Abstract
Background
Monitoring cellular immune responses is one prerequisite for the rational development of cancer vaccines.
Methods
Here, we describe an extensive effort to optimize and quantitatively validate an in vitro T cell culture method by determining the phenotype and function of both CD4+ and CD8+ T cells, including the measurement of the phenotype markers CCR7, CD45RA, CD28 and CD27 and of the functional markers IFN-γ, IL-2, MIP-1β, TNF-α and CD107a.
Results
Autologous PBMCs are potent stimulators that expand antigen-specific CD8+ T cells during short-term culture with the addition of IL-2 and IL-15 cytokines. Polyfunctional antigen-specific CD4+ and CD8+ T cells are detectable using this method.
Discussion
Our culture system represents a robust human T cell culture protocol which permits phenotypic, quantitative and qualitative evaluation of vaccine-induced CD4+ and CD8+ T cell responses.
Keywords: Immune monitoring, antigen-specific T cell, phenotype, polyfunctional analysis, CD4 T cell, CD8 T cell
INTRODUCTION
The discovery in the last several decades of human cancer antigens has ushered in a new era of antigen-specific cancer immunotherapy. These antigens include differentiation antigens expressed only on certain cell types/tumors, e.g. MART-1/Melan-A in melanoma, and cancer-testis antigens, such as NY-ESO-1, which are expressed on certain tumors but not in normal tissue, with the exception of the placenta and testes [1, 2]. Optimal antitumor activity is achieved by the induction of both CD4+ and CD8+ tumor-specific T cells [3-8].
A critical component of the success of such trials is the close monitoring of antigen-specific CD4+ and CD8+ responses to quantify the full phenotypic and functional characteristics of the T cells. This quantification allows for analysis of the effectiveness of the immunization strategy, correlation with any clinical benefit and, ultimately, development of an improved vaccine formulation that can be deployed clinically. Ideally, the most accurate form of T cell monitoring would involve ex vivo measurement without the need for in vitro manipulation. However, most vaccine and immune-based strategies produce relatively low frequencies of antigen-specific precursors in the peripheral blood [9, 10], which are difficult to accurately quantitate even with current technology. As such, in vitro expansion of these precursors is frequently necessary.
In terms of phenotype, four major subsets of human CD8+ T cells have been delineated with the help of two cell surface markers – CCR7 and CD45RA[11]. These are naïve cells (CD45RA+CCR7+), central memory cells (TCM, CD45RA-CCR7+), effector memory cells (TEM, CD45RA-CCR7-) and effector cells (TEMRA, CD45RA+CCR7-)[12-14]. Recently, two additional surface markers – CD27 and CD28 – have also proven to be useful in defining four additional subsets of CD8+CD45RA-CCR7- T cells that have different effector and cytolytic functions – EM1 (CD27+CD28+), EM2 (CD27+28-), EM3 (CD27-CD28-) and EM4 (CD27-CD28+)[15].
Until recently, T cell responses have largely been determined by measuring the expression of a single effector function, such as interferon (IFN)-γ production. However, there are increasing data that a single parameter does not reflect the full functional potential of a T cell[16]. Recent studies have demonstrated that polyfunctional T cells – which produce multiple cytokines and chemokines in response to antigen stimulation – are in fact associated with improved viral control in pre-clinical models of infectious diseases, in patients infected with human immunodeficiency virus (HIV) or immunized using vaccinia constructs [17, 18] and in cancer patients receiving vaccination or immunotherapy [8]. Flow cytometry is an important methodology that can simultaneously characterize multiple functions, enabling a broad assessment of the phenotype and functional capacity of T cell effector functions described above [19, 20]. Advances in the number of parameters that can be simultaneously evaluated now allow for even more extensive characterization of T cells at the single cell level.
In this paper, we have undertaken flow cytometry studies to determine the phenotypic and polyfunctional responses of antigen-specific T cells both before and after in vitro stimulation and expansion. To establish the parameters for optimal in vitro T cell stimulation, we have evaluated the ability of autologous peripheral blood mononuclear cells (PBMCs) to function as antigen-presenting cells (APCs) for T cell stimulation and expansion. We have examined four phenotype markers (CCR7, CD45RA, CD28 and CD27) and five functional markers (surface CD107a, IFN-γ, Interleukin (IL)-2, tumor necrosis factor (TNF)-α, macrophage inflammatory protein (MIP)-1β production) in T cells stimulated by several antigens (fluMP, Melan-A and NY-ESO-1).
MATERIALS AND METHODS
Blood donation from healthy donors and melanoma patients
Healthy donors provided blood samples for this study after giving informed consent, as participants in protocols approved by the Memorial Sloan-Kettering Cancer Center (MSKCC) Institutional Review Board (IRB). Five patients with completely resected high-risk melanoma whose blood cells were used in this study were enrolled after informed consent in IRB-approved trials at MSKCC or either a gp100 or tyrosinase DNA vaccine construct. Four patients were HLA-A*0201 positive and NY-ESO-1 serum antibody negative, while one patient was HLA-B*3501 and NY-ESO-1 serum antibody positive. A combination of pre- and post-vaccine blood samples were selected on the basis of a relatively high baseline frequency of the various tetramer-reactive CD8+ cells. PBMCs from healthy donors were stimulated with fluMP peptides while PBMCs from patients with a history of melanoma were stimulated with the MelanA and NY-ESO-1 peptides.
