Abstract
The current paradigm for cancer initiation and progression rests on the groundbreaking discoveries of oncogenes and tumor suppressor genes. This framework has revealed much about the role of genetic alterations in the underlying signaling pathways central to normal cellular function and to tumor progression. However, it is clear that single gene theories or even sequential acquisition of mutations underestimate the nature of the genetic and epigenetic changes in tumors, and do not account for the observation that many cancer susceptibility genes (e.g. BRCA1, APC) show a high degree of tissue specificity in their association with neoplastic transformation. Therefore, the cellular and tissue context itself must confer additional and crucial information necessary for mutated genes to exert their influence. A considerable body of evidence now shows that cell–cell and cell–extracellular matrix (ECM) interactions are essential organizing principles that help define the nature of the tissue context, and play a crucial role in regulating homeostasis and tissue specificity. How this context determines functional integrity, and how its loss can lead to malignancy, appears to have much to do with tissue structure and polarity.
Keywords: breast tissue, luminal epithelial cells, myoepithelial cells, breast cancer, 3D cultures
Introduction
Mechanisms that govern the maintenance of the differentiated state are inextricably linked with cancer. No-where is this more obvious than in the mammary gland, which can undergo repeated cycles through characteristic morphologic alterations: in puberty, there is branching morphogenesis; in pregnancy, expansion of the ductal tree is associated with massive cellular proliferation; in lactation, differentiation leads to milk production; and in involution, extensive apoptosis causes regression to the pre-pregnant state. These changes also occur, although less dramatically, during each estrus cycle, and in each phase, there are ample opportunities for the complex remodeling mechanisms to go wrong and be diverted toward pathways that lead to malignancy. Growth factors, hormones, cytokines, extracellular matrix (ECM) molecules, matrix metalloproteinases (MMPs), and morphogens all play crucial roles in development and remodeling of the gland (Daniel and Smith, 1999), and all play roles in breast cancer as well (Bissell and Radisky, 2001). How can we understand the mechanisms by which this myriad of molecules and pathways are coordinated, and what is the best way to study them?
The majority of studies of the mechanisms by which normal and cancer cells respond to extracellular signals have utilized cultures of isolated, cloned and immortal cells grown on rigid surfaces (2D culture conditions) (Bissell, 1981; Walpita and Hay, 2002). Although enormously useful for studying genetic interactions of mutated oncogenes and tumor suppressor genes, as well as fundamental cellular mechanisms that govern survival and cell renewal, these studies by necessity largely use growth, cell death, and adhesion of single cells or cells on monolayers as the final end points. However, work from many laboratories, including ours, has shown dramatic changes in morphology and function depending on context. More recently, comparative analysis of gene expression patterns of breast cancer cell lines and breast tumors suggests that tumorigenesis within the breast tissue results in distinct patterns of gene expression that reflect the influence of the tissue context on breast cancer development (Perou et al., 1999; 2000; Ross and Perou, 2001). Therefore, it is useful to model biological pathways and regulations under conditions that consider the microenvironment and the community of cells that form the organ.
The mammary gland presents an ideal model for investigating the relationship between tissue architecture and cellular function. The basic mammary structure is the acinus, in which milk is secreted into a central lumen by a continuous layer of luminal epithelial cells; these are surrounded by a layer of myoepithelial cells, and this double-layered structure is encased in the basement membrane (BM), the ECM that provides both structural support and contextual information. In this brief chapter, we will describe how three-dimensional (3D) ECM cultures can be used to model both normal and malignant mammary gland-specific cellular interactions, and how such investigations have provided valuable insights into the processes that govern both normal breast-specific functions and mechanisms of breast cancer progression.
Modeling the breast
As early as 1977, Emerman and Pitelka used “floating collagen gels” to show that normal breast tissue retains its differentiated unit tissue structure only when maintained in a deformable 3D ECM environment (Emerman et al., 1977; Emerman and Pitelka, 1977). Following this basic observation, we showed that primary mammary epithelial cells under these conditions can not only retain global and stage-specific metabolic function (Emerman et al., 1981), but could also regain functional differentiation as evidenced by de novo synthesis of milk proteins (Lee et al., 1984; 1985). Since then, we have developed additional 3D assays to investigate mammary-specific functions in rodent and human cells (Barcellos-Hoff et al., 1989; Petersen et al., 1992), and to distinguish normal and malignant behavior rapidly without the necessity of using whole animals. Simply by growing mammary epithelial cells in 3D gels of laminin-rich basement membrane (lrBM), normal and cancer cell behavior is rapidly and reproducibly distinguished (Fig. 1) (Petersen et al., 1992). Nontumorigenic cell lines respond to lrBM by arresting proliferation and undergoing a structural differentiation into polarized, acini-like structures. These structures are capable of production of an endogenous basement membrane, localization of cell-ECM receptors to the outer (basal) surface, and secretion of sialomucin into the central (apical) lumen. Under the same conditions, tumorigenic cell lines do not arrest growth or polarize, but instead grow into disorganized masses. This assay can be used to definitively establish mammary epithelial cells as “normal”, premalignant, and/or tumorigenic, even if the difference is very subtle when the cells are grown on 2D cultures.
