Abstract
Insect immune responses include prophenoloxidase (proPO) activation and Toll pathway initiation, which are mediated by serine proteinase cascades and regulated by serpins. Manduca sexta hemolymph proteinase 6 (HP6) is a component of both pathways. It cleaves and activates proPO activating proteinase 1 (PAP1) and hemolymph proteinase 8 (HP8), which activates proSpätzle. Inhibitors of HP6 could have the capability of regulating both of these innate immune proteinase cascade pathways. Covalent complexes of HP6 with serpin-4 and serpin-5 were previously isolated from M. sexta plasma using immunoaffinity chromatography with serpin antibodies. We investigated the inhibition of purified, recombinant HP6 by serpin-4 and serpin-5. Both serpin-4 and serpin-5 formed SDS-stable complexes with HP6 in vitro, and they inhibited the activation of proHP8 and proPAP1. Serpin-5 inhibited HP6 more efficiently than did serpin-4. Injection of serpin-5 into larvae resulted in decreased bacteria-induced antimicrobial activity in hemolymph and reduced the bacteria-induced expression of attacin, cecropin and hemolin genes in fat body. Injection of serpin-4 had a weaker effect on antimicrobial peptide expression. These results indicate that serpin-5 may regulate the activity of HP6 to modulate proPO activation and antimicrobial peptide production during immune responses of M. sexta.
Keywords: Serpin, clip domain proteinase, hemolymph, immunity, prophenoloxidase activation, Toll signaling pathway
1. Introduction
Activation of prophenoloxidase (proPO) leading to melanin synthesis (Gorman et al., 2007; Liu et al., 2007; Kanost and Gorman, 2008; Kan et al., 2008) and stimulation of the Toll signaling pathway to induce production of antimicrobial peptides/proteins (AMPs) (Ferrandon et al., 2007; Buchon et al., 2009; El Chamy et al., 2008; Shin et al., 2006; Kim et al., 2008; Roh et al., 2009; Wang et al., 2007; An et al., 2009; An et al., 2010) are innate immune responses in arthropods, which are initiated by serine proteinase cascades (Gorman et al., 2007; Liu et al., 2007; Kanost and Gorman, 2008; Kan et al., 2008). A series of serine proteinases is sequentially activated and amplify the initial signal to the terminal proteinase, proPO activating proteinases (PAPs, also named PPAFs or PPAEs) in the proPO activation cascade (Gorman et al., 2007; Kan et al., 2008; Kim et al., 2008; An et al., 2009; An et al., 2010) or spätzle processing enzymes in the Toll pathway (Kim et al., 2008; An et al., 2010; Jang et al., 2006; Jang et al., 2006).
Such extracellular serine proteinase cascades are often regulated by proteinase inhibitors from the serpin protein superfamily (Potempa et al., 1994; Gettins, 2002; Silverman and Lomas, 2007). Serpins are proteins of ~400 amino acid residues, including an exposed region termed the reactive center loop (RCL) (Gettins, 2002; Irving et al., 2000; Cabrita et al., 2007). The RCL, located 30 to 40 residues from the carboxyl terminus, includes the specificity determining region and participates in the initial interaction with the target proteinase. Upon binding to a target proteinase, serpin is cleaved at the scissile bond (P1- P1') in the RCL and subsequently undergoes a large conformational change, in which the RCL inserts into a β–sheet and becomes covalently linked to the target proteinase, which is therefore irreversibly inhibited (Gettins, 2002; Whisstock and Bottomley, 2006).
In insects, serpins are reported to participate in the regulation of immune responses, including melanization in Drosophila melanogaster (Ligoxygakis et al., 2002; De Gregorio et al., 2002; Scherfer et al., 2008; Ahmad et al., 2009; Tang et al., 2008), Anopheles gambiae (Michel et al., 2005; Michel et al., 2006; Abraham et al., 2005), Aedes aegypti (Zou et al., 2010), and Tenebrio molitor (Jiang et al., 2009), and Toll signaling pathway in D. melanogaster (Ahmad et al., 2009; Levashina et al., 1999), A. aegypti (Bian et al., 2005; Shin et al., 2006; Zou et al., 2008), and T. molitor (Jiang et al., 2009). In most of these cases, genetic evidence supports the involvement of serpins in regulating the immune pathways, but the proteinases the serpins inhibit have not been identified. An exception is the T. molitor system, in which the same proteinase cascade activates proPO and proSpätzle, and specific proteinase-serpin interactions for three steps in the pathway have been characterized biochemically (Jiang et al., 2009). In the tobacco hornworm, Manduca sexta, seven serpins have been identified so far (Kanost, 2007), and endogenous molecular targets for some of these serpins have been determined. Serpin-1 splicing isoform J (serpin-1J) regulates proPO activation in plasma by inhibiting PAPs (Gupta et al., 2005; Jiang et al., 2003), and it regulates the Toll pathway by inhibiting hemolymph proteinase-8 (HP8) (C. An and M. R. Kanost, submitted). Serpin-3 also inhibits PAPs to blocks proPO activation (Zhu et al., 2003). Serpin-6 can block proPO activation by inhibiting PAP3, and it also forms a covalent complex with HP8 (Zou and Jiang, 2005).
M. sexta serpin-4 and serpin-5 suppress proPO activation but they do not inhibit the PAPs, suggesting that they may regulate proteinases upstream of the PAPs in the proPO activation pathway (Tong et al., 2005; Tong and Kanost, 2005). Isolation of serpin-proteinase complexes from hemolymph by immunoaffinity chromatography with antibodies to serpin-4 or serpin-5 yielded complexes containing these serpins along with a clip domain proteinase, hemolymph proteinase-6 (HP6) (Tong et al., 2005). We recently determined that HP6, a putative ortholog of Drosophila persephone, becomes activated in response to microbial exposure and participates in proPO activation by activating proPAP1 (An et al., 2009). HP6 also activates HP8, which cleaves and activates proSpätzle, to stimulate expression of several antimicrobial hemolymph proteins (An et al., 2009; An et al., 2010). In this study, we used purified recombinant proteins to characterize the reactions of serpin-4 and serpin-5 with HP6, testing the hypothesis that these serpins inhibit the cleavage of proHP8 or proPAP1 by HP6, thereby down-regulating two innate immune responses, melanization and synthesis of antimicrobial proteins.