Media and cytokines
For in vitro T cell cultures, we used complete RPMI 1640 medium containing 1% penicillin/streptomycin (MediaTech, Herndon, VA), 50 μM β-mercaptoethanol (Gibco, Invitrogen, Grand Island, NY), and 1% L-glutamine (Gibco, Carlsbad, CA). The medium was supplemented with 10% pooled human serum (PHS; Gemini Bio-Products, Woodland CA), both heat-inactivated for 30 min at 56 °C. Sterile, lipopolysaccharide-, pyrogen-, and mycoplasma-free recombinant human cytokines IL-2 (Chiron, California, USA), IL-7 and IL-15 (R&D Systems, Minneapolis, MN) were used at concentrations of 10 IU/ml, 10 ng/ml and 10 ng/ml, respectively. All cytokines were supplied carrier-free by the manufacturer and were reconstituted in 1% human serum albumin (HSA) (25% HSA, NDC 63546-251-05, pharmaceutical grade, manufactured by Swiss Red Cross, distributed by Alpine Biologics, Orangeburg, NY) in phosphate buffered saline (PBS).
Collection, preparation and cryopreservation of PBMCs
Whole blood (100 ml) from healthy donors or patients with melanoma was collected in Vacutainer or Cell Preparation Tubes (CPT) containing sodium heparin (BD Vacutainer, Franklin Lakes, NJ). PBMCs were isolated from whole blood by centrifuging the CPT tubes at 800g for 25 min. The plasma was collected and retained for other experiments. The interface cells were harvested and washed twice with PBS with 10% fetal calf serum (FCS) at 500g and 450g for 10 minutes respectively. PBMCs were then resuspended in complete RPMI 1640 with 10% autologous plasma or PHS. For cryopreservation, PBMCs were resuspended in FCS with 10% dimethylsulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO), frozen at -80 °C for 2-3 days and then stored in liquid nitrogen.
Peptide synthesis
All peptides used in this study were synthesized by JPT Peptide Technologies (Berlin, Germany): Influenza matrix peptide (fluMP58-66) GILGFVFTL, Melan-A26-35 peptide ELAGIGILTV, NY-ESO-194-102 peptide MPFATPMEA and a pool of 17 NY-ESO-1 overlapping peptides (20-mer overlapped by 10 amino acid). Peptides were resuspended in DMSO/PBS at final concentrations of 10% (vol/vol) and stored at -20 °C. Peptides were thawed the day of the assay and diluted to the required concentration. They were never frozen or thawed more than once.
T-cell stimulation in vitro
Thawed PBMCs from healthy donors or melanoma patients were resuspended in 10% PHS RPMI 1640 medium and plated at 2.5 × 106 cells per well. Autologous PBMCs (5 × 106/ml) were pulsed with peptide (10 μg/ml) for 1 hour at room temperature, then irradiated with 30 Gy (Mark I Irradiator, JL Shepherd and Associates, San Fernando, CA) and cultured with the responder cells at 1:1 ratio. The culture medium was changed every 2-3 days during the in vitro stimulation. Cells were harvested at Day 10 and analyzed by tetramer staining and intracellular cytokine staining (ICS) for polyfunctionality. For kinetic experiments, cells were analyzed at various time points as indicated. Cell cultures with different cytokine combinations were treated as described above.
Tetramer staining
The following tetramers and fluorochrome-labeled antibodies were used: HLA-A*0201-PE labeled tetramers loaded with fluMP58-66 GILGFVFTL, Melan-A26-35 EAAGIGILTV; and HLA-B*3501-PE labeled tetramers loaded with NY-ESO-194-102 MPFATPMEA peptides (Tetramer Core, Lausanne Branch, Ludwig Institute of Cancer Research), PE-Cy7-CD3, APC-CD27, PerCPCy5.5-CD28 (BD, Pharmingen, San Jose, CA), APC-AF750-CD8 (eBioscience, San Diego, CA), ECD-CD45RA (Beckman Coulter Inc., Fullerton, CA) and FITC-CCR7 (R&D Systems, Minneapolis, MN). The optimal volume of each tetramer was titrated based on aliquots of a specific T cell line that was previously identified as a positive control for each tetramer [21, 22]. Cells were analyzed using a CYAN-ADP flow cytometer with Summit software (Dako Cytomation California Inc., Carpinteria, CA). Analysis was performed using FlowJo software (version 8.1; TreeStar, Inc.).
T cells were assayed for tetramer staining after the in vitro stimulation. Briefly, 5 × 105 cells were incubated with 0.5 μl of corresponding tetramer in 50 μl FACS buffer (PBS containing 1% bovine serum albumin (BSA), 0.05 mM EDTA, 0.01% sodium azide) at 37 °C for 15 min, followed by the surface antibodies for another 15 min at room temperature. The cells were then washed with FACS buffer once and resuspended in 300 μl FACS buffer for flow cytometric acquisition. Cells were considered positive for tetramer staining when they formed a clear population with mean fluorescence intensity that was at least 1 log above the negative tetramer control (Beckmann Coulter). Events (≥ 105) were collected after live gating on lymphocytes by forward and side scatter. 4',6-diamidino-2-phenylindole (DAPI, Invitrogen, Carlsbad, CA) was used to gate out dead cells for tetramer staining. Isotype controls included the appropriate fluorochrome conjugated or unconjugated mouse IgG1 or IgG2a (DAKO, Carpinteria, CA).