Fig. 1.
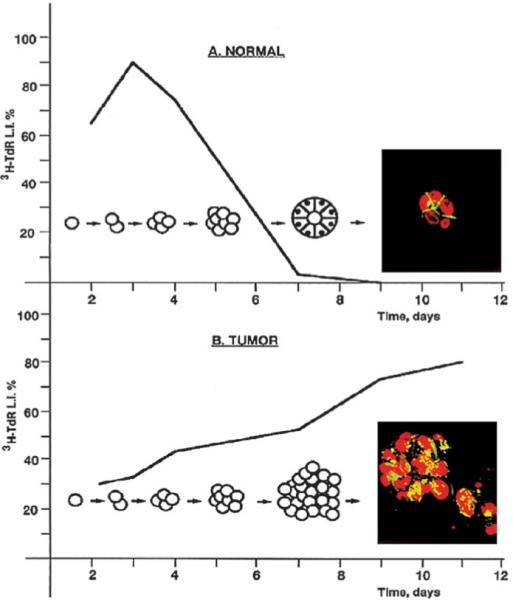
The 3D assay. Normal mammary epithelial cells, cultured in 3D gels of laminin-rich basement membrane (lrBM), arrest growth and organize into polar, acini-like structures (top). By contrast, mammary tumor cells continue to proliferate into disorganized masses (bottom). (Reproduced with modifications by permission of Cancer Biology from Weaver et al., 1995.)
Controlling the organization of the double-layered acinus
In contrast to normal mammary cells, breast tumor cells generally are unable to achieve a differentiated unit structure in 3D lrBM. How do nonmalignant cells recognize that they are to reorganize? Is three-dimensionality per se sufficient for this behavior or are specific components of the lrBM required? Recent work with heterotypic 3D cocultures demonstrates that the latter is the case (Gudjonsson et al., 2002). Using an assay in which luminal epithelial cells are cultured within collagen-I gels to form structures with reversed polarity (assessed by staining for sialomucin, epithelial specific antigen, and occludin; Fig. 2A), we found that addition of normal human myoepithelial cells corrected the inverse polarity and led to the formation of double-layered acini with central lumina. The polarizing component of the myoepithelial cells was found to be laminin-1, the specific component of lrBM able to substitute for myoepithelial cells for defining correct polarity. In contrast to normal breast tissue, breast tumor cells are characteristically apolar, even though breast cancers often contain myoepithelial cells. Further coculture experiments established that myoepithelial cells isolated from tumors did not produce laminin-1 (Fig. 2B), and were not competent to direct polarization of normal luminal epithelial cells (Gudjonsson et al., 2002).
Fig. 2.
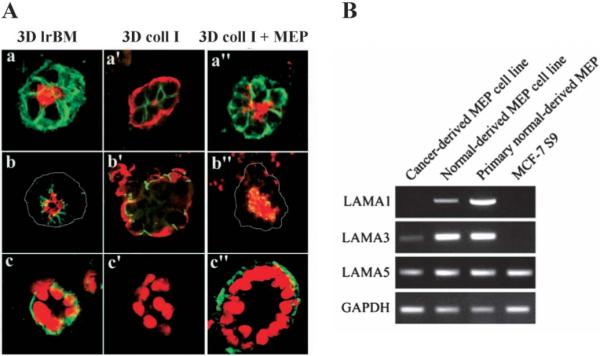
Myoepithelial cells (MEP) provide the organizational context to luminal epithelial cells. A Luminal cells form normal acini in lrBM, but inside-out acini in collagen I (coll I), and this inverse polarization is reversed by addition of human myoepithelial cells (MEP). a, a’, a”: apical marker sialomucin (red) and basal marker epithelial-specific antigen (green); b, b’, b”: occludin (green), which orients on the apical side of the cell–cell junctions, and sialomucin (red); c, c’, c”: nuclear stain (red) and cell-ECM receptor (and basal marker) β4-integrin (green). B Cancer-derived myoepithelial cells lack the expression of laminin-α1 chain, which is a component of laminin-1, but the same cells maintain expression of laminin-α3 and laminin-α5, components of laminin-5 and laminin-10/11, respectively. (Reproduced with modifications by permission of Journal of Cell Science from Gudjonsson et al., 2002.)
