Abstract
Organophosphates (OPs) exert their toxicity by inhibiting primarily acetylcholinesterase (AChE) and to a lesser extent butyrylcholinesterase (BChE). Binary mixtures of mammalian AChE and oximes of varying structure have been recently considered for treatment of OP poisoning as catalytic bioscavengers. In this study wild type human AChE and human AChE with residue mutations D134H, D134H_E202Q and D134H_F338A were characterized and investigated for inhibition by OPs and consequent oxime reactivation of phosphylated enzymes. The rationale for selecting these substitution positions was based on D134H being a naturally occurring single nucleotide polymorphism (SNP) in humans and that E202Q and F338A mutations slow aging of OP inhibited AChE's.
Inhibition of D134H by paraoxon and analogues of cyclosarin was 2-8 times slower than inhibition of wild type (wt), while reactivation of the paraoxon inhibited enzyme by 2PAM was 6 times faster. Both inhibition and reactivation of D134H_E202Q and D134H_F338A double mutants was up to two orders of magnitude slower than the wt indicating that introduction of the active center substitutions abolished fully the effect of the peripherally located D134H. These results indicate that selected residues outside the active center influence inhibition, reactivation and catalysis rates through longer range interactions.
Keywords: Acetylcholinesterase, organophosphates, oxime reactivation, catalysis, surface mutations
1. Introduction
To minimize the toxicity of organophosphate inhibitors (OP), binary mixtures of mammalian AChE and oximes of varying structure were recently considered as catalytic bioscavengers promoting the catalysis of the OP in plasma before it reacts with the target site in skeletal muscle or the nervous system [1]. Our goal is to generate site-specific mutations in AChE protein sequence in order to convert human AChE into catalytic scavenger that, when coupled with an oxime, will inactivate multiple OP molecules per one molecule of scavenger.
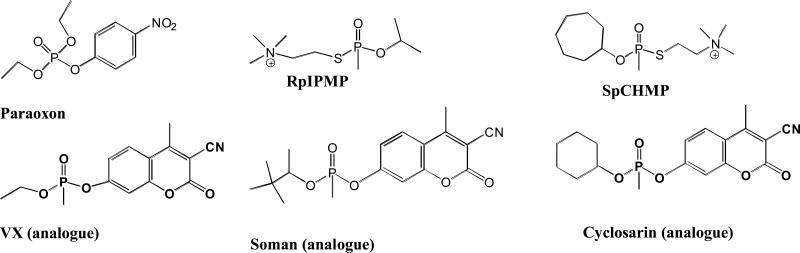
Human AChE wild type and D132H, D134H_E202Q, and D134H_F338A mutants were characterized and investigated for inhibition by OPs (an insecticide and nerve agent analogues; Figure 1) and reactivation of the phosphylated enzymes with 2PAM and HI6. The rationale for selecting these substitution positions was based on D134H being a naturally occurring SNP in humans and that E202Q and F338A mutations slow aging of OP inhibited AChE's [2,3].
Figure 1.
Structures of OP inhibitors used in this study.
2. Materials and methods
Nerve agent analogues of cyclosarin (methylphosphonic acid 3-cyano-4-methyl-2-oxo-2H-coumarin-7-yl ester cyclohexyl ester), VX (methylphosphonic acid 3-cyano-4-methyl-2-oxo-2H-coumarin-7-yl ester ethyl ester), soman (methylphosphonic acid 3-cyano-4-methyl-2-oxo-2H-coumarin-7-yl ester pinacolyl ester) were synthesized as previously described [4], as well as SP-Cycloheptyl methyl phosphonyl thiocholine (SPCHMP ) and RP-Isopropyl methyl phosphonyl thiocholine (RPIPMP) [5]. O,O-diethyl O-(4-nitrophenyl) phosphate (paraoxon) and the oxime 2PAM were purchased from Sigma-Aldrich (St. Louis, MO) . The oxime HI6 was purchased from US Biological (Swampscott, MA). Oximes were used in a range of 0.05-10 mM. Final concentrations of organic solvents were less than 1% in enzyme assays.
HEK-293 cells were transfected with the hAChE D134H DNA construct, prepared as described for the mouse AChE [6], with exception that hAChE constructs had FLAG peptide sequence encoded at the 5’ end of the construct. The stable cell clones were selected by treatment with G-418. The protein was purified in mg quantities by adsorption and desorption from an antiFLAG peptide antibody resin.
