Abstract
Background
Recent studies have examined the globalization of clinical research. These studies focused on adult trials, and the globalization of pediatric research has not been examined to date. We evaluated the setting of published studies conducted under the US Pediatric Exclusivity Program, which provides economic incentives to pharmaceutical companies to conduct drug studies in children.
Methods
Published studies containing the main results of trials conducted from 1998–2007 under the Pediatric Exclusivity Provision were included. Data were extracted from each study and described, including the therapeutic area of drug studied, number of patients enrolled, number of sites, and location where the study was conducted, if reported.
Results
Overall, 174 trials were included (sample size 8–27,065 patients); 9% did not report any information regarding the location or number of sites where the study was conducted. Of those that did report this information, 65% were conducted in at least one country outside the US, and 11% did not have any sites in the US. Fifty-four different countries were represented and 38% of trials enrolled patients in at least one site located in a developing/transition country, including more than one third of infectious disease, cardiovascular, and allergy/immunology trials.
Conclusions
The majority of published pediatric trials conducted under the Pediatric Exclusivity Provision included sites outside of the US, and over a third of trials enrolled patients in developing/transition countries. While there are many potential benefits to the globalization of pediatric research, this trend also raises certain scientific and ethical concerns which require further evaluation.
Keywords: clinical trials, globalization
Introduction
Globalization is a phenomenon of the past half century, and includes economic, technological, and sociocultural aspects (1). The trend of globalization has also spread to clinical research, and recent studies have examined the advantages, as well as ethical implications, of this trend (2). Glickman et al. found that the number of countries serving as clinical trial sites outside the US has more than doubled over the past 10 years (2). In addition, investigators found that approximately one third of clinical trials conducted by the 20 largest US based pharmaceutical companies are conducted solely outside the US, including many in developing countries (2). These studies focused on adult trials, and the globalization of pediatric research has not been examined to date. There are advantages to the globalization of research in children such as the ability to enroll more patients to adequately study treatments for rare and heterogeneous pediatric diseases, and opportunity to impact child health on a global scale. However, there are also additional ethical issues surrounding research conducted in children and the financial incentives provided by the Pediatric Exclusivity Provision (3).
The Pediatric Exclusivity Provision, passed by Congress in 1997, provides economic incentives in the form of 6 months of patent extension, or marketing exclusivity, to pharmaceutical companies to conduct safety and efficacy studies in children under guidelines established by the US Food and Drug Administration (4). The Best Pharmaceuticals for Children Act, passed in 2002, extended these incentives (5). These programs have encouraged drug studies in children and have resulted in more than 150 labeling changes to date (6). The characteristics and location of sites enrolling patients in trials completed for pediatric exclusivity have not been evaluated. The purpose of this study was to evaluate the globalization of pediatric research in published studies conducted under the Pediatric Exclusivity Program.
Methods
Selection of studies
The methodology used to identify studies for inclusion has been previously described (7). Clinical trials eligible for inclusion were any human pharmacokinetic, safety, or efficacy studies conducted in response to a written request issued by the US Food and Drug Administration as a part of the Pediatric Exclusivity Program. Initially, MEDLINE and EMBASE were searched for studies containing the main results of trials conducted under the Pediatric Exclusivity Program from 1998–2004 (and subsequently published from 1998–2005) using 3 separate search strategies: 1) generic name of product, all child (0–18 years), 1998–2005, and English language; 2) generic name of product, 1998–2005, and ages of trial participants; 3) generic name of product and key words from the study design. In addition, publications (including those submitted, in press, or already published) were requested for each trial from the pharmaceutical company who conducted the trial. This did not yield any additional studies. The trials identified made up the cohort analyzed in a previous study of pediatric exclusivity by our group (7). For the purposes of the present analysis, the above search strategy was repeated to update our data with studies conducted through 2007 and published through 2009. The search dates were focused to identify studies conducted from 2004–2007 to provide a one year overlap with the previous search strategy. Publications were not requested again from the pharmaceutical companies as this approach did not yield any additional included studies previously. In order to ensure we were not missing any studies not published in the English language, those published internationally, or not available in MEDLINE or EMBASE, we also searched the Cochrane Central Register of Controlled Trials for published results of trials during the entire study period not previously identified through the methodology noted above. Through this approach we identified an additional 9 studies. In fact, these additional studies were also available in MEDLINE, and were trials that had been conducted early on in the study period but not published until several years later and were thus missed in the initial search strategies.