Modifying the malignant phenotype
While a small number of breast cancer cell lines may still respond to a laminin-rich milieu by arresting growth and polarizing, we find that the majority of breast cancer cell lines continue to proliferate in a disorganized fashion when grown under these conditions (Wang et al., 2002). What signaling pathways have become aberrant in these tumor cells that makes them ignore signals that direct nonmalignant cells to arrest growth and become organized? An analysis of HMT-3522 T4–2 breast tumor cells (T4–2 cells) for surface expression of receptors for ECM and growth factors indicated aberrant levels of β1 integrin and epidermal growth factor receptor (EGFR), as compared to HMT-3522 S1 cells (S1 cells), the nontumorigenic counterpart of the T4–2 cells (Weaver et al., 1997). This suggested that, while the requisite ECM molecule (laminin-1) was present, and the EGF concentration was unchanged relative to the normal samples, the pathways downstream of β1 integrins and/or EGFR could be in overdrive due to excess receptor activity. If so, then down-modulation of receptor signaling could restore the responsiveness of the tumor cells to the 3D microenvironment. This indeed proved to be the case, as inhibition of ECM and growth factor receptors expressed by breast cancer cells successfully reverted the malignant phenotype (Fig. 3A) (Wang et al., 1998; Weaver et al., 1997). It is important to note that this phenotypic reversion occurs against a constant genetic background (Fig. 3B) and only in 3D cultures. Furthermore, “revertability” was not limited to HMT-3522 T4–2 cells. Analysis of additional cell lines, some of which were aggressive and metastatic, and each of which contained different sets of genetic deletions and amplifications, showed that reversion and/or apoptosis could be achieved by inhibiting ECM and growth factor signaling by one or two signaling inhibitors (Wang et al., 2002). Thus, in addition to the nonmetastatic T4 cells, three metastatic cell lines of very different origin and genetic composition, MCF-7, Hs578T, and MDA-MB-231, could be partially reverted by using single inhibitors against β1 integrin, MAPK, or PI3K, and nearly completely reverted (MDA-MB-231, MCF-7) or killed (Hs578T) by combined inhibition of β1 integrin and either PI3K or MAPK (Fig. 4; Wang et al., 2002).
Fig. 3.
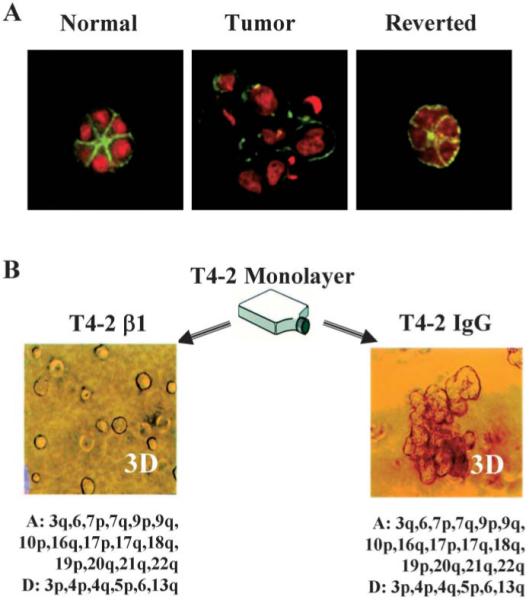
Reversion of HMT-3522 T4–2 cells. A Reversion by inhibition of EGFR using function-blocking antibodies. Green, actin; red, nuclei. B Phenotypic reversion by inhibition of β1 integrins occurs against a constant genetic background (A reproduced from Wang et al., 1998; B reproduced with modifications by permission of Journal of Cell Biology from Weaver et al., 1997; CGH data, Joe Gray and Bissell laboratories, manuscript in preparation).
Fig. 4.
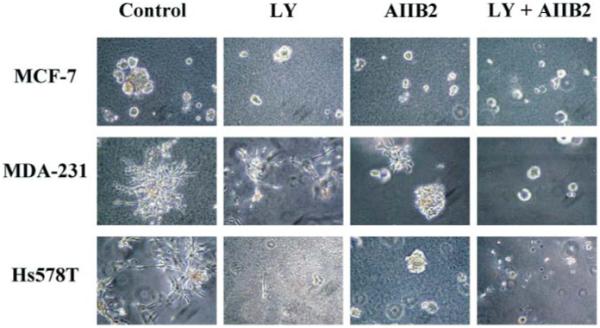
Inhibiting β1 integrin together with PI3K in 3D lrBM can revert or kill aggressive breast cancer cell lines. MCF-7, MDA-MB-231, and Hs578T cells were cultured in 3D lrBM either without inhibitor (control), with LY294002 (LY), an inhibitor of PI3–kinase, with AIIB2, an inhibitor of β1 integrins (AIIB2), or with both. (Reproduced with permission of Journal of National Cancer Institute from Wang et al, 2002.)