2. Material and methods
2.1. Insect Rearing
M. sexta eggs originally purchased from Carolina Biological Supplies were used to establish a laboratory colony and reared on an artificial diet as described previously (Dunn and Drake, 1983).
2.2. Production of recombinant proteins
Recombinant serpin-4 and serpin-5 were produced using a baculovirus expression system and purified as described previously (Tong and Kanost, 2005). Recombinant mutant proHP6 and wild type proHP8 were produced in Drosophila S2 cells and purified as reported recently (An et al., 2009). In mutant proHP6 (proHP6Xa), the cleavage activation site of proHP6 was changed from LDLH92 to IEGR92 to permit its activation by bovine Factor Xa. Recombinant proPAP1 was kindly provided by Dr. Haobo Jiang of Oklahoma State University.
2.3. Detection of SDS-stable serpin-proteinase complexes
ProHP6Xa was activated by bovine Factor Xa as described previously (An et al., 2009), and mixed with purified serpin-4 or serpin-5 at concentrations specified in figure legends. In control samples, proHP6Xa or factor Xa was omitted from the mixture. After incubation at room temperature for times specified in figure legends, the reaction mixtures were treated with SDS sample buffer at 95°C for 5 min and resolved by electrophoresis using NuPAGE 4–12% Bis-Tris gels (Invitrogen). Proteins were transferred to a nitrocellulose membrane and subjected to immunoblot analysis (An et al., 2010) using 1:2000 diluted antiserum against HP6 (Jiang et al., 2005) or serpin-4 or serpin-5 (Tong and Kanost, 2005) as primary antibodies.
2.4. Analysis of HP6Xa inhibition using proHP8 or proPAP1 as substrates
Activated HP6Xa (20 ng) was mixed with serpin-4 or serpin-5 at a molar ratio of 10:1 (serpin:HP6Xa). After incubation at room temperature for 10 min, 40 ng of proHP8 or proPAP1 was added to the reaction mixtures, and incubated at 37°C for 60 min. The mixtures were treated with SDS sample buffer and subjected to immunoblot analysis using 1:2000 diluted antiserum against M. sexta HP8 (Jiang et al., 2005) or PAP1 (Jiang et al., 1998).
2.5. Effects of serpin-4 and serpin-5 on expression of bacteria-induced hemolymph proteins in M. sexta
Day 0, fifth instar larvae were injected with serpin-4 or serpin-5 (200 μl/larva, 200 ng/μl) or bovine serum albumin (BSA) (200 μl/larva, 200 ng/μl) as a control. After 30 min, a subset of these larvae were injected again with Micrococcus luteus ATCC 4698 (Sigma, 50 μl/larva, 2 ng/μl). Twenty h later, fat body and hemolymph samples were collected. Total RNA samples were prepared from fat body, and cDNA was prepared as described previously (An et al., 2009). Cell-free hemolymph samples were heated at 95°C for 5 min to remove most high molecular weight proteins and then centrifuged at 10,000×g for 5 min. The supernatant was stored at −20°C. Assay of antimicrobial activity and quantitative real-time PCR were carried out as described previously (An et al., 2009).
3. Results
3.1. Recombinant serpin-4 and serpin-5 form SDS-stable complexes with HP6Xa
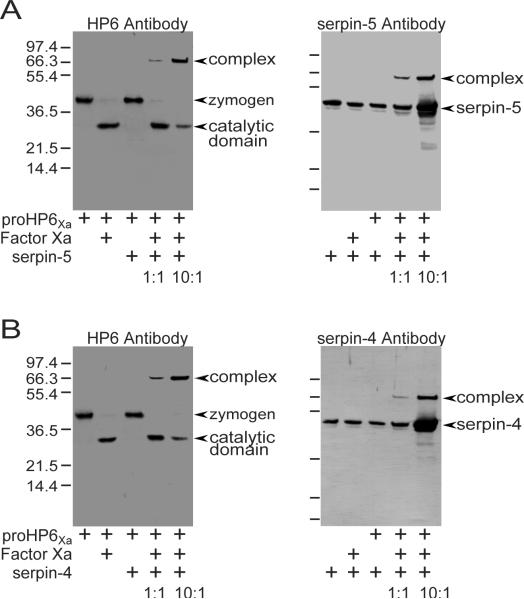
In a previous study, plasma proteinases that formed complexes with serpin-4 and serpin-5 were isolated from M. sexta hemolymph by immunoaffinity chromatography using serpin-4 or serpin-5 antibodies (Tong et al., 2005). HP6 was identified by immunoblotting, N-terminal sequencing, and MALDI-TOF mass fingerprint analysis as a component of one serpin-4 and serpin-5 complexes (Tong et al., 2005). To further investigate the interactions of these serpins with HP6, we have studied the inhibition reactions using purified, recombinant proteins. Serpin-4 or -5 mixed with factor Xa-activated HP6Xa formed higher molecular weight complexes identified by immunoblotting (Fig. 1). In the absence of factor Xa, anti-HP6 antiserum recognized the 39-kDa proHP6 zymogen. After activation with factor Xa, the 39-kDa band disappeared, and a 29-kDa band corresponding to the catalytic domain of HP6Xa was detected by the HP6 antiserum (Fig. 1A and 1B). When serpin-5 was mixed with active HP6Xa, a new immunoreactive band at ~66-kDa position (the expected size for a serpin-5:HP6Xa complex) was observed. The ~66-kDa band became more abundant when the molar ratio of serpin-5 to HP6Xa increased from 1:1 to 10:1 (Fig. 1A). This band was also recognized by serpin-5 antiserum, indicating it was composed of both HP6Xa and serpin-5. Similar results were observed when serpin-4 was mixed with active HP6Xa (Fig. 1B). In mixtures of serpin-4 and active HP6Xa, a ~66 kDa band was detected by both serpin-4 and HP6 antibodies, but this band was not visible in control mixtures lacking activated HP6Xa. These results confirm that serpin-4 and serpin-5 can form an SDS-stable complex with active HP6Xa, and indicate that these serpins are inhibitors of HP6.
