Abstract
Electrospray ionization produces multiply charged ions, thereby lowering the mass-to-charge ratio for peptides and small proteins to a range readily accessed by quadrupole ion trap, orbitrap, and ion cyclotron resonance (ICR) mass analyzers (m/z = 400–2,000). For Fourier transform mass analyzers (orbitrap and ICR), higher charge also improves signal-to-noise ratio, mass resolution, and mass accuracy. Addition of m-nitrobenzyl alcohol (m-NBA) or sulfolane has previously been shown to increase the charge states of proteins. Moreover, polar aprotic dimethylformamide (DMF) improves chromatographic separation of proteolytic peptides for mass analysis of solution-phase protein hydrogen/deuterium exchange for improved (78–96%) sequence coverage. Here, we show that addition of each of various modifiers (DMF, thiodiglycol, dimethylacetamide, dimethylsulfoxide, and N-methylpyrrolidone) can significantly increase the charge states of proteins up to 78 kDa. Moreover, incorporation of the same modifiers into reversed-phase liquid chromatography solvents improves sensitivity, charging, and chromatographic resolution for intact proteins.
Keywords: Charge state, Fourier transform mass spectrometry, ion cyclotron resonance, FTMS, FT-ICR, quadrupole ion trap, orbitrap
INTRODUCTION
Electrospray ionization (ESI)1 coupled with mass spectrometry (MS) is extensively used for analysis of large biopolymers2 and protein complexes.3 The principal advantage of ESI is to form and detect multiply charged analyte molecules. Low flow ESI such as microelectrospray4 and nanoelectrospray5 further enhances ESI through improved ionization efficiency and sensitivity. ESI facilitates the detection of large biomolecules with quadrupole and trapped-ion mass analyzers by lowering the mass-to-charge ratio. Moreover, the charge state distribution is affected by analyte gas-phase basicity,6–8 solution pH,9 solvent composition,6 droplet size,10 instrument parameters,11 and protein conformation.12–17
Buffers with strong gas-phase basicity such as triethyl ammonium acetate/bicarbonate18 or methyl ammonium acetate19 have been reported to decrease charging of proteins. High gas-phase basicity additives, such as diethylamine and other bases can significantly reduce protein charging by removing protons.20, 21 Ion/molecule22 and ion/ion reactions23 with neutral bases or anions can reduce protein charge: an advantage for low-resolution mass analyzers, by reducing the number of charge states and thus simplifying the mass spectrum. Higher skimmer voltage or capillary temperature11 can further promote charge reduction due to collisions between analyte ions and neutrals or an electrode.
Higher charge state is desirable for peptides and proteins because: (a) Ion signal-to-noise ratio, mass resolving power, and mass accuracy are proportional to charge state for the highest resolution mass analyzers for which detection is based on induced-charge (orbitrap24 and ion cyclotron resonance25, 26) and (b) For both electron capture dissociation (ECD)27 and electron transfer dissociation (ETD),28 MS/MS efficiency increases as the square of ion charge.29 Attempts to increase peptide/protein charge state have included variation in solvent30 and acid concentration,12 as well as disulfide bond reduction by dithiothreitol (DTT).31 The charge state distribution for test proteins shifts to lower m/z with decreased tip orifice diameter.32 Addition of m-nitrobenzyl alcohol (m-NBA) to create charge enhancement, or “super-charging” has been systematically studied by Williams et al. under denaturing conditions.33–36 Loo et al. have characterized enhanced charging of proteins and protein complexes by m-NBA, analogous molecules, and sulfolane in non-denaturing solvent with ammonium acetate buffer.37, 38 For example, sulfolane produces a higher average charge state (61%) than m-NBA (21%) for myoglobin, under non-denaturing conditions.38 Jensen and coworkers reported improved ETD MS/MS efficiency for peptide sequencing and identification by addition of m-NBA to the mobile phase for on-line LC ETD MS/MS of tryptic peptides: to our knowledge, the first such use of a “supercharging” modifier. However, in that report, signal-to-noise ratio decreased due to greater chromatographic peak width and consequently lower peak height.39
Here, we report the screening of dimethylformamide (DMF), dimethylacetamide (DMAA), N-methylpyrrolidone (NMP), dimethylsulfoxide (DMSO), and thiodiglycol (TDG) “super-charging” reagents. Compared with conventional solvents (water/acetonitrile/formic acid), some of the modifiers improved the signal-to-noise ratio (S/N) by enhanced charging of intact proteins. Furthermore, DMF and DMSO are LC-compatible and improve chromatographic resolution for intact proteins as well. Finally, we discuss physico-chemical properties that may contribute to charge enhancement.