Intracellular polyfunctional cytokine staining
Two million cultured T cells were harvested at Day 10 and resuspended in 1 ml 10% PHS RPMI medium. The cells were then stimulated with the addition of corresponding peptides for the first 2 hours at 37°C and then in the presence of 5 μg/ml each of Brefeldin A and monensin (BD Bioscience) for 4 hours. PE-Cy5-CD107a (5 ul/ml BD Pharmingen) was added prior to stimulation. The cells were harvested and washed with 2 ml FACS buffer once. A total of 106 cells were resuspended in 50 μl FACS buffer and stained with the following cell surface markers for 30 min at 4°C: Pacific blue-CD3, APC-AF750-CD8 (eBioscience) and ECD-CD4 (Beckman Coulter). After another wash with FACS buffer, the cells were fixed and permeabilized with 100 μl BD Cytofix/Cytoperm solution (BD Bioscience) for 20 min at 4°C, followed by two washes with 1× BD Perm/Wash (BD Bioscience) solution. Finally, the cells were stained with the following cytokine antibodies: APC-IL-2, PE-MIP-1β, PE-Cy7-TNF-α and FITC-IFN-γ (BD Pharmingen). Most samples were acquired on a CYAN flow cytometer, although several samples were also acquired on a BD FACSCalibur machine (BD Bioscience). Cell doublets were excluded using forward scatter versus pulsing with parameters. Gating for each cytokine or chemokine was based on a positive control sample stimulated with staphylococcal enterotoxin B (Sigma-Aldrich, St. Louis, MO) and on a negative control sample that was unstimulated.
Data analysis and statistics
The data analysis program Simplified Presentation of Incredibly Complex Evaluations (SPICE) (version 4.1.6) was kindly provided by M. Roederer, NIH, Bethesda, MD. It was used to analyze and generate graphical representations of T cell response detected by polychromatic flow cytometry. All values used for analyzing proportionate representation of responses are background subtracted. The test of equality of means between groups was based on the two-sample t-statistic. Group comparisons were performed using a stratified t-test, where the stratification variable was cell culture condition.
RESULTS
Autologous PBMCs function as potent stimulators to expand antigen-specific CD8+ T cells during short term in vitro culture
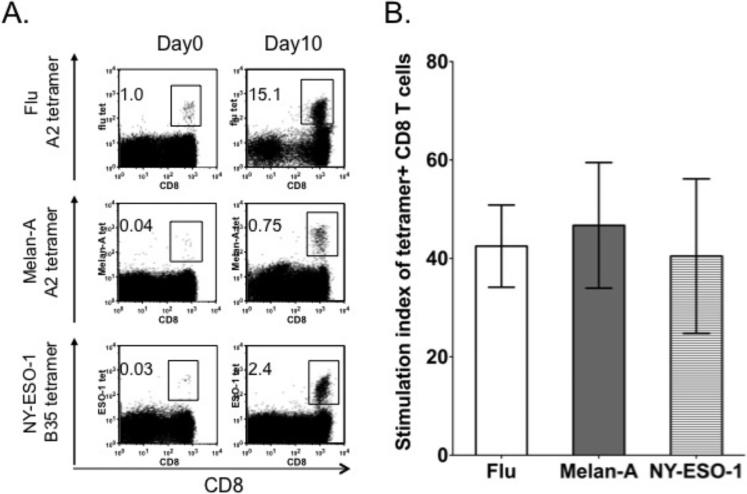
Thawed PBMCs were stimulated during a 10-day culture with irradiated autologous PBMCs pulsed with fluMP, Melan-A or NY-ESO-1 peptides respectively at a 1:1 ratio with the addition of IL-2 and IL-15 cytokines. PBMCs from healthy donors were stimulated with fluMP peptides while PBMCs from patients with a history of melanoma were stimulated with Melan-A or NY-ESO-1 peptides. Binding of the T cell population to fluMP/HLA-A*0201 tetramer, Melan-A/HLA-A*0201 tetramer and NY-ESO-1/HLA-B*3501 tetramers confirmed expansion of fluMP, melan-A and NY-ESO-1 antigen-specific T cells. The percentages of fluMP/HLA-A*0201 tetramer, Melan-A/HLA-A*0201 tetramer and NY-ESO-1/HLA-B*3501 tetramers were increased after the in vitro stimulation (Fig 1A). FluMP, Melan-A and NY-ESO-1 tetramer-reactive T cells underwent expansion from baseline by 42±8.4, 46±12.7 and 40±15.6 fold respectively (Fig 1B).
Figure 1. Autologous PBMCs function as potent stimulators to expand antigen-specific CD8+ T cells during short term in vitro culture.
PBMCs were stimulated with corresponding peptide-pulsed and irradiated autologous PBMCs at an effector:APC ratio of 1:1 with addition of cytokines IL-2 (10 IU/ml) and IL-15 (10 ng/ml). (A) Representative dot plots of flu, Melan-A and NY-ESO-1 antigen specific tetramers are shown at baseline (Day 0) and after in vitro stimulation (Day 10). (B) The stimulation index of tetramer-positive cells, calculated as the absolute number of tetramer-positive cells at the end of each stimulation over the number of tetramer-positive cells in the initial culture. The results represent three independent experiments and the error bars represent the standard error of the mean.
The combination of IL-2 and IL-15 cytokines and irradiation enhances the expansion of tetramer-reactive CD3+CD8+ T-cells
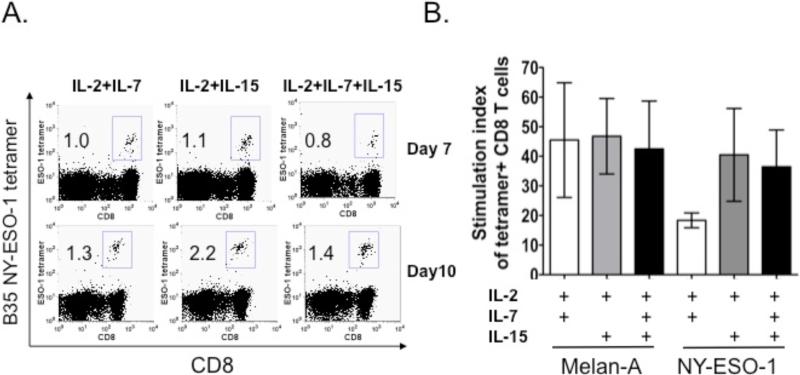
NY-ESO-1 specific T cells from a melanoma patient who was NY-ESO-1 seropositve were stimulated during a 10-day culture with irradiated autologous PBMCs pulsed with a pool of 20-mer overlapping peptides spanning the entire NY-ESO-1 protein in the presence of three different cytokine combinations: IL-2 + IL-7; IL-2 + IL-15 and; IL-2 + IL-7 + IL-15. The combination of IL-2 and IL-15 led to a greater expansion of NY-ESO-1 tetramer-reactive CD8+ T cells than the combination of IL-2 and IL-7 (Fig 2A and 2B). There was no further increase with the addition of IL-7 to IL-2 plus IL-15. A similar expansion pattern was observed for flu-MP tetramer-reactive T cells (data not shown) while there did not appear to be a significant difference in expansion between any of the cytokine conditions for Melan-A tetramer-reactive CD8+ T cells (Fig 2B).