Figure 1. SDS-stable complex formation between HP6Xa and serpin-5 (A) or serpin-4 (B).
ProHP6Xa (40 ng) was activated by factor Xa and then incubated for 10 min at room temperature with serpin-5 or serpin-4 at a molar ratio of 1:1 or 10:1 (serpins : proHP6Xa). In control reactions, proHP6Xa or factor Xa were omitted. The samples were subjected to SDS-PAGE and immunoblot analysis using antiserum against HP6 (left panel) or serpin (right panel). Bands representing a serpin-HP6 complex are marked by arrow heads. HP6 antibody recognized the 39-kDa proHP6Xa zymogen and a 29-kDa catalytic domain produced upon activation by factor Xa. Size and positions of molecular mass standards are indicated to the left of each blot.
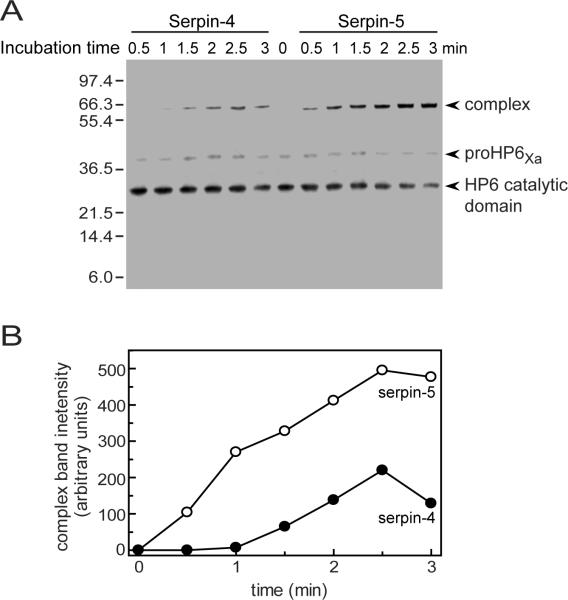
To examine the rate of complex formation between HP6Xa and serpin-4 or serpin-5, we mixed serpin-4 or -5 with active HP6Xa at a molar ratio of 10:1, and stopped the reaction at different times. As incubation time was extended, the 29-kDa band representing the catalytic domain of HP6Xa decreased in abundance while the intensity of ~66-kDa band representing the serpin-proteinase complex gradually increased in intensity (Fig. 2). The complex appeared more rapidly and accumulated to a greater extent for serpin-5 than for serpin-4. These results indicate serpin-5 interacted with active HP6Xa faster and more effectively than serpin-4.
Figure 2. Rate of formation of covalent complex between HP6 and serpin-4 or -5.
(A) Activated HP6Xa (40 ng) was incubated with serpin-4 (480 ng) or serpin-5 (460 ng) at room temperature for 0, 0.5, 1, 1.5, 2, 2.5 and 3 min. The reaction mixtures were separated by SDS-PAGE, followed by immunoblot analysis with antiserum against M. sexta HP6. The size and position of molecular weight standards are indicated on the left. (B) Quantitative analysis of 66-kDa band in (A) intensity by densitometry. Net intensity of the 66-kDa band corresponding to serpin-proteinase complex was analyzed using Carestream Molecular Imaging Software 5.0, and plotted against incubation time.
3.2. Serpin-4 and serpin-5 suppress activation of proHP8 or proPAP1 by HP6
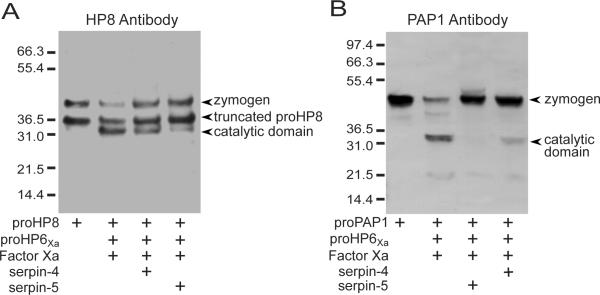
HP6 can proteolytically activate proHP8 and proPAP1 (An et al., 2009). Therefore, we tested a hypothesis that inhibition of HP6 by serpin-4 or serpin-5 would block its cleavage of these two natural substrates. When HP6Xa was incubated with proHP8, the 42-kDa band representing the proHP8 zymogen decreased in intensity, and a 34-kDa band corresponding to the catalytic domain of proHP8 appeared, consistent with previous results (An et al., 2009). When HP6Xa was pre-treated with serpin-4 or serpin-5, the processing of proHP8 was diminished, with lower intensity of the activated catalytic domain band and more residual proHP8 zymogen (Fig. 3A). Serpin-5 had a more pronounced effect than did serpin-4 in this inhibition of HP6Xa.
Figure 3. Serpin-4 and serpin-5 inhibit the activation of recombinant proHP8 (A) and proPAP1 (B) by HP6.
