-Yaniv J, Gros F. Characterization of a novel, low-molecular weight DNA-binding protein from Escherichia coli . Proc Natl Acad Sci USA. 1975;72:3428–3432. doi: 10.1073/pnas.72.9.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rouviere-Yaniv J, Yaniv M, Germond JE. E. coli DNA binding protein HU forms nucleosomelike structure with circular double-stranded DNA. Cell. 1979;17:265–274. doi: 10.1016/0092-8674(79)90152-1. [DOI] [PubMed] [Google Scholar]
- 3.Claret L, Rouviere-Yaniv J. Variation in HU composition during growth of Escherichia coli: the heterodimer is required for long term survival. J Mol Biol. 1997;273:93–104. doi: 10.1006/jmbi.1997.1310. [DOI] [PubMed] [Google Scholar]
- 4.Aitken A, Rouviere-Yaniv J. Amino and carboxy terminal sequences of the DNA-binding protein HU from the Cyanobacterium Synechocystis PCC 6701 (ATCC 27170). Biochem Biophys Res Commun. 1979;91:461–467. doi: 10.1016/0006-291x(79)91544-4. [DOI] [PubMed] [Google Scholar]
- 5.Azam TA, Ishihama A. Twelve species of the nucleoid-associated protein from Escherichia coli. Sequence recognition specificity and DNA binding affinity. J Biol Chem. 1999;274:33105–33113. doi: 10.1074/jbc.274.46.33105. [DOI] [PubMed] [Google Scholar]
- 6.Wery M, Woldringh C, Rouviere-Yaniv J. HU-GFP and DAPI co-localize on the Escherichia coli nucleoid. Biochimie (Paris) 2001;83:193–200. doi: 10.1016/s0300-9084(01)01254-8. [DOI] [PubMed] [Google Scholar]
- 7.Azam TA, Hiraga S, Ishihama A. Two types of localization of the DNA-binding proteins within the Escherichia coli nucleoid. Genes Cells. 2000;5:613–626. doi: 10.1046/j.1365-2443.2000.00350.x. [DOI] [PubMed] [Google Scholar]
- 8.Drlica K, Rouviere-Yaniv J. Histonelike proteins of bacteria. Microbiol Rev. 1987;51:301–319. doi: 10.1128/mr.51.3.301-319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pontiggia A, Negri A, Beltrame M, Bianchi ME. Protein HU binds specifically to kinked DNA. Mol Microbiol. 1993;7:343–350. doi: 10.1111/j.1365-2958.1993.tb01126.x. [DOI] [PubMed] [Google Scholar]
- 10.Pinson V, Takahashi M, Rouviere-Yaniv J. Differential binding of the Escherichia coli HU, homodimeric forms and heterodimeric form to linear, gapped and cruciform DNA. J Mol Biol. 1999;287:485–497. doi: 10.1006/jmbi.1999.2631. [DOI] [PubMed] [Google Scholar]
- 11.Boubrik F, Rouviere-Yaniv J. Increased sensitivity to gamma irradiation in bacteria lacking protein HU. Proc Natl Acad Sci U S A. 1995;92:3958–3962. doi: 10.1073/pnas.92.9.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li S, Waters R. Escherichia coli Strains Lacking Protein HU Are UV Sensitive due to a Role for HU in Homologous Recombination. J Bacteriol. 1998;180:3750–3756. doi: 10.1128/jb.180.15.3750-3756.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bramhill D, Kornberg A. A model for initiation at origins of DNA replication. Cell. 1988;54:915–918. doi: 10.1016/0092-8674(88)90102-x. [DOI] [PubMed] [Google Scholar]
- 14.Hwang DS, Kornberg A. Opening of the replication origin of Escherichia coli by DnaA protein with protein HU or IHF. J Biol Chem. 1992;267:23083–23086. [PubMed] [Google Scholar]
- 15.Lavoie BD, Shaw GS, Millner A, Chaconas G. Anatomy of a flexer-DNA complex inside a higher-order transposition intermediate. Cell. 1996;85:761–771. doi: 10.1016/s0092-8674(00)81241-6. [DOI] [PubMed] [Google Scholar]
- 16.Hodges-Garcia Y, Hagerman PJ, Pettijohn DE. DNA ring closure mediated by protein HU. J Biol Chem. 1989;264:14621–14623. [PubMed] [Google Scholar]
- 17.Swinger KK, Lemberg KM, Zhang Y, Rice PA. Flexible DNA bending in HU-DNA cocrystal structures. Embo J. 2003;22:3749–3760. doi: 10.1093/emboj/cdg351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stenzel TT, Patel P, Bastia D. The integration host factor of Escherichia coli binds to bent DNA at the origin of replication of the plasmid pSC101. Cell. 1987;49:709–717. doi: 10.1016/0092-8674(87)90547-2. [DOI] [PubMed] [Google Scholar]