Data Collection
Data extracted from each study included the therapeutic area of drug studied, number of patients enrolled, number of sites, and location where the study was conducted including number of countries, if reported (7). Countries where the trials were conducted were categorized as developing or transition nations based on the United Nations classification system (8). Data regarding the pharmaceutical company who sponsored the trial was also collected, and companies were categorized as being one of the 20 largest pharmaceutical companies vs. other (9).
Analysis
Trial characteristics were described using standard summary statistics. Categorical variables were reported as proportions, and continuous variables as medians and ranges. Due to the descriptive nature of the study, formal statistical comparisons were not made.
Results
Trial Characteristics
A total of 174 trials were included. Table 1 lists the therapeutic areas represented. The majority (64%, n=112) of trials focused on efficacy vs. safety or pharmacokinetic analysis. Data on trial location are displayed in Table 2. Of note, 9% did not report any information regarding the location or number of sites where the study was conducted. Of those that did report this information, two thirds were conducted in at least one country outside of the US, and 11% did not have any sites in the US. For the 79 trials with a study population of ≥ 100 patients, 87% enrolled patients in a site outside the US. Seventy-one percent (n=142) of trials were sponsored by one of the 20 largest pharmaceutical companies. Of these trials, 73% enrolled patients in a site outside the US vs. 46% of trials sponsored by smaller pharmaceutical companies.
Table 1.
Therapeutic areas and diseases/disorders represented
| n = 174 trials | |
|---|---|
| Central Nervous System/Psychiatric | 48 (28%) |
| Attention deficit hyperactivity disorder | |
| Psychiatric disorders | |
| Epilepsy | |
| Migraine headaches | |
| Neurogenic bladder | |
| Other | |
| Infectious Disease | 42 (24%) |
| HIV | |
| Hepatitis B | |
| Pneumonia | |
| Malaria | |
| Otitis media | |
| Tinea | |
| Other | |
| Cardiovascular | 29 (17%) |
| Arrhythmia | |
| Heart failure | |
| Hypertension | |
| Hyperlipidemia | |
| Other | |
| Allergy/Immunology | 20 (11%) |
| Allergic rhinitis | |
| Asthma | |
| Atopic dermatitis | |
| Juvenile rheumatoid arthritis | |
| Cancer | 11 (6%) |
| Refractory solid tumors | |
| Leukemia/lymphoma | |
| Stem cell transplant | |
| Nausea/vomiting associated with chemotherapy | |
| Endocrine | 10 (6%) |
| Diabetes | |
| Gynecomastia | |
| Obesity | |
| Other | |
| Gastrointestinal | 8 (5%) |
| Gastroesophageal reflux/esophagitis | |
| Anesthesia/Pain Management | 2 (1%) |
| Other | 4 (2%) |
Table 2.
Pediatric trial characteristics
| n = 174 | |
|---|---|
| Number of patients per trial | 107 (8 – 27,065) |
| Trials reporting any site/location data | 158 (91%) |
| Trials reporting data on number of sites | 157 (90%) |
| Single Center | 12 (8%) |
| Multicenter | 145 (92%) |
| Number of sites | 18 (2–101) |
| Trials reporting location data | 142 (82%) |
| US only | 50 (35%) |
| Multinational | 92 (65%) |
| Countries Represented | 54 |
| No US location | 16 (11%) |
| Trials reporting number of sites at each location | 90 (63%) |
| Trials reporting number of patients enrolled in each location | 0 (0%) |
| Number of trials involving developing/transition nation | 54 (38%) |
Percents were calculated for all major headings listed using the total number of trials (n=174). For subheadings indented under a major heading, percents were calculated using the number of trials in that specific heading as the denominator.