ProHP6Xa (20 ng) was activated by Factor Xa (50 ng), and then incubated at room temperature for 10 min with a 10-fold molar excess of serpin-4 or serpin-5, then incubated with proHP8 (40 ng) or proPAP1 (40 ng) at 37°C for 1 h. The mixtures were subjected to SDS-PAGE and immunoblotting using M. sexta HP8 (A) or PAP1 (B) antibodies. Bands representing zymogens and catalytic domains of proHP8 or proPAP1 are marked by arrows. Recombinant proHP8 contains two bands recognized by HP8 antiserum, a 42-kDa zymogen and 37-kDa truncated form (An et al., 2010). The sizes and positions of molecular weight standards are indicated on the left.
We performed a similar experiment to test the effects of serpin-4 and serpin-5 on activation of proPAP1 by HP6. The proPAP1 zymogen band at 44-kDa decreased in intensity when treated with HP6Xa, coinciding with the appearance of a 31-kDa band representing the PAP1 catalytic domain (Fig.3B) (An et al., 2009). When HP6Xa was pre-incubated with serpin-4 or serpin-5, very little activation of proPAP1 was detected, and this inhibition of HP6 was more complete with serpin-5 than with serpin-4.
3.3. Serpin-5 inhibited microbe-induced expression of AMP in M. sexta in vivo
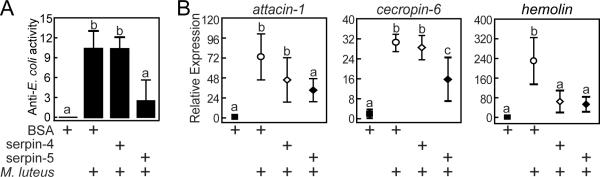
Microbial stimulation results in activation of proHP6, which then activates proHP8, and active HP8 cleaves proSpätzle to produce the active form of the Spätzle cytokine that induces the expression of antimicrobial peptide genes (An et al., 2009; An et al., 2010). Because serpin-4 and serpin-5 inhibit HP6 in vitro, we tested whether injection of serpin-4 or serpin-5 into larvae would result in reduced expression of acute phase response genes activated by this pathway. To test this hypothesis, we injected larvae with BSA (control) or with serpin-4 or serpin-5 and then 30 min later injected larvae with M. luteus to stimulate the antimicrobial response. After twenty hours we measured antimicrobial activity in plasma and bacteria-induced mRNA levels in fat body (Fig. 4). Injection of bacteria stimulated a strong increase in hemolymph antimicrobial activity, as assayed against E. coli. Pre-injection of larvae with serpin-5 significantly decreased this innate immune response, but injection of serpin-4 did not have this effect (Fig. 4A).
Figure 4. Effect of injection of serpin-5 on the expression of bacteria-induced hemolymph proteins.
Fifth instar day 0 larvae were injected with 40 μg of serpin-4, serpin-5, or BSA.. After 30 min, a subset of these larvae was injected with 100 ng of M. luteus. After 20 h, hemolymph was collected and fat body RNA samples were prepared from each insect, for assay of plasma antibacterial activity and mRNA levels for bacteria-induced hemolymph proteins. (A) Antimicrobial activity of plasma assayed against E. coli. (B) mRNA levels for indicated genes were assayed by quantitative RT-PCR relative to ribosomal protein S3 mRNA level as described in Materials and Methods. Bars represent mean ± S.D. (n = 3). Bars labeled with different letters are significantly different (one-way ANOVA followed by the Newman-Keuls test, P < 0.05).
Quantitative real-time PCR analysis revealed a significant increase in mRNA levels after injection of M. luteus for several genes encoding hemolymph proteins that function in the antibacterial response, including the antimicrobial peptides attacin-1 and cecropin-6 and a pattern recognition protein, hemolin (Fig. 4B). Pre-injection of larvae with serpin-5 significantly reduced the transcript levels for these innate immune proteins. In insects pre-injected with serpin-4, the level of mRNA for attacin-1 and cecropin-6 were not significantly different from those pre-injected with BSA. However, hemolin expression was reduced in larvae pre-injected with serpin-4. All together our results are consistent with a hypothesis that increasing the concentration of serpin-5 in hemolymph diminished up-regulation of attacin, cecropin, and hemolin after immune challenge due to inhibition of HP6. Serpin-4, a less efficient inhibitor of HP6, had a weaker (not statistically significant) effect on antimicrobial peptide gene expression but did strongly decrease hemolin expression. The latter observation may indicate that regulation of the hemolin gene differs somewhat from the antimicrobial peptides.
4. Discussion
In vertebrates and invertebrates, extracellular serine proteinase cascades mediate rapid defense responses upon wounding or microbial infection. Such proteinase cascades are regulated to prevent unnecessary activation and limit reaction to a short time at a discrete location. This regulation is performed, at least in part, by inhibitors of the serpin superfamily (Kanost, 1999; Silverman et al., 2001). Serpins inactivate proteinases irreversibly by forming covalent complexes. In M. sexta, serpin-1J, serpin-3, and serpin-6 regulate the proPO activation pathway by inhibiting PAPs (Jiang et al., 2003; Zhu et al., 2003; Zou and Jiang, 2005). Serpin-4 and serpin-5 can suppress proPO activation, but they do not inhibit PAPs, suggesting that they may inhibit proteinases upstream of PAPs in the pathway (Tong et al., 2005; Tong and Kanost, 2005). Complexes of serpin-4 and serpin-5 with the clip domain proteinase HP6 have been isolated from M. sexta plasma (Tong et al., 2005). HP6 has recently been shown to function as an activator of proPAP1 in the proPO pathway and also to activate proHP8, leading to induction of antimicrobial peptide synthesis (An et al., 2009). In this study, we used recombinant proteins to perform reconstitution experiments to further investigate the inhibition of HP6 by serpin-4 and serpin-5. This was made possible by the availability of a recombinant proHP6 mutant, which can be activated by bovine factor Xa (An et al., 2009).