- 19.Mukherjee A, Bhattacharyya G, Grove A. The C-terminal domain of HU-related histone-like protein Hlp from Mycobacterium smegmatis mediates DNA end-joining. Biochemistry. 2008;47:8744–8753. doi: 10.1021/bi800010s. [DOI] [PubMed] [Google Scholar]
- 20.Bonnefoy E, Rouvière-Yaniv J. HU and IHF, two homologous histone-like proteins of Escherichia coli, form different protein-DNA complexes with short DNA fragments. EMBO J. 1991;10:687–696. doi: 10.1002/j.1460-2075.1991.tb07998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamashev D, Balandina A, Rouviere-Yaniv J. The binding motif recognized by HU on both nicked and cruciform DNA. EMBO J. 1999;18:5434–5444. doi: 10.1093/emboj/18.19.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson RC, Bruist MF, Simon MI. Host protein requirements for in vitro site-specific DNA inversion. Cell. 1986;46:531–539. doi: 10.1016/0092-8674(86)90878-0. [DOI] [PubMed] [Google Scholar]
- 23.Micka B, Groch N, Heinemann U, Marahiel MA. Molecular cloning, nucleotide sequence, and characterization of the Bacillus subtilis gene encoding the DNA-binding protein HBsu. J Bacteriol. 1991;173:3191–3198. doi: 10.1128/jb.173.10.3191-3198.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross MA, Setlow P. The Bacillus subtilis HBsu protein modifies the effects of alpha/beta-type, small acid-soluble spore proteins on DNA. J Bacteriol. 2000;182:1942–1948. doi: 10.1128/jb.182.7.1942-1948.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prabhakar S, Annapurna PS, Jain NK, Dey AB, Tyagi JS, et al. Identification of an immunogenic histone-like protein (HLPMt) of Mycobacterium tuberculosis. Tuber Lung Dis. 1998;79:43–53. doi: 10.1054/tuld.1998.0004. [DOI] [PubMed] [Google Scholar]
- 26.Furugen M, Matsumoto S, Matsuo T, Matsumoto M, Yamada T. Identification of the mycobacterial DNA-binding protein 1 region which suppresses transcription in vitro. Microb Pathog. 2001;30:129–138. doi: 10.1006/mpat.2000.0416. [DOI] [PubMed] [Google Scholar]
- 27.Prabhakar S, Mishra A, Singhal A, Katoch VM, Thakral SS, et al. Use of the hupB gene encoding a histone-like protein of Mycobacterium tuberculosis as a target for detection and differentiation of M. tuberculosis and M. bovis. J Clin Microbiol. 2004;42:2724–2732. doi: 10.1128/JCM.42.6.2724-2732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller-Ortiz SL, Sepulveda E, Olsen MR, Jagannath C, Wanger AR, et al. Decreased infectivity despite unaltered C3 binding by a Delta hbhA mutant of Mycobacterium tuberculosis. Infect Immun. 2002;70:6751–6760. doi: 10.1128/IAI.70.12.6751-6760.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paci M, Pon CL, Losso MA, Gualerzi CO. Proteins from the prokaryotic nucleiod. High-resolution 1H NMR spectroscopic study of Escherichia coli DNA-binding proteins NS1 and NS2. Eur J Biochem. 1984;138:193–200. doi: 10.1111/j.1432-1033.1984.tb07899.x. [DOI] [PubMed] [Google Scholar]
- 30.Kamashev D, Rouviere-Yaniv J. The histone-like protein HU binds specifically to DNA recombination and repair intermediates. EMBO J. 2000;19:6527–6535. doi: 10.1093/emboj/19.23.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shindo H, Furubayashi A, Shimizu M, Miyake M, Imamoto F. Preferential binding of E.coli histone-like protein HU alpha to negatively supercoiled DNA. Nucleic Acids Res. 1992;20:1553–1558. doi: 10.1093/nar/20.7.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kar S, Choi EJ, Guo F, Dimitriadis EK, Kotova SL, et al. Right-handed DNA supercoiling by an octameric form of histone-like protein HU: modulation of cellular transcription. J Biol Chem. 2006;281:40144–40153. doi: 10.1074/jbc.M605576200. [DOI] [PubMed] [Google Scholar]