These studies demonstrate that microenvironment-initiated signals can be modified so as to induce tumor cells to return to differentiation or apoptotic pathways, results that suggest unexplored avenues to new approaches for treatment of breast cancer. New, directed therapies such as Herceptin® and Gleevec® that target signaling pathways are an initial step in this direction, but optimal development of therapies tailored to specific groups of breast cancer patients will require a greater understanding of the key ECM components/receptors in the microenvironment of tumor cells and signaling pathways that operate in 3D that permit and participate in their unregulated proliferation and resistance to apoptosis.
Reconnecting signaling pathways
Nonmalignant and malignant breast cells (both primary and cell lines) can be distinguished from each other, easily and rapidly (within 6 days), by culturing in 3D lrBM. In studying the basis of these phenotypic differences, we discovered that growth factor and adhesion pathways are “connected” in 3D, i.e., that inhibition of one signaling pathway leads to down-modulation of the other (Bissell et al., 1999; Wang et al., 1998), and that this phenomenon does not occur in 2D (Fig. 5).
Fig. 5.
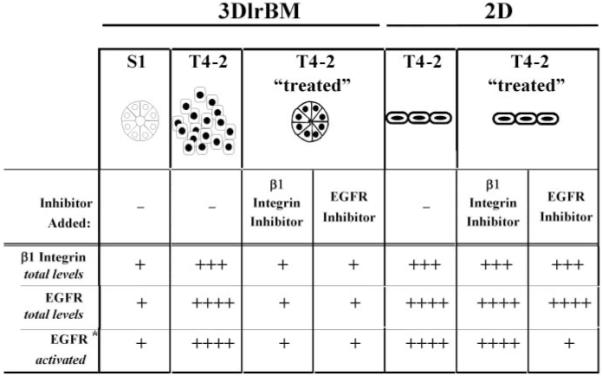
β1 integrin and EGFR protein levels and signal activation are coordinately modulated in HMT-3522 cells cultured in 3D lrBM but not in 2D. When 3D T4–2 cells are treated with functional inhibitors of either β1 integrin or EGFR (T4–2 treated) to revert the cells, endogenous β1 and EGFR protein levels down-modulate coordinately. This phenomenon does not occur in 2D. (Reproduced with permission of Cancer Research from Bissell et al, 1999.)
We have found that cross talk between ECM signaling pathways extended its effects to modulation of functional expression of the Cocksackie adenovirus receptor (CAR). Our investigations of HMT-3522 cells in 3D lrBM show that CAR mRNA and protein expression is higher in tumorigenic T4–2 cells than in the normal S1 cells, although CAR expression levels are similar when the two cell types are grown on 2D. Thus, CAR expression represents another pathway that becomes cross-modulated during reversion (Michael Korn and Bissell laboratories, submitted for publication).
Just as inhibition of abnormally active signaling pathways can restore responsiveness to 3D conditions, so abnormal or excessive reactivation of signaling pathways can be sufficient to block or reverse normal organization. These relationships were tested in HMT-3522 S1 cells by overexpression of EGFR/ErbB1. When these cells were stably transfected with EGFR, and then placed in 3D conditions, they did not properly reorganize, and they expressed higher levels of other surface receptors such as β1 integrins (Wang et al., 1998). It is interesting to note that when EGFR was activated conditionally in another human mammary epithelial cell line, MCF10A, which also form acini in 3D lrBM (Petersen et al., 1992), its activation had no effect, whereas homodimerization and activation of ErbB2 in 3D led to reinitiation of cell proliferation, to a block of apoptotic mechanisms that allowed lumen formation and to loss of acinar structure (Fig. 6) (Muthuswamy et al., 2001).
Fig. 6.
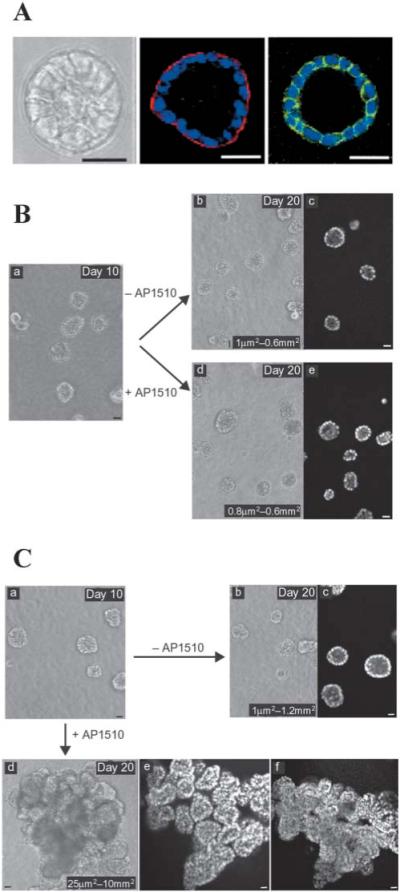
Selective alteration of signaling pathways disrupts organization of 3D structures. A MCF10A cells form growth-arrested, polarized acinar structures in 3D lrBM. a, phase contrast; b, collagen IV (red)/nuclei (blue); c, beta-catenin (green)/nuclei (blue). Scale bars: 50 mm. B MCF-10A cells in which ErbB1/EGFR could be autoactivated by addition of AP1510 show no alteration in acinar structure (b) relative to untreated cultures (a). C MCF-10A cells in which ErbB2 could be activated by addition of AP1510 showed loss of polarized organization and developed multi-acinar structures with filled lumina indicating that the combination of loss of growth and loss of apoptotic regulation creates ductal carcinoma in situ (DCIS)-like structures. (Reproduced with modifications by permission of Nature Cell Biology from Muthuswamy et al., 2002.)