Both serpin-4 and serpin-5 formed SDS-stable complexes with recombinant HP6. However, the reaction with serpin-5 was more rapid, and more complete (Fig. 2). This result is consistent with the observation that cleavage of proHP8 and proPAP1 by HP6 was inhibited more strongly by serpin-5 than by serpin-4 (Fig. 3) and indicates that serpin-5 is a more efficient inhibitor of HP6. We hypothesize that a hemolymph proteinase that is inhibited by serpin-4 or serpin-5 may also cleave its natural substrate at a similar sequence (Arg-Ile or Lys-Ile). We compared the predicted zymogen activation cleavage sites of 25 M. sexta hemolymph serine proteinases (Jiang et al., 2005) and found that HP5, HP8, HP12, HP15, PAP1, PAP2, and PAP3 are activated by cleavage of Arg-Ile or Lys-Ile (Fig. 5). An Asn residue at the P2 position at the activation sites of HP15, PAP2, and PAP3 also matches that in the RCL of serpin-4. In contrast, serpin-5 has a P2 Asp, which also occurs in the activation site of HP5, HP8, HP12, and PAP1. These observations are consistent with our results indicating that HP6, which cleaves and activates HP8 and PAP1(An et al., 2009), is more efficiently inhibited by serpin-5 than serpin-4. The RCL sequence of serpin-5 more closely mimics the sequence of the natural substrates of HP6.
Figure 5. Sequence similarities of the region containing the scissile bond in serpin-4 or serpin-5 and zymogen activation sites of M. sexta serine proteinases.
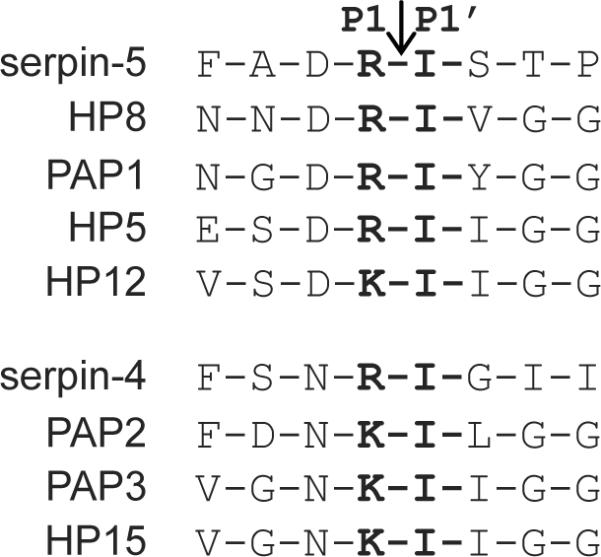
The P1- P1' scissile bond is indicated by an arrow.
The concentration of serpin-4 and serpin-5 in hemolymph of naïve larvae is approximately 1–3 μg/ml, and both increase to 6–8 μg/ml by 24 h after injection of bacteria (Tong and Kanost, 2005). We injected serpin-4 or serpin-5 (40 μg) into naive M. sexta larvae, increasing their concentration approximately 40-fold, a manipulation analogous to a genetic overexpression experiment. Animals with this high concentration of serpin-5 mounted a reduced immune response to injection of M. luteus, detected as reduced antibacterial activity of plasma, and significantly lower bacteria-induced mRNA levels for antimicrobial peptides attacin-1, cecropin-6 and a pattern recognition protein, hemolin (Fig. 4). Serpin-4 did not strongly affect induction of antibacterial activity or expression of attacin and cecropin. Results presented in this study indicate that serpin-5 can regulate antimicrobial peptide expression in M. sexta by inhibiting HP6, which cleaves and activates proHP8, an activating proteinase of the Toll ligand Spätzle (An et al., 2009; An et al., 2010). Serpin-4, a less efficient inhibitor of HP6, might contribute to this regulation to a lesser degree. In control reactions, we determined that serpin-4 and serpin-5 do not inhibit HP8 (Fig. S1). Other serpins known to function as regulators of the Toll signaling pathway for AMP expression in insects include serpin43Ac, serpin5, and serpin77Ba in D. melanogaster (Levashina et al., 1999; Green et al., 2000; Robertson et al., 2003) and serpin2 in A. aegypti (Bian et al., 2005; Shin et al., 2006), although which proteinases these serpins inhibit has not yet been determined. In T. molitor, the specific proteinases inhibited by serpin55, serpin40, and serpin48 to regulate activation of the Toll pathway have been indentified through biochemical analysis (Jiang et al., 2009), but none of these are orthologous with M. sexta HP6. M. sexta serpin-1J can inhibit HP8, also leading to reduced activation of Spätzle and diminished antibacterial response (C. An and M. R. Kanost, unpublished results).