Figure 2. The combination of IL-2 and IL-15 cytokines enhances the expansion of tetramer-reactive CD3+CD8+ T cells.
PBMCs were stimulated with the corresponding peptide-pulsed autologous PBMCs with irradiation. Different combinations of cytokines IL-2 (10 IU/ml), IL-7 (10 ng/ml) and IL-15 (10 ng/ml) were added to the cell culture as indicated. (A) Representative dot plots of NY-ESO-1 antigen tetramer-positive CD3+CD8+ T cells are shown at Day 7 and Day 10 after in vitro stimulation. (B) The stimulation index of tetramer-positive cells for Melan-A and NY-ESO-1 peptide stimulation under different cytokine conditions. These results are the summary of three experiments: for the Melan-A peptide condition, PBMCs from several patients were repeated three times while; for the NY-ESO-1 peptide condition, PBMCs from one patient were used for three separate experiments. For NY-ESO-1 peptide stimulation, IL-2 plus IL-15 resulted in greater stimulation than IL-2 plus IL-7. The addition of IL-7 to IL-2 and IL-15 did not result in further stimulation. Melan-A peptide-stimulated expansion appeared to be similar across each of the three cytokine conditions.
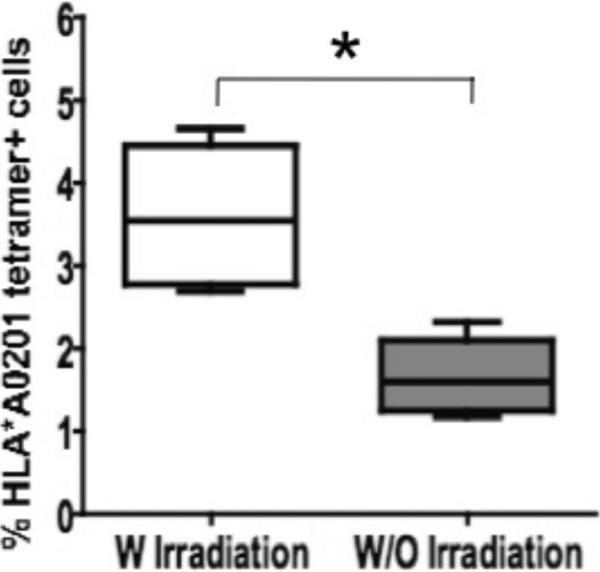
We also explored if irradiation of the peptide-pulsed autologous PBMCs prior to culture with effector cells affected the expansion of tetramer-reactive CD8+ cells. Irradiated Melan-A peptide-pulsed PBMCs produced a significantly greater expansion of Melan-A tetramer-reactive CD8+ T cells than PBMCs that were pulsed without subsequent irradiation (Fig. 3). Irradiation had a similar impact on the expansion of fluMP and NY-ESO-1 antigen-specific CD8+ T cells (data not shown).
Figure 3. Irradiation enhances the expansion of Melan-A tetramer-positive CD3+CD8+ T cells.
The difference in the expansion of Melan-A tetramer-positive CD3+CD8+ T cells between irradiated and non-irradiated Melan-A peptide-pulsed APCs was significant (p=0.013).
Different combinations of cytokines in culture expand similar effector and central memory CD4+ and CD8+ T cell populations
We next determined whether different cytokine combinations in the culture medium favored specific effector and central memory T cell populations. Flow cytometry confirmed that T cells could be separated into four subsets – naïve, TCM, TEM and TEMRA – based on CD45RA and CCR7 labeling. The proportion of CD4+ and CD8+ T cells belonging to each of these four populations was similar in the three different culture conditions with IL-2, IL-7 and/or IL-15 after 10-day culture (data not shown).
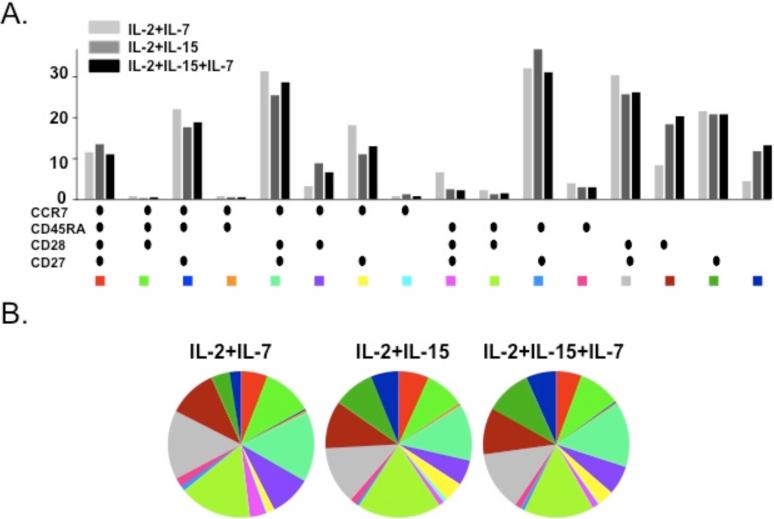
We also examined whether the cytokines used for in vitro stimulation affected the profile of tetramer-reactive CD4+ and CD8+ T cells after 10-day culture. The proportions of fluMP, Melan-A and NY-ESO-1 tetramer-positive cells bearing effector and central memory phenotypes were similar irrespective of culture cytokine conditions (Fig 4A, 4B). Finally, it has been suggested that CD27 and CD28 can further identify subpopulations within the four effector/central memory groups. In the CD8+ CD45RACCR7- population, four separate subsets – EM1, EM2, EM3 and EM4 – could be identified and exhibited similar frequencies among the three different culture conditions.