EXPERIMENTAL
Materials
Dimethylformamide, dimethylacetamide, N-methylpyrrolidone, dimethylsulfoxide, thiodiglycol, bovine ubiquitin (8.5 kDa), equine cytochrome c (12.8 kDa), chicken lysozyme (14.3 kDa), equine myoglobin (16.8 kDa), bovine carbonic anhydrase (29 kDa), and transferrin (78 kDa) were obtained from Sigma-Aldrich (St. Louis, MO, USA). HPLC grade water and acetonitrile were purchased from J. T. Baker (Philipsburg, NJ).
Sample Preparation
Protein samples (10 μM) were first prepared in conventional ESI solution: H2O/acetonitrile/formic acid, 50/50/0.5 (v/v/v). All protein samples were then diluted to 1 μM in conventional ESI solution with varying concentrations of DMF, DMAA, DMSO, TDG, NMP, and m-NBA to yield the same final protein concentration for ESI FT-ICR MS.
Direct Infusion, Liquid Chromatography, and Mass Spectrometry
Direct infusion was performed with an Advion Biosystems TriVersa Nanomate (Ithaca, NY).40 Liquid chromatography was performed with a Jasco HPLC/SFC instrument (Jasco, Easton, MD). Sample injection (10 μL of 500 fmol/μL protein mixture) was performed with a LEAP robot (HTS PAL, Leap Technologies, Carrboro, NC). Reversed-phase liquid chromatography (RPLC) was performed with conventional (A = H2O/acetonitrile/formic acid, 95/5/0.5 v/v; B = acetonitrile/H2O/formic acid, 95/5/0.5, v/v) and modified (A = H2O/acetonitrile/DMSO/formic acid, 90/5/5/0.5 v/v; B = acetonitrile/H2O/DMSO/formic acid, 90/5/5/0.5 v/v). Separation was conducted with either a Jupiter C18 or C5 column (Jupiter™, Phenomenex, Torrance, CA, USA; 1 × 50 mm, 5 μm particle size, 300 A pore size) at 50 μL/min. Column effluent was split 1/1000 for efficient microelectrospray ionization.4
Mass spectrometry was carried out with a hybrid 14.5 T LTQ FT-ICR mass spectrometer (LTQ-FT, Thermo Fisher Corp., Bremen, Germany).41 External ion accumulation42 was performed in the front end linear ion trap with a target ion population of one million charges for each FT-ICR measurement. Ions were then transferred (~1 ms transfer period) through three octopole ion guides (2.2 MHz, 250 Vp-p) to a capacitively coupled43 closed cylindrical ICR cell (55 mm i.d.) for analysis. Data was collected with Xcalibur 2.0 software (Thermo Fisher). FT-ICR mass spectra were acquired from m/z 390–2000 at high mass resolving power (m/Δm50% = 200,000 at m/z 400, in which Δm50% denotes mass spectral peak full width at half-maximum peak height). The total data acquisition period for each sample was 3 min for direct infusion and 80 min for LC/MS.