In reactivation experiments wild type and mutant enzymes (40 nM) were inhibited for 10-30 minutes until inhibition was greater than 95%. Inhibited enzyme and the control were passed through two consecutive Sephadex G-50 spin columns to remove excess unreacted inhibitor. Inhibited and control enzymes were incubated with oxime and time courses of recovery of hAChE activity were measured in aliquots of reactivation and control reaction mixtures by spectrophotometric Ellman assay (at 22°C, in 100 mM phosphate buffer, pH 7.4, containing 1 mM ATCh as substrate and 333 μM DTNB as final concentrations) Detailed description of experimental procedures and calculations is given elsewhere [6]. Inhibition kinetic experiments were conducted as described before [7] using final OP concentrations between 10 nM and 50μM.
3. Results and discussion
Inhibition of human D134H with paraoxon and an analogue of cyclosarin was 2-8 times slower than that of human wt (Table 1), whereas inhibition by soman and analogues of VX was not affected by D134H substitution. Introduction of additional substitutions in two double mutants further slowed inhibition up to two orders of magnitude.
Table 1.
Relative inhibition rates of hD134H wt; hD134H_E202Q and hD134H_F338A.
| Enzyme | Paraoxon | VX analogue | Cyclosarin analogue | Soman analogue |
|---|---|---|---|---|
| hAChE wt 1 | 1 | 1 | 1 | 1 |
| hD134H | 0.4 | 1 | 0.1 | 1 |
| hD134H_E202Q | 0.5 | 0.2 | 0.01 | 0.04 |
| hD134H_F338A | 0.1 | 1 | 0.2 | 0.3 |
The second order inhibition rate constants of hAChE wt with paraoxon and analogues of VX, cyclosarin and soman were 330*104, 290*104, 580*104 and 60*104 M-1 min-1, respectively. Inhibition by SPCHMPTCh, RPIPMPTCh was not measured.
2PAM reactivation of paraoxon inhibited human D134H is, on the other hand, 6 times faster than human wt (Table 2). Our previous study indicated that differential occupation of the acyl pocket in mouse AChE, by covalent ligands of varying geometry, had distinct effects on spectra of fluorophores covalently attached on the AChE surface in the general spatial vicinity of residue D134 [8]. In order to determine whether the acyl pocket stabilized ethoxy substituent on phosphorus in diethylphosphorylated hAChE was primarily responsible for the observed enhancement of reactivation rates, the enzymes were inhibited with an excess of racemic VX analogue. Inhibition was assumed to yield wt and D134H AChEs conjugated primarily by the SP VX enantiomer with methylphosphonyl substituent of the conjugate stabilized in the acyl pocket and the ethoxy substituent oriented towards the choline binding site. Reactivation of both wt and D134H hAChE SP VX cojugates with 2 PAM showed similar reactivation rates. This suggests that the 2PAM reactivation rate enhancement observed in the diethylphosphorylated (paraoxon inhibited) D134H mutant may be linked with improved stabilization of the ethoxy substituent in the mutant acyl pocket and is consistent with the observed three-fold enhancement of the HI6 reactivation rate (Table 2). To verify this hypothesis further 2PAM reactivation of the RPIPMP inhibited enzyme conjugates (identical to RP Sarin conjugated AChE) was studied but yielded no effect of D134H mutation. Thus, our experiments implicate differential flexibility of the acyl pocket in D134H substituted hAChE, but the actual mechanism of the 2PAM reactivation enhancement remains unclear.
Table 2.
Relative reactivation rates of OP inhibited hD134H; hD134H_E202Q and hD134H_F338A.
| hAChE | OP | OXIME | Relative Reactivation Rate |
|---|---|---|---|
| wt 1 | Paraoxon | 2PAM | 1 |
| D134H | 6 | ||
| D134H_E202Q | 0.1 | ||
| D134H_F338A | 1 | ||
| wt 2 | HI6 | 1 | |
| D134H | 3 | ||
| wt 3 | SP CHMPTCh | 2PAM | 1 |
| D134H | 2 | ||
| wt 4 | HI6 | 1 | |
| D134H | 0.3 | ||
| D134H_E202Q | 0.02 | ||
| D134H_F338A | 0.002 | ||
| wt 5 | VX analogue | 2PAM | 1 |
| D134H | 1 | ||
| wt 6 | RP IPMPTCh | 2PAM | 1 |
| D134H | 1 |
The second order rate constants for reactivation of OP inhibited wt hAChE by oximes were:
120 M-1min-1 (paraoxon inhibited hAChE reactivated by 2PAM)
45 M-1min-1 (paraoxon inhibited wt hAChE reactivated by HI6)
275 M-1min-1 (SPCHMPTCh inhibited wt hAChE reactivated by 2PAM)
13000 M-1min-1(SPCHMPTCh inhibited wt hAChE reactivated by HI6)
490 M-1min-1(VX inhibited wt hAChE reactivated by 2PAM)
270 M-1min-1(RPIPMPTCh inhibited wt hAChE reactivated by 2PAM).
Reactivation of cyclosarin and soman inhibited hAChE was not measured.