Countries/Regions represented
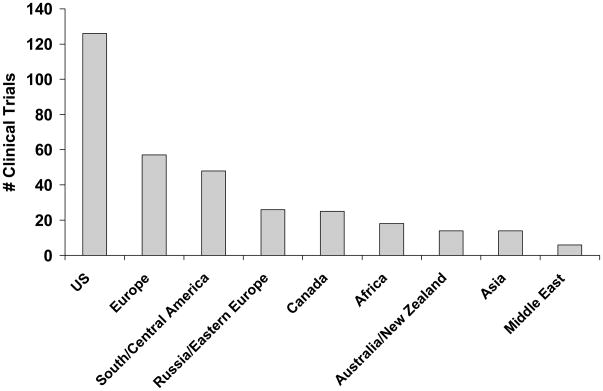
Fifty-four different countries were represented in the clinical trials (Table 3), although 16 (11%) of the trials who reported data on location reported only region or continent and not the specific country where the trial took place. The number of trials involving various geographical regions is displayed in Figure 1. Sixty-three percent of trials reported the number of sites in each region or country, and no trials reported the number of patients enrolled in each country.
Table 3.
Countries represented in pediatric clinical trials**
| North America | |
| United States | Canada |
| South and Central America* | |
| Brazil | Chile |
| Peru | Argentina |
| Venezuela | Columbia |
| Panama | Costa Rica |
| Mexico | Guatemala |
| Ecuador | |
| Europe and Russia | |
| United Kingdom | France |
| Italy | Spain |
| Belgium | Netherlands |
| Norway | Finland |
| Sweden | Denmark |
| Germany | Austria |
| Portugal | Switzerland |
| Russia* | Ukraine* |
| Belarus* | Czech Republic |
| Croatia* | Poland |
| Hungary | Serbia* |
| Estonia | Latvia |
| Lithuania | Slovakia |
| Bulgaria | Romania |
| Asia | |
| Thailand* | India* |
| Phillipines* | |
| Africa* | |
| Gabon | Kenya |
| Uganda | South Africa |
| Australia/New Zealand | |
| Australia | New Zealand |
| Middle East | |
| Israel | Egypt |
| Lebanon | Turkey |
indicates country or all countries in specified region are classified as developing/transition in United Nations classification system
specific countries were not listed for all trials included, see Table 1.
Figure 1.
Number of pediatric clinical trials with patients enrolled in at least one site in the region specified
Over a third (38%) of trials enrolled patients in at least one site located in a developing/transition country. The proportion of trials enrolling patients in at least one site in a developing/transition country in the different therapeutic areas is displayed in Table 4. Trials in the infectious disease area were mostly likely to include patients from at least one site in a developing/transition country, in addition to more than a third of cardiovascular and allergy/immunology trials. Sixteen different pharmaceutical companies conducted trials in which patients were enrolled in at least one developing/transition country, with 4 large pharmaceutical companies accounting for 52% of these trials. Of trials who enrolled patients in at least one developing/transition country, 26% were primarily pharmacokinetic or safety analyses (vs. efficacy) trials compared with 36% of trials overall.
Table 4.
Proportion of pediatric clinical trials with patients enrolled in at least one site in a developing/transition country by therapeutic area
| Therapeutic Area | n | % |
|---|---|---|
| Infectious Disease | 22/42 | (52%) |
| Cardiovascular | 12/29 | (41%) |
| Allergy/Immunology | 9/20 | (45%) |
| Endocrine | 3/10 | (30%) |
| Central Nervous System | 6/48 | (13%) |
| Gastrointestinal | 1/8 | (13%) |
| Cancer | 0/11 | (0%) |
| Anesthesia | 0/2 | (0%) |
| Other | 2/4 | (50%) |
Discussion
This analysis shows that the majority of published pediatric trials conducted by pharmaceutical companies for the US Food and Drug Administration for 6 months of market exclusivity include sites outside of the US, with 11% conducted exclusively outside the US. Over a third of trials enrolled patients in developing/transition countries.