To provide a more global picture of the role that 3D lrBM plays in the control of gene expression in normal and tumor cells, we have performed genome-wide expression analyses using cDNA microarrays, of S1 and T4–2 cells, grown in 2D and 3D lrBM. We found very little overlap in gene expression differences between nontumorigenic and tumorigenic cells in 2D as compared to 3D (Fig. 7): of 8000 genes, 166 transcripts showed statistically significant differences between S1 and T4–2 when cells were cultured in 2D, while 445 showed altered expression when the cells were cultured in 3D (Fig. 7B). Of these, only 15 genes were expressed differentially in both S1 and T4–2 cells between 2D and 3D conditions, and 6 of these 15 genes showed altered expression in opposite directions (Fig. 7). (For example, the gene denoted by #4265 was overexpressed in T4–2 in 3D compared to S1 in 3D, but was downregulated in T4–2 in 2D compared to S1 in 2D culture.) These observations provide a quantitative sense of how many signaling pathways are wired differently in 2D and 3D, and suggest that studying the gene expression patterns of cancer cell lines in the 3D context will likely provide an additional set of clues about which pathways are disrupted in these cells.
Fig. 7.
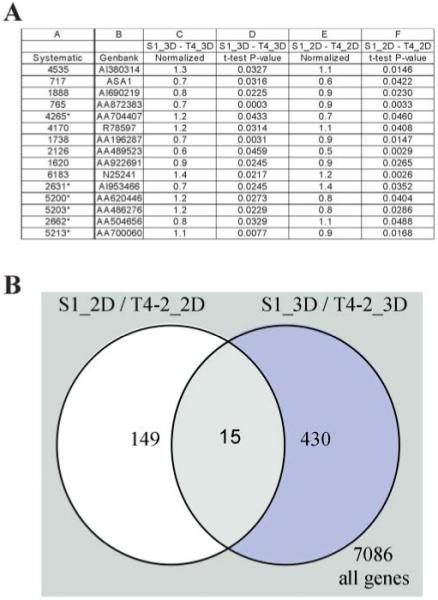
Gene expression differences between tumorigenic and nontumorigenic cell lines are influenced by 3D lrBM. HMT-3522 cell lines S1 (nontumorigenic) and T4–2 (tumorigenic) were compared for global gene expression, when grown in 2D or in 3D lrBM, using 8k human cDNA arrays. Three cultures and four slides, including a dye swap, were scanned in per experiment. Slides were scanned in using Genepix®, and data was normalized (per slide). Genes that change significantly (t-test, P<0.05) were determined using GeneSpring®. A Table showing a list of genes that change in both 2D and 3D when nontumorigenic and tumorigenic cells are compared. A normalized ratio of > 1 represents a higher expression in tumorigenic T4–2 cells; * indicates genes that change in opposite direction in 2D vs. 3D lrBM (Rizki and Bissell, unpublished data). B Venn diagram shows a comparison of the list of genes that change significantly between S1 and T4–2 in 2D vs. in 3D.
Controlling apoptotic pathways
Just as 3D lrBM can stimulate mammary epithelial cells to structurally differentiate, so the microenvironmental context also controls apoptotic response in this cell type. Previously, we showed that attachment to BM components was necessary to prevent apoptotic cell death (Boudreau et al., 1995; 1996), a process that could be distinguished from the universal requirement for attachment to prevent anoikis (Frisch and Screaton, 2001). While generic cell-ECM attachment appears to delay apoptosis in virtually all cell types, specific attachment of mammary epithelial cells to laminin-containing BM is required for homeostasis for extended periods (more than 10 days). More recently, we have shown that context also determines apoptotic sensitivity to chemotherapeutic agents and immune modulators, regardless of the genetic makeup, the rate of growth, or the malignancy status of the cell. Specifically, we found that resistance to apoptosis strictly correlated with ECM-induced polarity in 3D (Fig. 8; Weaver et al., 2002). While nonmalignant S1 cells and malignant T4–2 cells are sensitive to a variety of apoptotic stimuli when grown on 2D, they behave very differently in 3D: S1 cells or reverted T4–2 cells become resistant to the apoptotic stimuli, but non-reverted T4–2 cells continue to be sensitive. Disrupting polar S1 structures by incubation with function-blocking antibodies against E-cadherin or by disrupting hemidesmosomes with dominant negative β4 integrin in 3D lrBM resulted in loss of resistance to apoptosis. These data clearly establish that testing cancer drugs for an effect on apoptosis of cells grown in 3D is likely to produce very different results than drug testing with 2D cultures, and that combining information from 2D and 3D cultures with clinical data could provide a rich surrogate for finding new drugs against human tumors.