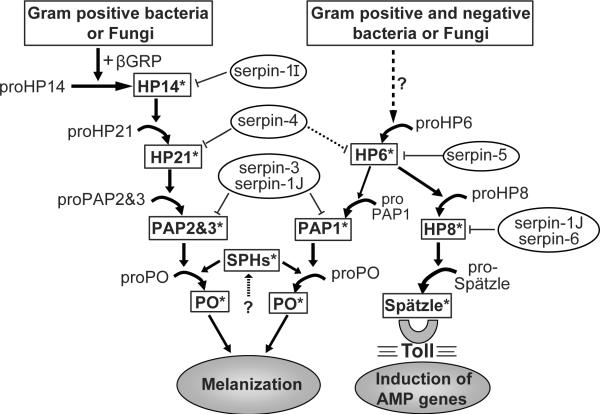
Based on the results from current and previous studies, we propose a model for the regulation of the proPO activation cascade and Toll signaling pathway by serpins in M. sexta (Fig. 6). Upon fungal or Gram-positive bacterial challenge, proHP14 becomes active in the presence of β-1,3-glucan recognition protein (Wang and Jiang, 2006). HP14 can then activate proHP21, which in turn activates proPAP2 or proPAP3 (Gorman et al., 2007; Wang and Jiang, 2007). PAP2 and PAP3 activate proPO in the presence of active SPHs (Yu et al., 2003; Wang and Jiang, 2004). In this pathway, HP14 can be inhibited by serpin-1I (Wang and Jiang, 2006), HP21 is inhibited by serpin-4 (Tong et al., 2005), and PAP2 and PAP3 are regulated by serpin-1J and serpin-3 (Jiang et al., 2003; Zhu et al., 2003). Another activation pathway occurs when M. sexta larvae are exposed to Gram-positive or Gram-negative bacteria or fungi. Unknown serine proteinase(s) are activated, one of which then activates proHP6. Active HP6 then processes proHP8 and proPAP1 to initiate a branched pathway (An et al., 2009). Activated HP8 cleaves pro-spätzle to produce active Spätzle (An et al., 2010), which binds a Toll receptor to initiate a signaling pathway resulting in expression of AMPs. Activated PAP1 can activate proPO in the presence of SPHs (Yu et al., 2003). Serpin-5 inhibits HP6 (data in this paper), and HP8 is regulated by serpin-1J and serpin-6 (C. An and M. R. Kanost, submitted) (Zou and Jiang, 2005). PAP1 is inhibited by serpin-3 and serpin-1J (Gupta et al., 2005; Zhu et al., 2003). Future research is required to identify components of the pathway upstream of HP6, to learn how this branch of the pathway is initiated in response to infection. Also, the D. melanogaster ortholog of HP6, called Persephone, can be activated by microbial proteinases, and whether this is also the case for HP6 requires further investigation.
Figure 6. A model for the regulation of M. sexta proPO activation cascade and Toll-like pathway by serpins.
Arrows indicate activation of downstream components or steps. Dashed arrows indicate potentially more than one step. Arrows labeled with “?” indicate steps that have not been experimentally verified. Regulation of proteinases by serpins is indicated. A dotted line represents weak inhibition of HP6 by serpin-4..
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health Grants GM41247. This is contribution 10-259-J from the Kansas Agricultural Experiment Station. We thank Dr. Haobo Jiang for purified proPAP1, Dr. Youren Tong for serpin samples, and Dr. Maureen Gorman and Dr. Emily R. Ragan for helpful comments.
The abbreviations used are
- AMP
antimicrobial peptide/protein
- HP6
hemolymph proteinase-6
- HP8
hemolymph proteinase-8
- PO and proPO
phenoloxidase and its precursor
- PAP
proPO activating proteinase
- SDS-PAGE
SDS-polyacrylamide gel electrophoresis
- Serpin
serine proteinase inhibitor
- SPH
serine proteinase homologue.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham EG, Pinto SB, Ghosh A, Vanlandingham DL, Budd A, Higgs S, et al. An immune-responsive serpin, SRPN6, mediates mosquito defense against malaria parasites. Proc.Natl.Acad.Sci.U.S.A. 2005;102:16327–16332. doi: 10.1073/pnas.0508335102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad ST, Sweeney ST, Lee JA, Sweeney NT, Gao FB. Genetic screen identifies serpin5 as a regulator of the toll pathway and CHMP2B toxicity associated with frontotemporal dementia. Proc.Natl.Acad.Sci.U.S.A. 2009;106:12168–12173. doi: 10.1073/pnas.0903134106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An C, Ishibashi J, Ragan EJ, Jiang H, Kanost MR. Functions of Manduca sexta Hemolymph Proteinases HP6 and HP8 in Two Innate Immune Pathways. J.Biol.Chem. 2009;284:19716–19726. doi: 10.1074/jbc.M109.007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An C, Jiang H, Kanost MR. Proteolytic activation and function of the cytokine Spätzle in the innate immune response of a lepidopteran insect, Manduca sexta. FEBS J. 2010;277:148–162. doi: 10.1111/j.1742-4658.2009.07465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian G, Shin SW, Cheon HM, Kokoza V, Raikhel AS. Transgenic alteration of Toll immune pathway in the female mosquito Aedes aegypti. Proc.Natl.Acad.Sci.U.S.A. 2005;102:13568–13573. doi: 10.1073/pnas.0502815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Poidevin M, Kwon HM, Guillou A, Sottas V, Lee BL, et al. A single modular serine protease integrates signals from pattern-recognition receptors upstream of the Drosophila Toll pathway. Proc.Natl.Acad.Sci.U.S.A. 2009;106:12442–12447. doi: 10.1073/pnas.0901924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrita LD, Irving JA, Pearce MC, Whisstock JC, Bottomley SP. Aeropin from the extremophile Pyrobaculum aerophilum bypasses the serpin misfolding trap. J.Biol.Chem. 2007;282:26802–26809. doi: 10.1074/jbc.M705020200. [DOI] [PubMed] [Google Scholar]
- De Gregorio E, Han SJ, Lee WJ, Baek MJ, Osaki T, Kawabata S, et al. An immune-responsive Serpin regulates the melanization cascade in Drosophila. Dev.Cell. 2002;3:581–592. doi: 10.1016/s1534-5807(02)00267-8. [DOI] [PubMed] [Google Scholar]
- Dunn PE, Drake D. Fate of bacteria injected into naive and immunized larvae of the tobacco hornworm, Manduca sexta. J.Invertebr.Pathol. 1983;41:77–85. [Google Scholar]