These findings are similar to recent studies of globalization focusing on clinical trials conducted in adults (2). Several possible reasons for this trend have been examined, including 1) lower labor and infrastructure costs; 2) the availability of a larger pool of research subjects which reduces trial and drug development timelines; and 3) fewer and less time-consuming regulatory requirements (10–12). Scientific advantages of globalization include clinical innovation and collaboration with investigators in other countries, and evaluation of safety and efficacy of therapies in a more diverse population which may lead to a broader impact on global health.
Additional and related factors may play a role in the globalization of pediatric trials. Pediatric diseases are often more rare and heterogeneous, making recruitment of an adequate number of patients in a timely manner more difficult (13). Expanding the pool of eligible patients by recruiting from multiple sites and locations is therefore desirable. Additional regulatory requirements for research involving children may also increase the time and resources necessary to conduct pediatric trials, and these may also be minimized by recruiting patients in counties with fewer regulatory requirements (13, 14). Conducting pediatric trials in an efficient manner allows pharmaceutical companies to more readily take advantage of the economic benefits of 6 months of patent extension for performing these studies in children under the Pediatric Exclusivity Provision. These economic incentives are significant. It is estimated that the extensions in patent protection have resulted in more than $14 billion in profits to pharmaceutical companies (6).
However, in addition to the potential benefits of globalization, several concerns have also been raised. The purpose of the Pediatric Exclusivity Program is to encourage pediatric drug studies so that medications marketed in the US may be properly labeled for use in children. The scientific validity of extrapolation of results from trials conducted in other countries is not known. Treatment effects may be dependent on baseline event rates, background therapy, access to health care resources, and adherence to therapy, all of which may differ across countries (15). Genetic differences may also impact drug effect and limit the applicability of study results from one population to another (16). Differences in training of investigators and research infrastructure may also impact study results (10, 17).
Ethical concerns regarding potential exploitation of children and families in developing nations have also been raised. In some cases, the appropriateness of the research question being examined and purpose of conducting the study in a developing country has been questioned (15). In our analysis we found that trials involving drugs in the therapeutic area of infections diseases, including studies of anti-retroviral and anti-malaria medications, were the most likely to enroll patients in a developing/transition nation. This is logical given the disproportionate impact of HIV and malaria in countries where these trials were conducted. However, we also found that more than a third of cardiovascular and allergy/immunology trials enrolled patients in developing countries, including studies of therapies for atopic dermatitis and juvenile rheumatoid arthritis. It is unclear whether treatments such as these will become readily available in these countries once approved, or whether they would be prohibitively expensive even if made available. The Declaration of Helsinki outlines the expectation that all patients enrolled in a clinical trial should have access to the best proven therapy identified in the study when the trial has concluded (18). On the other hand, regarding the type of trials conducted, we found a proportionate number of pharmacokinetic and safety vs. efficacy analyses were conducted in developing/transition nations, suggesting that these countries are not being exploited in terms of disproportionate exposure of their population to the early stages of drug development/testing.
Concern for inducement of children and families in the developing world to participate in trials has also been raised. Participation in the study may be the only access a family has to medical care, and compensation for trial participation can be significant, in come cases reported to exceed the annual salary of those in developing countries (10). This may be exacerbated in cultures where children are expected to contribute to the economic stability of the family, so that participation of the child in a clinical trial is seen as a means to fulfill the child’s economic duty to the family (14). Finally, traditional methods of informed consent are often not possible in developing countries due to widespread illiteracy, and in particular, the concept of child assent may be difficult to define in countries where children are traditionally not considered to have any decision making capacity independent of the family or community (2, 10, 14).