Fig. 8.
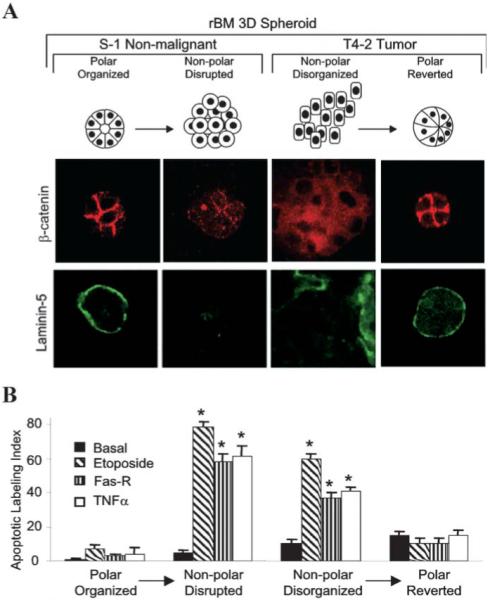
Polarized mammary structures are resistant to apoptosis induction. S1 acini within 3D lrBM were treated with E-cadherin blocking antibody to perturb polarity. T4–2 structures within 3D lrBM were treated with beta-1 integrin inhibitory antibody to restore polarity. A Confocal microscopy of beta-catenin and laminin-5. S1 and reverted T4–2 acini have junctionally organized beta-catenin and basally secreted laminin-5, in contrast to disrupted S1 and disorganized T4–2 colonies. B Apoptotic index of the cell cultures in (A), either untreated (basal), or treated with etoposide, Fas-R, or TNF-α. (Reproduced with modification by permission Cancer Cell from Weaver et al, 2002.)
Adding complexity with 3D cocultures
While much has been learned using 3D monocultures of mammary epithelial cells, closer approximations of the normal and malignant breast microenvironment will require inclusion of other cell types. To that end, we have developed more complex models to dissect heterotypic interactions. Coculture of luminal epithelial and myoepithelial cells can be used to identify the structural principles of the normal, double-layered acinus (Figs. 2 and 9A) (Gudjonsson et al., 2002). Similarly, coculture of carcinoma and stromal fibroblast cells can be used to model tumors (Fig. 9B) (Ronnov-Jessen et al., 1995). Future studies will be required to combine yet additional cell types and possibly even to reconstitute the entire breast organ architecture.
Fig. 9.
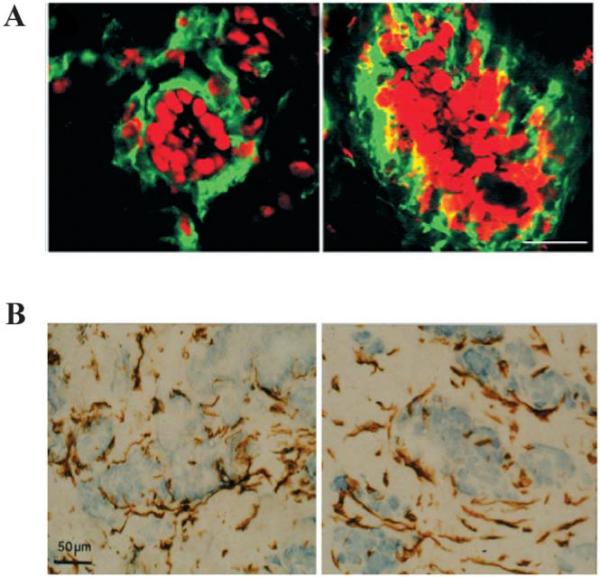
Heterotypic 3D cultures recapitulate in vivo structures. A Coculture of human mammary luminal and myoepithelial cells in collagen I (left), compared to histologic section of normal human breast tissue (right). Green is a myoepithelial marker Thy-1; red is nuclear staining (reproduced with modifications by permission of Journal of Clinical Investigation from Gudjonsson et al., 2002). B Coculture of human luminal epithelial and stromal fibroblast cells in collagen I (left), compared to histologic section of human breast cancer (right). Staining for vimentin and counterstaining of nuclei with hematoxylin (reproduced with modifications by permission of Journal of Cell Science from Ronnov-Jessen, 1995).