- El Chamy L, Leclerc V, Caldelari I, Reichhart JM. Sensing of `danger signals' and pathogen-associated molecular patterns defines binary signaling pathways `upstream' of Toll. Nat.Immunol. 2008;9:1165–1170. doi: 10.1038/ni.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandon D, Imler JL, Hetru C, Hoffmann JA. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat.Rev.Immunol. 2007;7:862–874. doi: 10.1038/nri2194. [DOI] [PubMed] [Google Scholar]
- Gettins PG. Serpin structure, mechanism, and function. Chem.Rev. 2002;102:4751–4804. doi: 10.1021/cr010170+. [DOI] [PubMed] [Google Scholar]
- Gorman MJ, Wang Y, Jiang H, Kanost MR. Manduca sexta hemolymph proteinase 21 activates prophenoloxidase-activating proteinase 3 in an insect innate immune response proteinase cascade. J.Biol.Chem. 2007;282:11742–11749. doi: 10.1074/jbc.M611243200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C, Levashina E, McKimmie C, Dafforn T, Reichhart JM, Gubb D. The necrotic gene in Drosophila corresponds to one of a cluster of three serpin transcripts mapping at 43A1.2. Genetics. 2000;156:1117–1127. doi: 10.1093/genetics/156.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Wang Y, Jiang H. Manduca sexta prophenoloxidase (proPO) activation requires proPO-activating proteinase (PAP) and serine proteinase homologs (SPHs) simultaneously. Insect Biochem.Mol.Biol. 2005;35:241–248. doi: 10.1016/j.ibmb.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving JA, Pike RN, Lesk AM, Whisstock JC. Phylogeny of the serpin superfamily: implications of patterns of amino acid conservation for structure and function. Genome Res. 2000;10:1845–1864. doi: 10.1101/gr.gr-1478r. [DOI] [PubMed] [Google Scholar]
- Jang IH, Chosa N, Kim SH, Nam HJ, Lemaitre B, Ochiai M, et al. A Spätzle-processing enzyme required for toll signaling activation in Drosophila innate immunity. Dev.Cell. 2006;10:45–55. doi: 10.1016/j.devcel.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Jiang H, Wang Y, Gu Y, Guo X, Zou Z, Scholz F, et al. Molecular identification of a bevy of serine proteinases in Manduca sexta hemolymph. Insect Biochem.Mol.Biol. 2005;35:931–943. doi: 10.1016/j.ibmb.2005.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Wang Y, Kanost MR. Pro-phenol oxidase activating proteinase from an insect, Manduca sexta: a bacteria-inducible protein similar to Drosophila easter. Proc.Natl.Acad.Sci.U.S.A. 1998;95:12220–12225. doi: 10.1073/pnas.95.21.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Wang Y, Yu XQ, Zhu Y, Kanost M. Prophenoloxidase-activating proteinase-3 (PAP-3) from Manduca sexta hemolymph: a clip-domain serine proteinase regulated by serpin-1J and serine proteinase homologs. Insect Biochem.Mol.Biol. 2003;33:1049–1060. doi: 10.1016/s0965-1748(03)00123-1. [DOI] [PubMed] [Google Scholar]
- Jiang R, Kim EH, Gong JH, Kwon HM, Kim CH, Ryu KH, et al. Three pairs of protease-serpin complexes cooperatively regulate the insect innate immune responses. J.Biol.Chem. 2009;284:35652–35658. doi: 10.1074/jbc.M109.071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan H, Kim CH, Kwon HM, Park JW, Roh KB, Lee H, et al. Molecular control of phenoloxidase-induced melanin synthesis in an insect. J.Biol.Chem. 2008;283:25316–25323. doi: 10.1074/jbc.M804364200. [DOI] [PubMed] [Google Scholar]
- Kanost MR. Serpins in a Lepidopteran Insect, Manduca sexta. In: Silverman GA, Lomas DA, editors. Molecular and cellular aspects of the serpinopathies and disorders in serpin activity. World Scientific Publishing Co.; Hackensack, NJ: 2007. pp. 229–241. [Google Scholar]
- Kanost MR, Gorman MJ. Phenoloxidases in insect immunity. In: Beckage NE, editor. Insect Immunity. Academic Press; San Diego: 2008. pp. 69–96. [Google Scholar]
- Kanost MR. Serine proteinase inhibitors in arthropod immunity. Dev.Comp.Immunol. 1999;23:291–301. doi: 10.1016/s0145-305x(99)00012-9. [DOI] [PubMed] [Google Scholar]
- Kim CH, Kim SJ, Kan H, Kwon HM, Roh KB, Jiang R, et al. A three-step proteolytic cascade mediates the activation of the peptidoglycan-induced toll pathway in an insect. J.Biol.Chem. 2008;283:7599–7607. doi: 10.1074/jbc.M710216200. [DOI] [PubMed] [Google Scholar]
- Levashina EA, Langley E, Green C, Gubb D, Ashburner M, Hoffmann JA, et al. Constitutive activation of toll-mediated antifungal defense in serpin-deficient Drosophila. Science. 1999;285:1917–1919. doi: 10.1126/science.285.5435.1917. [DOI] [PubMed] [Google Scholar]
- Ligoxygakis P, Pelte N, Ji C, Leclerc V, Duvic B, Belvin M, et al. A serpin mutant links Toll activation to melanization in the host defence of Drosophila. EMBO J. 2002;21:6330–6337. doi: 10.1093/emboj/cdf661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Jiravanichpaisal P, Cerenius L, Lee BL, Soderhall I, Soderhall K. Phenoloxidase is an important component of the defense against Aeromonas hydrophila infection in a crustacean, Pacifastacus leniusculus. J.Biol.Chem. 2007;282:33593–33598. doi: 10.1074/jbc.M706113200. [DOI] [PubMed] [Google Scholar]
- Michel K, Budd A, Pinto S, Gibson TJ, Kafatos FC. Anopheles gambiae SRPN2 facilitates midgut invasion by the malaria parasite Plasmodium berghei. EMBO Rep. 2005;6:891–897. doi: 10.1038/sj.embor.7400478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel K, Suwanchaichinda C, Morlais I, Lambrechts L, Cohuet A, Awono-Ambene PH, et al. Increased melanizing activity in Anopheles gambiae does not affect development of Plasmodium falciparum. Proc.Natl.Acad.Sci.U.S.A. 2006;103:16858–16863. doi: 10.1073/pnas.0608033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potempa J, Korzus E, Travis J. The serpin superfamily of proteinase inhibitors: structure, function, and regulation. J.Biol.Chem. 1994;269:15957–15960. [PubMed] [Google Scholar]