Human D134H_E202Q and D134H_F338A non-aging double mutants did not reactivate any faster than the wt or the hD134H mutant (Table 2). In fact, of all tested enzymes the one most compromised with mutations was D134H_E202Q mutant. It showed both inhibition and reactivation rates about 20 times slower in comparison to wt and D134H (Tables 1 and 2). The characteristics of double mutants mostly arose from E202Q mutation that has slow phosphonylation and reactivation rates. We were thus unable to combine resistance to aging (coming from E202Q or F338A substitutions) and enhanced reactivation (coming from D134H substitution) in the same double mutant hAChE protein.
In summary, our initial study shows that selected surface residue substitutions in the AChE molecule located remotely from the active center affect its catalytic parameters, OP inhibition and oxime reactivation and may be useful in designing oxime-hAChE couples for catalytic OP hydrolysis. However, combining diverse properties of single site mutations to form multiple site hAChE mutants does not result in a simple summation of ΔG values, rather there is a coupling of energy values that appear linked.
Acknowledgement
This study was supported by fellowships from IIE and The Fulbright Program, and the Scientific and Technical Research Council of Turkey to Tuba Kucukkilinc and by NIH NINDS U01NS58046 to Palmer Taylor.
Abbreviations
- OPs
organophosphates
- AChE
acetylcholinesterase
- hAChE
human AChE
- BChE
butyrylcholinesterase
- SNP
single nucleotide polymorphism
- wt
wild type
- ATCh
acetylthiocholine
- DTNB
5,5’-dithiobis-(2-nitrobenzoic acid)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Taylor P, Kovarik Z, Reiner E, Radic Z. Acetylcholinesterase: converting a vulnerable target to a template for antidotes and detection of inhibitor exposure. Toxicology. 2007;233:70–78. doi: 10.1016/j.tox.2006.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashani Y, Radic Z, Tsigelny I, Vellom DC, Pickering NA, Quinn DM, Doctor BP, Taylor P. Amino acid residues controlling reactivation of organophosphonyl conjugates of acetylcholinesterase by mono- and bisquaternary oximes. J. Biol. Chem. 1995;270:6370–6380. doi: 10.1074/jbc.270.11.6370. [DOI] [PubMed] [Google Scholar]
- 3.Shafferman A, Ordentlich A, Barak D, Stein D, Ariel N, Velan B. Aging of phosphylated human acetylcholinesterase: catalytic processes mediated by aromatic and polar residues of the active centre. Biochem. J. 1996;318:833–840. doi: 10.1042/bj3180833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amitai G, Adani R, Yacov G, Yishay S, Teitlboim S, Tveria L, Limanovich O, Kushnir M, Meshulam H. Asymmetric fluorogenic organophosphates for the development of active organophosphate hydrolases with reversed stereoselectivity. Toxicology. 2007;233:187–198. doi: 10.1016/j.tox.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Berman HA, Leonard K. Chiral reactions of acetylcholinesterase probed with enantiomeric methylphosphonothioates. Noncovalent determinants of enzyme chirality. J. Biol. Chem. 1989;264:3942–3950. [PubMed] [Google Scholar]
- 6.Kovarik Z, Radic Z, Berman HA, Simeon-Rudolf V, Reiner E, Taylor P. Mutant cholinesterases possessing enhanced capacity for reactivation of their phosphonylated conjugates. Biochemistry. 2004;43:3222–3229. doi: 10.1021/bi036191a. [DOI] [PubMed] [Google Scholar]
- 7.Kovarik Z, Radić Z, Berman HA, Simeon-Rudolf V, Reiner E, Taylor P. Acetylcholinesterase active centre and gorge conformations analysed by combinatorial mutations and enantiomeric phosphonates. Biochem. J. 2003;373:33–40. doi: 10.1042/BJ20021862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi J, Boyd AE, Radic Z, Taylor P. Reversibly bound and covalently attached ligands induce conformational changes in the omega loop, Cys69-Cys96, of mouse acetylcholinesterase. J. Biol. Chem. 2001;276:42196–42204. doi: 10.1074/jbc.M106896200. [DOI] [PubMed] [Google Scholar]