Conclusion: Tumors as Organs
The complex microenvironment of cells in organs is produced and remodeled by interactions among several cell types. Therefore, cancer is initiated and then progresses in a complex signaling microenvironment. While breast cancer is traditionally considered to originate from luminal epithelial cells, components of the developing carcinoma can subsequently differentiate toward myofibroblast phenotypes (Ronnov-Jessen et al., 1996). That this is important to our understanding of breast cancer progression is demonstrated by observations that myofibroblasts derived from breast tumors exert tumor-promoting effects (Airola and Fusenig, 2001; Dimanche-Boitrel et al., 1994; Fukumura et al., 1998; Gilles et al., 1997; Petersen et al., submitted), while myoepithelial cells can suppress growth of normal and tumor breast epithelial cells, and the presence of residual normal myoepithelial cells in tumors is often associated with a favorable prognosis (Pechoux et al., 1999; Radice et al., 1997; Slade et al., 1999; Sternlicht et al., 1997; Zou et al., 1994).
As a consequence of these types of studies, we have proposed that each breast tumor is a unique and complex organ that constantly evolves not only because of specific mutations and genomic instability within individual cancer cells, but also because of dynamic changes in tumor cell interactions with their microenvironment, which consists of soluble factors, the ECM, the immune modulators, normal cells and phenotypically heterogeneous tumor cells (Bissell and Radisky, 2001). It follows that investigations into the mechanisms that control breast cancer progression and identification of clinically useful reagents will be accelerated if experiments are performed either in animals in vivo or in model systems that recapitulate the physiologic microenvironment. Since the former is costly and complex and in any case not easily recapitulated in humans, physiologic three-dimensional model systems using human cells to create the right context are an obvious choice.
Acknowledgements
The work summarized in this article was supported by funds from the United States Department of Energy, Office of Biological and Environmental Research (DE-AC03 SF0098 to M.J.B.), the National Cancer Institute (CA64786-02 to M.J.B. and CA57621 to Zena Werb and M.J.B.). O.W.P. was supported by grants from The Danish Research Council, The Novo Nordisk Foundation, The Thaysen Foundation, Friis Fonden, The Meyer Foundation, The Danish Cancer Society. D.R. was supported by a Distinguished Hollaender Postdoctoral Fellowship from the DOE, the American Cancer Society, and A.R. was supported by a Postdoctoral Fellowship from the DOD-BCRP.
Contributor Information
Valerie M. Weaver, Department of Pathology and Institute for Medicine and Engineering, University of Pennsylvania, PA, USA
Ole W. Petersen, Structural Cell Biology Unit, Institute of Medical Anatomy, The Panum Institute, Copenhagen, Denmark
References
- Airola K, Fusenig NE. Differential stromal regulation of MMP-1 expression in benign and malignant keratinocytes. J Invest Dermatol. 2001;116:85–92. doi: 10.1046/j.1523-1747.2001.00223.x. [DOI] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ. The differentiated state of normal and malignant cells or how to define a “normal” cell in culture. Int Rev Cytol. 1981;70:27–100. doi: 10.1016/s0074-7696(08)61130-4. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Radisky D. Putting tumours in context. Nature Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Weaver VM, Lelievre SA, Wang F, Petersen OW, Schmeichel KL. Tissue structure, nuclear organization, and gene expression in normal and malignant breast. Cancer Res. 1999;59:1757s–1763s. discussion 1763s–1764s. [PubMed] [Google Scholar]
- Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau N, Werb Z, Bissell MJ. Suppression of apoptosis by basement membrane requires three-dimensional tissue organization and withdrawal from the cell cycle. Proc Natl Acad Sci USA. 1996;93:3509–3513. doi: 10.1073/pnas.93.8.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel CW, Smith GH. The mammary gland: a model for development. J Mammary Gland Biol Neoplasia. 1999;4:3–8. doi: 10.1023/a:1018796301609. [DOI] [PubMed] [Google Scholar]
- Dimanche-Boitrel MT, Vakaet L, Jr., Pujuguet P, Chauffert B, Martin MS, Hammann A, Van Roy F, Mareel M, Martin F. In vivo and in vitro invasiveness of a rat colon-cancer cell line maintaining E-cadherin expression: an enhancing role of tumor-associated myofibroblasts. Int J Cancer. 1994;56:512–521. doi: 10.1002/ijc.2910560410. [DOI] [PubMed] [Google Scholar]
- Emerman JT, Bartley JC, Bissell MJ. Glucose metabolite patterns as markers of functional differentiation in freshly isolated and cultured mouse mammary epithelial cells. Exp Cell Res. 1981;134:241–250. doi: 10.1016/0014-4827(81)90481-x. [DOI] [PubMed] [Google Scholar]