- Robertson AS, Belorgey D, Lilley KS, Lomas DA, Gubb D, Dafforn TR. Characterization of the necrotic protein that regulates the Toll-mediated immune response in Drosophila. J.Biol.Chem. 2003;278:6175–6180. doi: 10.1074/jbc.M209277200. [DOI] [PubMed] [Google Scholar]
- Roh KB, Kim CH, Lee H, Kwon HM, Park JW, Ryu JH, et al. Proteolytic cascade for the activation of the insect toll pathway induced by the fungal cell wall component. J.Biol.Chem. 2009;284:19474–19481. doi: 10.1074/jbc.M109.007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherfer C, Tang H, Kambris Z, Lhocine N, Hashimoto C, Lemaitre B. Drosophila Serpin-28D regulates hemolymph phenoloxidase activity and adult pigmentation. Dev.Biol. 2008;323:189–196. doi: 10.1016/j.ydbio.2008.08.030. [DOI] [PubMed] [Google Scholar]
- Shin SW, Bian G, Raikhel AS. A toll receptor and a cytokine, Toll5A and Spz1C, are involved in toll antifungal immune signaling in the mosquito Aedes aegypti. J.Biol.Chem. 2006;281:39388–39395. doi: 10.1074/jbc.M608912200. [DOI] [PubMed] [Google Scholar]
- Silverman GA, Bird PI, Carrell RW, Church FC, Coughlin PB, Gettins PG, et al. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J.Biol.Chem. 2001;276:33293–33296. doi: 10.1074/jbc.R100016200. [DOI] [PubMed] [Google Scholar]
- Silverman GA, Lomas DA. Molecular and Cellular Aspects of the Serpinopathies and Disorders in Serpin Activity. World Scientific Publishing Co.; Hackensack, NJ: 2007. [Google Scholar]
- Tang H, Kambris Z, Lemaitre B, Hashimoto C. A serpin that regulates immune melanization in the respiratory system of Drosophila. Dev.Cell. 2008;15:617–626. doi: 10.1016/j.devcel.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Jiang H, Kanost MR. Identification of plasma proteases inhibited by Manduca sexta serpin-4 and serpin-5 and their association with components of the prophenoloxidase activation pathway. J.Biol.Chem. 2005;280:14932–14942. doi: 10.1074/jbc.M500532200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Kanost MR. Manduca sexta serpin-4 and serpin-5 inhibit the prophenoloxidase activation pathway: cDNA cloning, protein expression, and characterization. J.Biol.Chem. 2005;280:14923–14931. doi: 10.1074/jbc.M500531200. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cheng T, Rayaprolu S, Zou Z, Xia Q, Xiang Z, et al. Proteolytic activation of pro-spätzle is required for the induced transcription of antimicrobial peptide genes in lepidopteran insects. Dev.Comp.Immunol. 2007;31:1002–1012. doi: 10.1016/j.dci.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jiang H. Reconstitution of a branch of the Manduca sexta prophenoloxidase activation cascade in vitro: snake-like hemolymph proteinase 21 (HP21) cleaved by HP14 activates prophenoloxidase-activating proteinase-2 precursor. Insect Biochem.Mol.Biol. 2007;37:1015–1025. doi: 10.1016/j.ibmb.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jiang H. Interaction of beta-1,3-glucan with its recognition protein activates hemolymph proteinase 14, an initiation enzyme of the prophenoloxidase activation system in Manduca sexta. J.Biol.Chem. 2006;281:9271–9278. doi: 10.1074/jbc.M513797200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jiang H. Prophenoloxidase (proPO) activation in Manduca sexta: an analysis of molecular interactions among proPO, proPO-activating proteinase-3, and a cofactor. Insect Biochem.Mol.Biol. 2004;34:731–742. doi: 10.1016/j.ibmb.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Whisstock JC, Bottomley SP. Molecular gymnastics: serpin structure, folding and misfolding. Curr.Opin.Struct.Biol. 2006;16:761–768. doi: 10.1016/j.sbi.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Yu XQ, Jiang H, Wang Y, Kanost MR. Nonproteolytic serine proteinase homologs are involved in prophenoloxidase activation in the tobacco hornworm, Manduca sexta. Insect Biochem.Mol.Biol. 2003;33:197–208. doi: 10.1016/s0965-1748(02)00191-1. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Wang Y, Gorman MJ, Jiang H, Kanost MR. Manduca sexta serpin-3 regulates prophenoloxidase activation in response to infection by inhibiting prophenoloxidase-activating proteinases. J.Biol.Chem. 2003;278:46556–46564. doi: 10.1074/jbc.M309682200. [DOI] [PubMed] [Google Scholar]
- Zou Z, Jiang H. Manduca sexta serpin-6 regulates immune serine proteinases PAP-3 and HP8. cDNA cloning, protein expression, inhibition kinetics, and function elucidation. J.Biol.Chem. 2005;280:14341–14348. doi: 10.1074/jbc.M500570200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z, Shin SW, Alvarez KS, Bian G, Kokoza V, Raikhel AS. Mosquito RUNX4 in the immune regulation of PPO gene expression and its effect on avian malaria parasite infection. Proc.Natl.Acad.Sci.U.S.A. 2008;105:18454–18459. doi: 10.1073/pnas.0804658105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z, Shin SW, Alvarez KS, Kokoza V, Raikhel AS. Distinct Melanization Pathways in the Mosquito Aedes aegypti. Immunity. 2010;32:41–53. doi: 10.1016/j.immuni.2009.11.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.