Due to these issues, it has been questioned whether there is adequate oversight of trials conducted outside of the US. The Declaration of Helsinki, and guidelines put forth by the Council for International Organization of Medical Sciences and US National Bioethics Advisory Commission do address basic ethical principles for the conduct of human research regardless of location, and certain guidelines address pediatric research in particular (18–20). However, it has been reported that more than half of adult trials conducted in developing countries do not receive any local official review (21). Lack of transparency is also evident. It has been previously shown that only half of studies completed for pediatric exclusivity are published in the peer-reviewed literature (7). In the present study, we found that 9% of the published trials did not report any information regarding the study location. Of those who did report this information, 11% reported only the region or continent where the study took place and not the specific country, and no trials reported the number of patients enrolled in each country. These are similar to findings recently published regarding adult trials (2).
Limitations
This analysis was limited to trials conducted under the Pediatric Exclusivity Provision and published in the peer-reviewed literature. Previous studies have documented that approximately half of studies completed under this program have not been published, although this trend has improved in recent years (7). Therefore, our analysis may represent an underestimate of the extent of globalization of pediatric trials as information regarding the location of unpublished studies was unavailable. The trend toward publication of more studies over time also precluded us from being able to accurately assess changes or trends in globalization over time. Finally, we were unable to assess specific operational details, data quality, and oversight of these trials. Further evaluation and understanding of these issues will give a more complete picture of the potential impact of globalization.
Conclusions
We found that the majority of published pediatric trials conducted under the Pediatric Exclusivity Provision included sites outside of the US, and over a third of trials enrolled patients in developing/transition countries. There are many potential benefits to the globalization of pediatric research including a shortened timeline/reduced cost for drug development and testing, fostering global clinical innovation, and improving access to therapies and the health of children worldwide. However, this trend also raises certain scientific and ethical concerns. Many of the recommendations set forth by Glickman et al. regarding globalization of adult trials could also be applied/adapted to pediatric trials to address these concerns, including: requiring sponsors/investigators to describe how proposed pediatric trial populations match intended markets for the drug being tested, requiring all pediatric trials conducted under the Pediatric Exclusivity Provision be published in the peer reviewed literature and include specific data regarding the setting and location of the trial, creating formal mechanisms of global regulatory oversight with input from all stakeholders, developing programs to facilitate training of investigators in the developing world in pediatric specific research methodologies and research ethics, and making greater use of centralized review boards with representation to adequately address global issues of international studies.
Acknowledgments
Dr. Pasquali receives grant support (KL2 RR024127-02) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and from the American Heart Association (AHA) Mid-Atlantic Affiliate Clinical Research Program.
Dr. Benjamin receives support from the United States Government for his work in pediatric and neonatal clinical pharmacology (1R01HD057956-02, 1R01FD003519-01, 1U10-HD45962-06, 1K24HD058735-01, and Government Contract HHSN267200700051C), the non profit organization Thrasher Research Foundation for his work in neonatal candidiasis (http://www.thrasherresearch.org), and from industry for neonatal and pediatric drug development (http://www.dcri.duke.edu/research/coi.jsp).
Dr. Smith receives support from the United States Government for his work in pediatric and neonatal clinical pharmacology (1K23HD060040-01) and from industry for neonatal and pediatric drug development (http://www.dcri.duke.edu/research/coi.jsp).
Dr. Li receives grant support from Genzyme and Sanofi Aventis and consulting fees/honoraria from Pfizer, Eli Lilly, and PTC Bio.
References
- 1.Summary of the Annual Review of Developments in Globalization and Regional Integration in the Countries of the ESCWA Region by the United Nations Economic and Social Commission for Western Asia. [Accessed January 5, 2010]; Available at: http://www.escwa.un.org/information/publications/edit/upload/grid-02-2.pdf.