RESULTS AND DISCUSSION
Direct Infusion
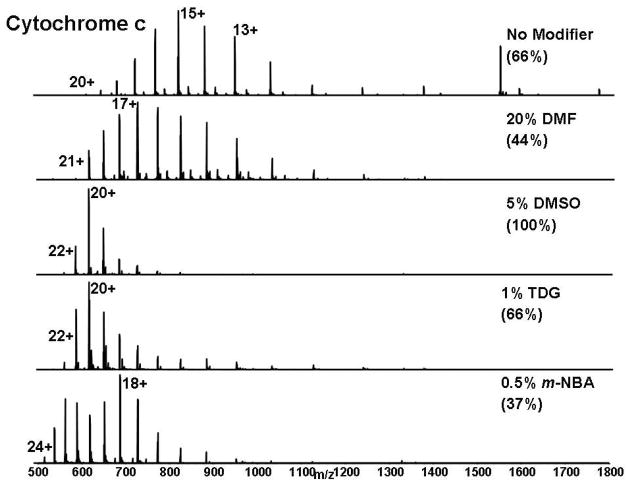
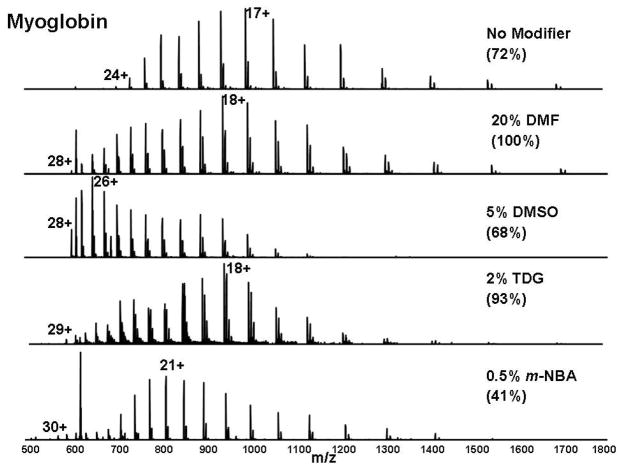
The addition of a relatively low proportion of an appropriate “modifier” reagent to the conventional electrospray denaturing solvent mixture shifts both the average and the maximum charge state of an intact protein to lower m/z, as seen in Figure 1 for cytochrome c. The most abundant charge state shifts from 15+ with conventional electrospray solvent mixture to 17+, 20+, 20+ and 18+ on addition of DMF, DMSO, TDG or m-NBA. Note that DMSO achieved 3 times higher S/N, partly by confining the charge state distribution to a narrower m/z range. In contrast, m-NBA reduced the S/N by 20%. The average charge state shifts from 13.6 (for conventional ESI solvent mixture) to 16, 19.5, 18.6 and 19.1 on addition of DMF, DMSO, TDG or m-NBA. Thus, DMSO improves S/N and increases the average and most abundant charge states to yield lower m/z. The same DMSO effects are observed for myoglobin (Figure 2) and carbonic anhydrase (Supporting Information Figure S-1).
Figure 1.
Representative mass spectra of cytochrome c from solutions containing the listed modifiers, each at concentration optimal for “supercharging”. The highest peak in each spectrum is reported as a percentage of the highest peak in the DMSO spectrum.
Figure 2.
Representative positive-ion electrospray FT-ICR mass spectra of myoglobin (1 μM), displayed as in Figure 1. The base peak shifts from 17+ with conventional solvents to 26+ with DMSO and 21+ with m-NBA. The highest peak in each spectrum is reported as a percentage of the highest peak in the DMF spectrum.
Liquid Chromatography
For high-throughput top down proteomics it is imperative to test the chromatographic separation performance for various modifiers with a Jupiter C18 column for conventional and modified solvents (e.g., 5% DMSO or 0.5% m-NBA or 10% DMF added to both mobile phases). (A stepwise gradient (Supporting Information, Figure S-2) resulted in a total run time of 100 min.) For all four solvent systems, proteins eluted in the order of their molecular weight; but with longer shorter retention time and improved separation with DMSO. Addition of m-NBA, as reported for tryptic peptides,39 shortened the retention times for all proteins, as did DMSO (Supporting Information, Figure S-3). DMF, as we previously reported,44 increased the protein retention time relative to conventional mobile phase solvents.
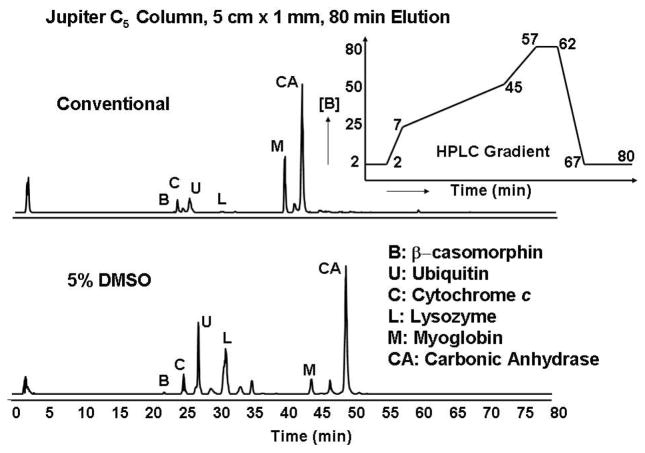
We further optimized chromatographic separation by use of a Jupiter C5 chromatographic column. Figure 3 presents a total ion chromatogram for protein standards separated by conventional and 5% DMSO-modified solvents. Proteins elute in the same order with 5% DMSO, but with improved chromatographic separation and higher peak capacity relative to conventional solvents. As for direct infusion data, enhanced charging and better sensitivity were observed with DMSO.