- 33.Pettijohn DE. Prokaryotic DNA in nucleoid structure. CRC Crit Rev Biochem. 1976;4:175–202. doi: 10.3109/10409237609105458. [DOI] [PubMed] [Google Scholar]
- 34.Dame RT. The role of nucleoid-associated proteins in the organization and compaction of bacterial chromatin. Mol Microbiol. 2005;56:858–870. doi: 10.1111/j.1365-2958.2005.04598.x. [DOI] [PubMed] [Google Scholar]
- 35.Swinger KK, Rice PA. Structure-based analysis of HU-DNA binding. J Mol Biol. 2007;365:1005–1016. doi: 10.1016/j.jmb.2006.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar S, Farhana A, Hasnain SE. In-vitro helix opening of M. tuberculosis oriC by DnaA occurs at precise location and is inhibited by IciA like protein. PLoS ONE. 2009;4:e4139. doi: 10.1371/journal.pone.0004139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sassetti CM, Boyd DH, Rubin EJ. Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol. 2003;48:77–84. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- 38.Ghosh S, Rasheedi S, Rahim SS, Banerjee S, Choudhary RK, et al. A novel method for enhancing solubility of the expressed proteins in E. coli. Biotechniques. 2004;37:418–424. doi: 10.2144/04373ST07. [DOI] [PubMed] [Google Scholar]
- 39.Prakash P, Aruna B, Sardesai AA, Hasnain SE. Purified recombinant hypothetical protein coded by open reading frame Rv1885c of Mycobacterium tuberculosis exhibits a monofunctional AroQ class of periplasmic chorismate mutase activity. J Biol Chem. 2005;280:19641–19648. doi: 10.1074/jbc.M413026200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A detailed description of method employed for multiple sequence alignment, calculation of dissociation constant and estimation of secondary structure of protein.
(0.05 MB DOC)
A) Sequence alignment of HU protein from M. tuberculosis (M._tb_HU), E. coli (E._coli), S. enterica (S._enterica_Hup) and B. subtilis (B._subtilis_HU) using Clustal W programme shows conservation of N terminal region. Completely conserved residues are shaded green, identical residues are shaded yellow, similar residues are shaded cyan and different residues are white. B) Sequence alignment between M. tuberculosis HupB (M._tb_HU) and sea urchin Histone H1 (Sea_Urchin_H1-g) revealed conservation of amino acids at C terminal region. The rectangular shaded boxes represent conserved PAKK and KAAK amino acid residues.
(0.66 MB PDF)
Amino acid sequence alignment of HupBMtb from M. tuberculosis (M.tb) with M. bovis, M. bovis BCG, M. ulcerans, M. marinum, M. leprae, M. kansasii, M. smegmatis, M. avium, M. parascrofula, M. gilvium, M. vanbaalenii and M. abscessus. Completely conserved residues are shaded green, identical residues are shaded yellow, similar residues are shaded cyan and different residues are white.
(1.35 MB PDF)
Plots indicating the half maximal saturation of A) 2- nucleotide gap DNA (high salt), B) 2- nucleotide gap DNA (low salt), C) Cruciform DNA (high salt), D) 1- nucleotide gap DNA (low salt), E) nick DNA (high salt), in presence of indicated HupBMtb. Respective Kd values are shown as inset in each panel.
(1.89 MB EPS)
Plots indicating the half maximal saturation of A) nick DNA B) 1- nucleotide gap DNA, C) 2- nucleotide gap DNA, D) Cruciform DNA, in presence of indicated HupBMtbN in low salt conditions. Respective Kd values are shown as inset in each panel.
(1.54 MB EPS)
Far UV CD spectrum analysis of HupBMtb and HupBMtbN was carried out as described in supplementary methods and shown by arrowheads. HupBMtb has 8.3% as α helix, 50.8% as β sheet, 7.6% as turn and 33.3% as random, however HupBMtbN has 16.9% as α helix, 35.2% as β sheet, 16.9% as turn and 31.1% as random.
(1.36 MB EPS)