- 2.Glickman SW, McHutchinson JG, Peterson ED, et al. Ethical and Scientific Implications of the Globalization of Clinical Research. N Engl J Med. 2009;360:816–23. doi: 10.1056/NEJMsb0803929. [DOI] [PubMed] [Google Scholar]
- 3.Li JS, Eisenstein EL, Grabowski HG, et al. Economic Return of Clinical Trials Performed Under the Pediatric Exclusivity Program. JAMA. 2007;297:480–8. doi: 10.1001/jama.297.5.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Pediatric Exclusivity Provision, January 2001 Status Report to Congress. [Accessed January 5, 2010]; Available at www.fda.gov/cder/pediatric.
- 5. [Accessed January 5, 2010];Best Pharmaceuticals for Children Act. Available at: www.fda.gov/cder/pediatric.
- 6. [Accessed January 5, 2010];Pediatric Exclusivity Labeling Changes. Available at: http://www.fda.gov/cder/pediatric/labelchange.htm.
- 7.Benjamin DK, Smith PB, Murphy MD, et al. Peer-Reviewed Publication of Clinical Trials Completed for Pediatric Exclusivity. JAMA. 2006;296:1266–73. doi: 10.1001/jama.296.10.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Composition of macro geographical (continental) regions, geographical sub-regions, and selected economic and other groupings. [Accessed January 5, 2010]; Available at: http://unstats.un.org/unsd/methods/m49/m49regin.htm#developed.
- 9.Truelove C. 21st Annual report: belt tightens on big pharma. Med Ad News. 2007;26:4–7. [Google Scholar]
- 10.Nundy S, Gulhati CM. A New Colonialism? Conducting Clinical Trials in India. N Engl J Med. 2005;352(16):1633–6. doi: 10.1056/NEJMp048361. [DOI] [PubMed] [Google Scholar]
- 11.DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22:151–85. doi: 10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- 12.Yusuf S. Randomized clinical trials: Slow death by a thousand unnecessary polices? CMAJ. 2004;171:889–92. doi: 10.1503/cmaj.1040884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanders SP. Conducting pediatric cardiovascular trials. Am Heart J. 2001;142:218–223. doi: 10.1067/mhj.2001.117064. [DOI] [PubMed] [Google Scholar]
- 14.Vreeman RC, Nyandiko WM, Meslin EM. Pediatric Assent For A Study Of Antiretroviral Therapy Dosing For Children In Western Kenya: A Case Study In International Research Collaboration. J Empir Res Hum Res Ethics. 2009;4:3–16. doi: 10.1525/jer.2009.4.1.3. [DOI] [PubMed] [Google Scholar]
- 15.Stough WG, Zannad F, Pitt B, Goldstein S. Globalization of cardiovascular clinical research: The balance between meeting medical needs and maintaining scientific standards. Am Heart J. 2007;154:232–8. doi: 10.1016/j.ahj.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein DB, Tate SK, Sisodiya SM. Pharmacogenetics goes genomic. Nat Rev Genet. 2003;4:937–47. doi: 10.1038/nrg1229. [DOI] [PubMed] [Google Scholar]
- 17.Bhutta ZA. Ethics in international health research: a perspective from the developing world. Bull World Health Organ. 2002;80:114–20. [PMC free article] [PubMed] [Google Scholar]
- 18.World Medical Association. [Accessed January 5, 2010];Declaration of Helsinki: ethical principles for medical research involving human subjects. Available at: http://www.wma.net/e/policy/pdf/17c.pdf.
- 19.Council for International Organizations of Medical Sciences (CIOMS) [Accessed January 5, 2010];International ethical guidelines for biomedical research involving human subjects. Available at: http://www.cioms.ch/frame_guidelines_nov_2002.htm. [PubMed]
- 20.Shapiro HT, Meslin EM. Ethical issues in the design and conduct of clinical trials in developing countries. N Engl J Med. 2001;345:139–42. doi: 10.1056/NEJM200107123450212. [DOI] [PubMed] [Google Scholar]
- 21.Hyder AA, Wali SA, Khan AN, Teoh NB, Kass NE, Dawson L. Ethical review of research: a perspective from developing country researchers. J Med Ethics. 2004;30:68–72. doi: 10.1136/jme.2002.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]