- Emerman JT, Enami J, Pitelka DR, Nandi S. Hormonal effects on intracellular and secreted casein in cultures of mouse mammary epithelial cells on floating collagen membranes. Proc Natl Acad Sci USA. 1977;74:4466–4470. doi: 10.1073/pnas.74.10.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman JT, Pitelka DR. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977;13:316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK, Seed B. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- Gilles C, Polette M, Seiki M, Birembaut P, Thompson EW. Implication of collagen type I-induced membrane-type 1–matrix metalloproteinase expression and matrix metalloproteinase-2 activation in the metastatic progression of breast carcinoma. Lab Invest. 1997;76:651–660. [PubMed] [Google Scholar]
- Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci. 2002;115:39–50. doi: 10.1242/jcs.115.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EY, Lee WH, Kaetzel CS, Parry G, Bissell MJ. Interaction of mouse mammary epithelial cells with collagen substrata: regulation of casein gene expression and secretion. Proc Natl Acad Sci USA. 1985;82:1419–1423. doi: 10.1073/pnas.82.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EY, Parry G, Bissell MJ. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J Cell Biol. 1984;98:146–155. doi: 10.1083/jcb.98.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol. 2001;3:785–792. doi: 10.1038/ncb0901-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechoux C, Gudjonsson T, Ronnov-Jessen L, Bissell MJ, Petersen OW. Human mammary luminal epithelial cells contain progenitors to myoepithelial cells. Dev Biol. 1999;206:88–99. doi: 10.1006/dbio.1998.9133. [DOI] [PubMed] [Google Scholar]
- Perou CM, Jeffrey SS, van de, Rijn M, Rees CA, Eisen MB, Ross DT, Pergamenschikov A, Williams CF, Zhu SX, Lee JC, Lashkari D, Shalon D, Brown PO, Botstein D. Distinctive gene expression patterns in human mammary epithelial cells and breast cancers. Proc Natl Acad Sci USA. 1999;96:9212–9217. doi: 10.1073/pnas.96.16.9212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci USA. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radice GL, Ferreira-Cornwell MC, Robinson SD, Rayburn H, Chodosh LA, Takeichi M, Hynes RO. Precocious mammary gland development in P-cadherin-deficient mice. J Cell Biol. 1997;139:1025–1032. doi: 10.1083/jcb.139.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev. 1996;76:69–125. doi: 10.1152/physrev.1996.76.1.69. [DOI] [PubMed] [Google Scholar]
- Ronnov-Jessen L, Petersen OW, Koteliansky VE, Bissell MJ. The origin of the myofibroblasts in breast cancer. Recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth muscle cells. J Clin Invest. 1995;95:859–873. doi: 10.1172/JCI117736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross DT, Perou CM. A comparison of gene expression signatures from breast tumors and breast tissue derived cell lines. Dis Markers. 2001;17:99–109. doi: 10.1155/2001/850531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade MJ, Coope RC, Gomm JJ, Coombes RC. The human mammary gland basement membrane is integral to the polarity of luminal epithelial cells. Exp Cell Res. 1999;247:267–278. doi: 10.1006/excr.1998.4340. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Kedeshian P, Shao ZM, Safarians S, Barsky SH. The human myoepithelial cell is a natural tumor suppressor. Clin Cancer Res. 1997;3:1949–1958. [PubMed] [Google Scholar]
- Walpita D, Hay E. Studying actin-dependent processes in tissue culture. Nat Rev Mol Cell Biol. 2002;3:137–141. doi: 10.1038/nrm727. [DOI] [PubMed] [Google Scholar]
- Wang F, Hansen RK, Radisky D, Yoneda T, Barcellos-Hoff MH, Petersen OW, Turley EA, Bissell MJ. Phenotypic Reversion or Death of Cancer Cells by Altering Signaling Pathways in Three-Dimensional Contexts. J Natl Cancer Inst. 2002;94:1494–1503. doi: 10.1093/jnci/94.19.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, Lupu R, Bissell MJ. Reciprocal interactions between beta1–integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci USA. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Howlett AR, Langton-Webster B, Petersen OW, Bissell MJ. Seminars in Cancer Biology. 1995;6:175–184. doi: 10.1006/scbi.1995.0021. [DOI] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Leliévre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, Werb Z, Bissell MJ. beta4 Integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2(3):205–216. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z, Anisowicz A, Hendrix MJ, Thor A, Neveu M, Sheng S, Rafidi K, Seftor E, Sager R. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994;263:526–529. doi: 10.1126/science.8290962. [DOI] [PubMed] [Google Scholar]