Figure 4. The phenotypic profiles of fluMP, Melan-A and NY-ESO-1 tetramer-positive CD3+CD8+ cells are not affected by different cytokine conditions in culture medium.
Multiparameter flow cytometry was performed to analyze the phenotype of tetramer-positive CD3+CD8+ cells after a 10-day in vitro culture with MelanA peptide stimulation using the markers CCR7, CD45RA, CD27 and CD28 under three different cytokine conditions in the growth medium: IL-2 + IL-7, IL-2 + IL-15 and IL-2 + IL-7 + IL-15. Figure 4A represents a bar chart of the different phenotypic combinations under the three different cytokine conditions. Figure 4B is a pie chart representing the different phenotypic combinations for each cytokine condition.
In addition, we also examined the frequencies of the four EM subpopulations at baseline and at Days 10 and 14. These frequencies remained approximately the same at each time-point, with the EM1 subset comprising approximately 50% of the population. The similar proportions at all three time-points suggest that there was no preferential expansion of any subpopulation.
Frequency of tetramer-positive CD3+CD8+ T cells peaks at Days 10 while a longer culture time supports the expansion of effector memory (CCR7-CD45RA-) T cells
We proceeded to study the kinetics of antigen-specific T cell expansion by using PBMCs from melanoma patients. The frequency of Melan-A and NY-ESO-1 tetramer-reactive CD8+ T cells peaked at Day 10 and appeared to decline after Day 15 (data not shown). These results are consistent with our previous study, in which the peptide-pulsed K562-A*0201 cell line was used as the APC for cell culture.
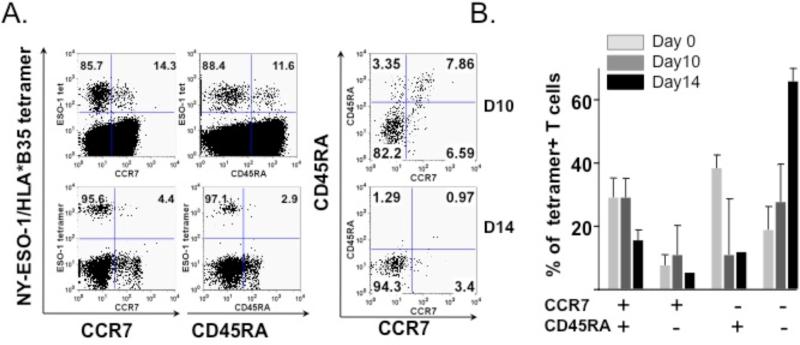
We noted that beyond 10 days of in vitro culture, the proportion of TEM (CCR7-CD45RA-) cells at Day 14 had increased compared to the baseline and Day 10 values. Dot plots from a representative experiment (Fig 5A), as well as the average percentage of CCR7-CD45RA- tetramer-reactive CD8+ T cells from four independent experiments, are shown (Fig. 5B). Overall, these observations support an ideal cell culture time of 10 days, with a longer culture time favoring the expansion of the TEM population but a lower level of overall T cell expansion.
Figure 5. Longer culture time leads to expansion of the CD8+ CCR7-CD45RA- subset.
Multiparameter flow cytometry with the markers CCR7 and CD45RA was performed to analyze the phenotype of tetramer-positive CD3+CD8+ cells after 10 and 14 days of in vitro culture. (A) A representative dot plot indicates an increase in the population of CCR7-CD45RA- NY-ESO-1 tetramer-positive CD3+CD8+ T cells between Days 10 and 14 of in vitro culture. (B) This chart represents an average of CCR7/CD45RA subsets for fluMP, Melan-A and NY-ESO-1 tetramer-positive CD3+CD8+ T cells at Day 0, 10 and 14 of in vitro culture. The proportion of CCR7-CD45RA- cells increases with the length of culture time.
Antigen specific CD4+ and CD8+ T cells are polyfunctional
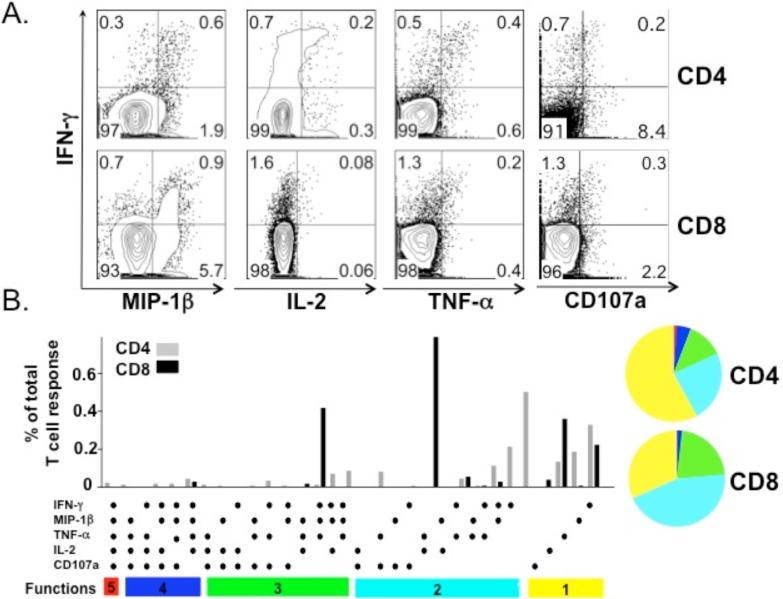
One advantage of using autologous APCs is the potential ability to assess both CD4+ and CD8+ T cell responses. PBMCs from a NY-ESO-1 serum antibody positive patient were analyzed by intracellular polyfunctional cytokine staining after a 10-day culture. Both CD4+ and CD8+ IFN-γ+ T cells that were polyfunctional were detected (Fig 6A). These IFN-γ+ T cells also secreted TNF-α, MIP-1β and IL-2. The total frequencies of NY-ESO-1 specific CD4+ and CD8+ T cells were calculated by summing the frequencies of CD4+ and CD8+ T cells within each unique combination of functions. Thus, each responding cell was only counted once. The representative pie and bar charts summarize the results of three independent experiments (Fig 6B).