Figure 3.
Elution profiles for 6 standards for a Jupiter C5 column with conventional (top) and 5% DMSO-modified (bottom) electrospray solvents. The gradient elution profile is shown in the inset.
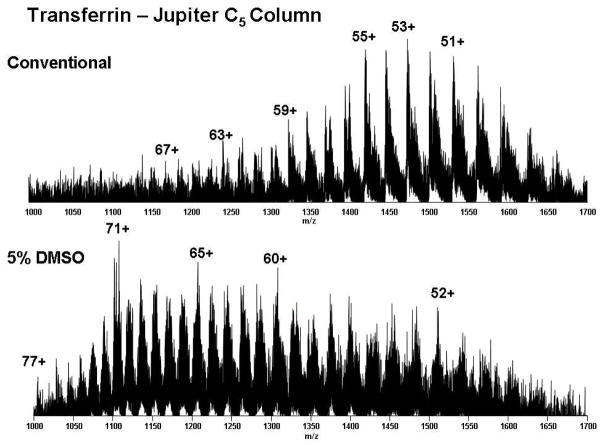
Figure 4 shows the charge state distributions of transferrin as it elutes from the Jupiter C5 column with conventional and DMSO-modified solvents. The base peak shifts dramatically from 53+ to 71+ with DMSO. The highest charge state of transferrin shifts from 67+ to 77+ by DMSO incorporation into the mobile phase. Retention time for transferrin increases from 35 min to 37 min with DMSO, as we also found for other protein standards (Figure 3).
Figure 4.
Charge state distribution for transferrin (78 kDa) eluted from a Jupiter C5 column with conventional and 5% DMSO-modified solvents. The gradient profile is as in Figure 3.
Physico-Chemical Properties
The total number of charges, Z, on a spherical droplet of radius, R, at the point of Rayleigh instability is predicted to be:
| [1] |
in which e is the elementary charge, ε0 is permittivity of a vacuum and γ is surface tension.45 Hence, the charge state distribution should shift to lower m/z (higher charge) as the solvent surface tension increases. Relative to acetonitrile (27.6 mN/m) all of the presently tested solvents have higher surface tension than conventional ESI solvents and exhibit enhanced charging, consistent with the Rayleigh equation. The modifiers could also affect the ESI droplet size and thus the desolvation process. Similarly, DMAA (32.4 mN/m) yielded lower positive charge states than DMF (35.2 mN/m), DMSO (42.9 mN/m), TDG (54.0 mN/m) and m-NBA (50±5 mN/m). Moreover, the somewhat higher gas-phase basicity for DMAA (877.0 KJ/mol) and NMP (891.6KJ/mol)46 relative to DMF (856.6 KJ/mol) which may contribute to their lower charging due to proton abstraction.
In ESI, solvents with higher vapor pressure evaporate first. All of the tested solvents have significantly higher boiling point and lower vapor pressure than water (100 °C) thereby increasing the lifetime of the ESI droplets and causing droplet heating, leading to changes in protein conformation as reported by Williams and coworkers.47 Also, the altered distribution of analyte within the droplet48 could cause enhanced charging with these polar modifiers (e.g., due to higher surface concentration). Further work is in progress to better understand charge enhancement, improved LC separation, and applicability to higher molecular weight proteins.
Supplementary Material
Acknowledgments
The authors thank Christopher Hendrickson for insightful discussions. This work was supported by NIH (R01 GM78359), NSF Division of Materials Research through DMR-06-54118, and the State of Florida.