Figure 6. Polyfunctional NY-ESO-1 specific CD4+ and CD8+ T cell responses.
Representative ICS and chemokine staining of both CD4+ and CD8+ T cells responding to NY-ESO-1 pooled peptides from a NY-ESO-1 seropositive patient after a 10-day culture with IL-2 and IL-15. (A) Representative dot plots indicate that NY-ESO-1 specific CD4+ and CD8+ T cells secrete IL-2, MIP-1β, IFN-γ and TNF-α. (B) Functional composition of the CD4+ and CD8+ T cell response. All possible combinations of four selected functional responses are shown on the x-axis. Responses are grouped and color-coded. Each slice of the pie charts represents the fraction of the total response that is positive for a given number of functions.
DISCUSSION
In this paper, we present a protocol for the in vitro culture of human T cells obtained from peripheral blood with stimulation by peptide-pulsed autologous irradiated PBMCs. Our data support that this is a robust and reproducible system that reliably expands the frequency of precursor T cells present in the peripheral blood proportionally.
Many other T cell culture methods have been described in the literature. Notable examples include a similar protocol using autologous PBMCs as the APC, as reported by Jackson et al [23]. An important difference from our protocol is the fact that the peptide-pulsed autologous PBMCs were not irradiated. Instead, peptide was added to the entire PBMC population without subsequent irradiation. The authors did note that irradiation of peptide-pulsed PBMCs facilitated T cell expansion but only if <50% of the entire cell population was irradiated, which would be consistent with our observation. The mechanism by which irradiation of the peptide-pulsed APCs leads to increased T cell expansion is unclear but is presumably related to augmentation of antigen-presentation and/or the generation of a mitogenic signal.
Other T cell culture protocols include the use of cell lines to act as APCs, such as the K562/A*0201 cell line, as previously reported by our group [24]. While the use of a cell line has the theoretical benefit of offering a “standardized” APC with known frequency and characteristics, disadvantages include the fact that only T cells from individuals with the same HLA type as the HLA molecule expressed on the cell line can be assayed. Maintaining multiple cell lines for routine immune monitoring may be challenging even for research laboratories. Patient PBMCs may also mount responses to the multiple allogenic epitopes present on the foreign cell line, which may mask or overwhelm the expansion of the low-frequency antigen-specific T cell population of interest.
Finally, other T cell culture protocols have utilized autologous dendritic cells. While dendritic cells serve as professional APCs that can present antigen more efficiently than non-professional APCs, the need to first generate and expand them in vitro from precursors in the peripheral blood prior to use in an assay is both cumbersome and time-consuming but may also be impractical in many situations where only a limited number of patient PBMCs are available for manipulation and analysis.
In our series of experiments, we demonstrated that culture with IL-2 and IL-15 led to greater T cell expansion than with IL-2 and IL-7 and was not further enhanced by the addition of IL-7. These results are in broad agreement with the findings of other investigators, who have demonstrated that several cytokines other than or in addition to IL-2 alone (IL-7, IL-12 and IL-15) can enhance T cell proliferation in various culture systems [25] [26] [27].
More importantly, we also verified that the proportion of effector/ memory CD4+ and CD8+ T cells – as defined by the markers CCR7 and CD45RA – was not affected by the different cytokine culture conditions. Similarly, the proportion of effector/memory populations in tetramer-reactive CD8+ T cells was not influenced by the cytokine milieu. Finally, we further subdivided the CD8+CCR7-CD45RA- with the markers CD27 and CD28 and determined that the proportion of these subpopulations was similarly unaffected by cytokine conditions.
Our experiments also demonstrated that we were able to generate NY-ESO-1 specific polyfunctional CD4+ and CD8+ responses in a melanoma patient who was NY-ESO-1 seropositive. These T cells were capable of producing multiple cytokines, consisting of various combinations of IFN-γ, TNF-α, MIP-1β, IL-2 and CD107a. We did note that the staining pattern of these combinations differ from that of other T cell culture systems, where IL-2 and TNF-α production are often more abundant. One possible explanation is that the polyfunctional cytokine profile of a spontaneous response against a tumor-specific antigen such as NY-ESO-1 may differ from the response produced by viral antigens or by other strategies, e.g. prime-boost vaccination.