Footnotes
SUPPORTING INFORMATION AVAILABLE
Additional information as noted in the text. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Science. 1989;246:64–71. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
- 2.Wunschel DS, Pasa-Tolic L, Feng B, Smith RD. J Am Soc Mass Spectrom. 2000;11:333–337. doi: 10.1016/s1044-0305(99)00156-7. [DOI] [PubMed] [Google Scholar]
- 3.Loo JA. Mass Spectrom Rev. 1997;16:1–23. doi: 10.1002/(SICI)1098-2787(1997)16:1<1::AID-MAS1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 4.Emmett MR, Caprioli RM. J Am Soc Mass Spectrom. 1994;5:605–613. doi: 10.1016/1044-0305(94)85001-1. [DOI] [PubMed] [Google Scholar]
- 5.Wilm MS, Mann M. Int J Mass Spectrom Ion Processes. 1994;136:167–180. [Google Scholar]
- 6.Iavarone AT, Jurchen JC, Williams ER. J Am Soc Mass Spectrom. 2000;11:976–985. doi: 10.1016/S1044-0305(00)00169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogorzalek Loo RR, Smith RD. J Mass Spectrom. 1995;30:339–347. [Google Scholar]
- 8.Williams ER. J Mass Spectrom. 1996;31:831–842. doi: 10.1002/(SICI)1096-9888(199608)31:8<831::AID-JMS392>3.0.CO;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang G, Cole RB. Org Mass Spectrom. 1994;29:419–427. [Google Scholar]
- 10.Benkestock K, Sundqvist G, Edlund PO, Roeraade J. J Mass Spectrom. 2004;39:1059–1067. doi: 10.1002/jms.685. [DOI] [PubMed] [Google Scholar]
- 11.Thomson BA. J Am Soc Mass Spectrom. 1997;8:1053–1058. [Google Scholar]
- 12.Chowdhury SK, Katta V, Chait BT. J Am Chem Soc. 1990;112:9012–9013. [Google Scholar]
- 13.Loo JA, Ogorzalek Loo RR, Udseth HR, Edmonds CG, Smith RD. Rapid Commun Mass Spectrom. 1991;5:101–105. doi: 10.1002/rcm.1290050303. [DOI] [PubMed] [Google Scholar]
- 14.Konermann L, Douglas DJ. Rapid Commun Mass Spectrom. 1998;12:435–442. doi: 10.1002/(SICI)1097-0231(19980430)12:8<435::AID-RCM181>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 15.Dobo A, Kaltashov IA. Anal Chem. 2001;73:4763–4773. doi: 10.1021/ac010713f. [DOI] [PubMed] [Google Scholar]
- 16.Gumerov DR, Dobo A, Kaltashov IA. Eur J Mass Spectrom. 2002;8:123–129. [Google Scholar]
- 17.Samalikova M, Matecko I, Muller N, Grandori R. Anal Bioanal Chem. 2004;378:1112–1123. doi: 10.1007/s00216-003-2339-6. [DOI] [PubMed] [Google Scholar]
- 18.Lemaire D, Marie G, Serani L, Laprevote O. Anal Chem. 2001;73:1699–1706. doi: 10.1021/ac001276s. [DOI] [PubMed] [Google Scholar]
- 19.Kaltashov IA, Mohimen A. Anal Chem. 2005;77:5370–5379. doi: 10.1021/ac050511+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verkerk UH, Peschke M, Kebarle P. J Mass Spectrom. 2003;38:618–631. doi: 10.1002/jms.475. [DOI] [PubMed] [Google Scholar]
- 21.Catalina MI, Van den Heuvel RH, Van Duijn E, Heck AJ. Chemistry. 2005;11:960–968. doi: 10.1002/chem.200400395. [DOI] [PubMed] [Google Scholar]
- 22.Mcluckey SA, Van Berkel GJ, Glish GL. J Am Chem Soc. 1990;112:5668–5670. [Google Scholar]
- 23.Pitteri SJ, McLuckey SA. Mass Spectrom Rev. 2005;24:931–958. doi: 10.1002/mas.20048. [DOI] [PubMed] [Google Scholar]
- 24.Hu Q, Noll RJ, Li H, Makarov A, Hardman M, Cooks RG. J Mass Spectrom. 2005;40:430–443. doi: 10.1002/jms.856. [DOI] [PubMed] [Google Scholar]