IL-2, TNF-α, MIP-1β and IFN-γ represent a relatively simple set of cytokines and chemokines that can be used to define a vaccine-elicited response. In addition, T cells mediate cytolytic activity through the release of perforin or granzymes. CD107a is an indirect measure of degranulation and provides further insight into important effector pathways[28]. While immunization with vaccinia virus has been shown to induce polyfunctional CD8 T cell responses, a recent study by our group showed that tumor antigen-specific T cells are also polyfunctional[8]. There are increasing data that such polyfunctional T cell responses, which correlate with the “quality” of the response, are associated with improved disease control in such chronic infections as HIV, tuberculosis and Hepatitis C [17, 18]. Murine experiments involving various vaccine formulations against Leishmania major have correlated the development of a polyfunctional response with protection against subsequent challenge while, in humans, vaccinia vaccination, which is protective against smallpox, has been shown to generate a polyfunctional virus-specific CD8+ response.
Based on these observations, it is thought that vaccination strategies that can elicit polyfunctional responses are desirable and may ultimately be associated with increased protection/efficacy. While this concept has yet to be rigorously proven in human cancers, there are nevertheless clear implications for monitoring in any immune-based strategy in cancer trials. The ability to generate polyfunctional T cell responses will allow for correlation with clinical outcome to determine if a particular subset of T cells with a specific combination of effector functions is associated with clinical benefit. Such polyfunctional responses have the potential to become a surrogate biomarker by which the effectiveness of cancer immunotherapy can be judged.
Utilizing the T cell culture methodology described in this paper, our group has subsequently provided monitoring for 15 patients with metastatic melanoma receiving therapy with ipilimumab, a monoclonal antibody against cytotoxic T-lymphocyte antigen-4, a negative regulator of immune function. Our data indicate that patients who derive clinical benefit from ipilimumab experience an increase in NY-ESO-1 specific polyfunctional cytokine responses [29].
In summary, we have presented a protocol for human T cell culture using peptide-pulsed irradiated autologous PBMCs as APCs. We believe that the use of autologous PBMCs offers theoretical and practical advantages over other systems that employ cell lines or expanded dendritic cells. To our knowledge, we are also the first to demonstrate that our T cell culture system is able to efficiently expand low-frequency precursors without biasing their baseline proportions and that our system is capable of monitoring antigen-specific polyfunctional T cell responses, which may play an important role in future immune monitoring.
Acknowledgement
We thank Dr. M. Roederer for providing the SPICE software, Dr. Immanuel Luescher from the Tetramer Core, Lausanne Branch, Ludwig Institute of Cancer Research for providing all tetramers used in these experiments, Dr. Bo Dupont and Alice Yeh from his laboratory for performing HLA analysis on one patient and Dr. Miguel Perales, who provided editorial advice. This work was supported by Swim Across America, the Experimental Therapeutics Center of MSKCC and Ludwig Foundation. JDW was supported by a Damon Runyon-Lilly Clinical Investigator Award.
Abbreviations
- CTL
cytotoxic T lymphoctye
- PBMC
peripheral blood mononuclear cell
REFERENCES
- 1.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 2.Gnjatic S, Nishikawa H, Jungbluth AA, Gure AO, Ritter G, Jager E, Knuth A, Chen YT, Old LJ. NY-ESO-1: review of an immunogenic tumor antigen. Adv Cancer Res. 2006;95:1–30. doi: 10.1016/S0065-230X(06)95001-5. [DOI] [PubMed] [Google Scholar]
- 3.Romero P, Valmori D, Pittet MJ, Zippelius A, Rimoldi D, Levy F, Dutoit V, Ayyoub M, Rubio-Godoy V, Michielin O, et al. Antigenicity and immunogenicity of Melan-A/MART-1 derived peptides as targets for tumor reactive CTL in human melanoma. Immunol Rev. 2002;188:81–96. doi: 10.1034/j.1600-065x.2002.18808.x. [DOI] [PubMed] [Google Scholar]
- 4.Janetzki S, Panageas KS, Ben-Porat L, Boyer J, Britten CM, Clay TM, Kalos M, Maecker HT, Romero P, Yuan J, et al. Results and harmonization guidelines from two large-scale international Elispot proficiency panels conducted by the Cancer Vaccine Consortium (CVC/SVI). Cancer Immunol Immunother. 2008;57:303–315. doi: 10.1007/s00262-007-0380-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jager E, Nagata Y, Gnjatic S, Wada H, Stockert E, Karbach J, Dunbar PR, Lee SY, Jungbluth A, Jager D, et al. Monitoring CD8 T cell responses to NY-ESO-1: correlation of humoral and cellular immune responses. Proc Natl Acad Sci U S A. 2000;97:4760–4765. doi: 10.1073/pnas.97.9.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jager E, Karbach J, Gnjatic S, Neumann A, Bender A, Valmori D, Ayyoub M, Ritter E, Ritter G, Jager D, et al. Recombinant vaccinia/fowlpox NY-ESO-1 vaccines induce both humoral and cellular NY-ESO-1-specific immune responses in cancer patients. Proc Natl Acad Sci U S A. 2006;103:14453–14458. doi: 10.1073/pnas.0606512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolchok JD, Yuan J, Houghton AN, Gallardo HF, Rasalan TS, Wang J, Zhang Y, Ranganathan R, Chapman PB, Krown SE, et al. Safety and immunogenicity of tyrosinase DNA vaccines in patients with melanoma. Mol Ther. 2007;15:2044–2050. doi: 10.1038/sj.mt.6300290. [DOI] [PubMed] [Google Scholar]
- 8.Perales MA, Yuan J, Powel S, Gallardo HF, Rasalan TS, Gonzalez C, Manukian G, Wang J, Zhang Y, Chapman PB, et al. Phase I/II Study of GM-CSF DNA as an Adjuvant for a Multipeptide Cancer Vaccine in Patients With Advanced Melanoma. Mol Ther. 2008 doi: 10.1038/mt.2008.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coulie PG, van der Bruggen P. T-cell responses of vaccinated cancer patients. Curr Opin Immunol. 2003;15:131–137. doi: 10.1016/s0952-7915(03)00009-8. [DOI] [PubMed] [Google Scholar]
- 10.Zippelius A, Batard P, Rubio-Godoy V, Bioley G, Lienard D, Lejeune F, Rimoldi D, Guillaume P, Meidenbauer N, Mackensen A, et al. Effector Function of Human Tumor-Specific CD8 T Cells in Melanoma Lesions: A State of Local Functional Tolerance. Cancer Res. 2004;64:2865–2873. doi: 10.1158/0008-5472.can-03-3066. [DOI] [PubMed] [Google Scholar]
- 11.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 12.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 13.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 14.Sauce D, Rufer N, Mercier P, Bodinier M, Remy-Martin J-P, Duperrier A, Ferrand C, Herve P, Romero P, Lang F, et al. Retrovirus-mediated gene transfer in polyclonal T cells results in lower apoptosis and enhanced ex vivo cell expansion of CMV-reactive CD8 T cells as compared with EBV-reactive CD8 T cells. Blood. 2003;102:1241–1248. doi: 10.1182/blood-2002-11-3407. [DOI] [PubMed] [Google Scholar]
- 15.Romero P, Zippelius A, Kurth I, Pittet MJ, Touvrey C, Iancu EM, Corthesy P, Devevre E, Speiser DE, Rufer N. Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J Immunol. 2007;178:4112–4119. doi: 10.4049/jimmunol.178.7.4112. [DOI] [PubMed] [Google Scholar]
- 16.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 17.Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR, Asher TE, Douek DC, Harari A, Pantaleo G, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J Exp Med. 2007;204:1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duvall MG, Precopio ML, Ambrozak DA, Jaye A, McMichael AJ, Whittle HC, Roederer M, Rowland-Jones SL, Koup RA. Polyfunctional T cell responses are a hallmark of HIV-2 infection. Eur J Immunol. 2008;38:350–363. doi: 10.1002/eji.200737768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maecker HT, Rinfret A, D'Souza P, Darden J, Roig E, Landry C, Hayes P, Birungi J, Anzala O, Garcia M, et al. Standardization of cytokine flow cytometry assays. BMC Immunol. 2005;6:13. doi: 10.1186/1471-2172-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horton H, Thomas EP, Stucky JA, Frank I, Moodie Z, Huang Y, Chiu YL, McElrath MJ, De Rosa SC. Optimization and validation of an 8-color intracellular cytokine staining (ICS) assay to quantify antigen-specific T cells induced by vaccination. J Immunol Methods. 2007;323:39–54. doi: 10.1016/j.jim.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chattopadhyay PK, Melenhorst JJ, Ladell K, Gostick E, Scheinberg P, Barrett AJ, Wooldridge L, Roederer M, Sewell AK, Price DA. Techniques to improve the direct ex vivo detection of low frequency antigen-specific CD8+ T cells with peptide-major histocompatibility complex class I tetramers. Cytometry A. 2008;73:1001–1009. doi: 10.1002/cyto.a.20642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wooldridge L, Lissina A, Cole DK, van den Berg HA, Price DA, Sewell AK. Tricks with tetramers: how to get the most from multimeric peptide-MHC. Immunology. 2009;126:147–164. doi: 10.1111/j.1365-2567.2008.02848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson HM, Dimopoulos N, Chen Q, Luke T, Yee Tai T, Maraskovsky E, Old LJ, Davis ID, Cebon J, Chen W. A robust human T-cell culture method suitable for monitoring CD8+ and CD4+ T-cell responses from cancer clinical trial samples. J Immunol Methods. 2004;291:51–62. doi: 10.1016/j.jim.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Yuan J, Gallardo HF, Rasalan T, Ranganathan R, Wang J, Zhang Y, Panageas K, Stan R, Young JW, Houghton AN, Wolchok JD. In vitro expansion of Ag-specific T cells by HLA-A*0201-transfected K562 cells for immune monitoring. Cytotherapy. 2006;8:498–508. doi: 10.1080/14653240600868262. [DOI] [PubMed] [Google Scholar]
- 25.Foster AE, Leen AM, Lee T, Okamura T, Lu A, Vera J, Atkinson R, Bollard CM, Dotti G, Rooney CM. Autologous designer antigen-presenting cells by gene modification of T lymphocyte blasts with IL-7 and IL-12. J Immunother. 2007;30:506–516. doi: 10.1097/CJI.0b013e318046f3b1. [DOI] [PubMed] [Google Scholar]
- 26.Quintarelli C, Dotti G, De Angelis B, Hoyos V, Mims M, Luciano L, Heslop HE, Rooney CM, Pane F, Savoldo B. Cytotoxic T lymphocytes directed to the preferentially expressed antigen of melanoma (PRAME) target chronic myeloid leukemia. Blood. 2008;112:1876–1885. doi: 10.1182/blood-2008-04-150045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melenhorst JJ, Solomon SR, Shenoy A, Hensel NF, McCoy JP, Jr., Keyvanfar K, Barrett AJ. Robust expansion of viral antigen-specific CD4+ and CD8+ T cells for adoptive T cell therapy using gene-modified activated T cells as antigen presenting cells. J Immunother. 2006;29:436–443. doi: 10.1097/01.cji.0000211302.52503.93. discussion 365-436. [DOI] [PubMed] [Google Scholar]
- 28.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 29.Yuan J, Gnjatic S, Li H, Powel S, Gallardo HF, Ritter E, Ku GY, Jungbluth AA, Segal NH, Rasalan TS, et al. CTLA-4 blockade enhances polyfunctional NYESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci U S A. 2008;105:20410–20415. doi: 10.1073/pnas.0810114105. [DOI] [PMC free article] [PubMed] [Google Scholar]