- 25.Marshall AG, Hendrickson CL, Jackson GS. Mass Spectrom Rev. 1998;17:1–35. doi: 10.1002/(SICI)1098-2787(1998)17:1<1::AID-MAS1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 26.Marshall AG, Hendrickson CL. Rapid Commun Mass Spectrom. 2001;15:232–235. doi: 10.1002/(SICI)1097-0231(19990815)13:15<1639::AID-RCM691>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 27.Zubarev RA, Kelleher NL, McLafferty FW. J Am Chem Soc. 1998;120:3265–3266. [Google Scholar]
- 28.Syka JE, Coon JJ, Schroeder MJ, Shabanowitz J, Hunt DF. Proc Natl Acad Sci U S A. 2004;101:9528–9533. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zubarev RA, Horn DM, Fridriksson EK, Kelleher NL, Kruger NA, Lewis MA, Carpenter BK, McLafferty FW. Anal Chem. 2000;72:563–573. doi: 10.1021/ac990811p. [DOI] [PubMed] [Google Scholar]
- 30.Loo JA, Udseth HR, Smith RD. Biomed Environ Mass Spectrom. 1988;17 doi: 10.1002/bms.1200150409. [DOI] [PubMed] [Google Scholar]
- 31.Loo JA, Edmonds CG, Udseth HR, Smith RD. Anal Chem. 1990;62:693–698. doi: 10.1021/ac00206a009. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Cole RB. Anal Chem. 2003;75:5739–5746. doi: 10.1021/ac0301402. [DOI] [PubMed] [Google Scholar]
- 33.Iavarone AT, Jurchen JC, Williams ER. Anal Chem. 2001;73:1455–1460. doi: 10.1021/ac001251t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iavarone AT, Williams ER. Int J Mass Spectrom. 2002;219:63–72. [Google Scholar]
- 35.Iavarone AT, Williams ER. J Am Chem Soc. 2003;125:2319–2327. doi: 10.1021/ja021202t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iavarone AT, Williams ER. Anal Chem. 2003;75:4525–4533. doi: 10.1021/ac034144i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lomeli SH, Yin S, Ogorzalek Loo RR, Loo JA. J Am Soc Mass Spectrom. 2009;20:593–596. doi: 10.1016/j.jasms.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lomeli SH, Peng IX, Yin S, Ogorzalek Loo RR, Loo JA. J Am Soc Mass Spectrom. 2010;21:127–131. doi: 10.1016/j.jasms.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kjeldsen F, Giessing AM, Ingrell CR, Jensen ON. Anal Chem. 2007;79:9243–9252. doi: 10.1021/ac701700g. [DOI] [PubMed] [Google Scholar]
- 40.Zhang S, Van Pelt CK, Henion JD. Electrophoresis. 2003;24:3620–3632. doi: 10.1002/elps.200305585. [DOI] [PubMed] [Google Scholar]
- 41.Schaub TM, Hendrickson CL, Horning S, Quinn JP, Senko MW, Marshall AG. Anal Chem. 2008;80:3985–3990. doi: 10.1021/ac800386h. [DOI] [PubMed] [Google Scholar]
- 42.Senko MW, Hendrickson CL, Emmett MR, Shi SDH, Marshall AG. J Am Soc Mass Spectrom. 1997;8:970–976. [Google Scholar]
- 43.Beu SC, Laude DA. Anal Chem. 1992;64:177–180. [Google Scholar]
- 44.Valeja SG, Emmett MR, Marshall AG. 57th Amer. Soc. Mass Spectrom. Annual Conf. on Mass Spectrometry & Allied Topics; Philadelphia, PA. May 31-June 5 2009. [Google Scholar]
- 45.Rayleigh L. Philos Mag. 1882;14:184–186. [Google Scholar]
- 46.Lide DR. CRC Handbook of Chemistry and Physics. 90. 2009–2010. [Google Scholar]
- 47.Sterling HJ, Williams ER. J Am Soc Mass Spectrom. 2009;20:1933–1943. doi: 10.1016/j.jasms.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cech NB, Enke CG. Anal Chem. 2000;72:2717–2723. doi: 10.1021/ac9914869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.