Abstract
Elevated blood glucose is a key initiator of mechanisms leading to diabetic neuropathy. Increases in glucose induce acute mitochondrial oxidative stress in dorsal root ganglion (DRG) neurons, the sensory neurons normally affected in diabetic neuropathy, whereas Schwann cells are largely unaffected. We propose that activation of an antioxidant response in DRG neurons would prevent glucose-induced injury. In this study, mild oxidative stress (1 μM H2O2) leads to the activation of the transcription factor Nrf2 and expression of antioxidant (phase II) enzymes. DRG neurons are thus protected from subsequent hyperglycemia-induced injury, as determined by activation of caspase 3 and the TUNEL assay. Schwann cells display high basal antioxidant enzyme expression and respond to hyperglycemia and mild oxidative stress via further increases in these enzymes. The botanical compounds resveratrol and sulforaphane activate the antioxidant response in DRG neurons. Other drugs that protect DRG neurons and block mitochondrial superoxide, identified in a compound screen, have differential ability to activate the antioxidant response. Multiple cellular targets exist for the prevention of hyperglycemic oxidative stress in DRG neurons, and these form the basis for new therapeutic strategies against diabetic neuropathy. Antioxid. Redox Signal. 11, 425–438.
Introduction
One of the most common complications of diabetes mellitus is diabetic neuropathy, affecting ~60% of the 20 million people with diabetes in the United States (6, 7). The consequences of diabetic neuropathy, including chronic pain or loss of sensation, recurrent foot ulcerations, and amputation, are responsible for a significant loss of quality of life and high economic impact (6, 7). Currently, no effective treatment is known for diabetic neuropathy beyond tight glycemic control (71).
Although multiple mechanisms underlie the pathogenesis of diabetic neuropathy, studies indicate that glucose-induced oxidative stress is a key mediator in this process (8, 9, 22, 52, 77, 82). Peripheral nervous system DRG neurons, but not Schwann cells, generate excessive reactive oxygen species (ROS) in the mitochondria when exposed to elevated glucose concentrations (19, 59, 79). ROS form additional free radical compounds that, over time, damage lipids, proteins, and nucleic acids (22, 74). These ROS-induced modifications promote DRG neuron injury and impair nerve function, leading to the signs and symptoms of diabetic neuropathy (8, 9, 22, 68, 86).
Many cells respond to ROS by activating an antioxidant response (49, 73). Endogenous antioxidant enzymes important for the detoxification of ROS in the nervous system include superoxide dismutase (SOD) (88), heme oxygenase (HO-1) (24, 43, 54), catalase (61), glutathione S-transferase (GST) (32, 44), and NAD(P)H:quinone oxidoreductase-1 (NQO1) (37, 49, 50). The genes coding many cellular antioxidant enzymes, such as GST and NQO1, contain a promoter–enhancer sequence known as the antioxidant response element (ARE). The ARE regulates transcriptional activity of these enzymes, known as phase II enzymes, after cellular exposure to ROS (27, 49). Activation of the ARE is primarily under the control of the transcription factor Nrf2 (34, 48). The ARE has not been characterized in DRG neurons and Schwann cells; however, we previously observed the ability of these neurons to generate intracellular antioxidants and increase antioxidant enzyme expression in the face of hyperglycemia (79).
Our research focuses on understanding how glucose-mediated oxidative stress leads to injury in cells of the peripheral nervous system and on discovering treatments to prevent this injury cascade (75, 78–81, 84). We anticipate that treatments that diminish oxidative stress or neuronal susceptibility to oxidative stress or both could provide new therapeutic options for diabetic neuropathy (78). Exogenous antioxidants such as vitamin C, vitamin E, SOD mimetics, and α-lipoic acid provide modest protection against the development of diabetic neuropathy (75). However, a more robust protection may result from exploiting the endogenous anti-oxidant response. Botanical compounds that activate Nrf2 are actively being tested for their therapeutic efficacy in other diseases involving oxidative damage, including cancer and cardiovascular disease (2, 14, 28, 40, 49, 69). We recently identified a panel of compounds that prevent glucose-induced mitochondrial oxidative stress and injury in DRG neurons (78), but their effects on Nrf2 are not known.
The current study explores the antioxidant response in DRG neurons after oxidative stress and compares this with relatively glucose-insensitive Schwann cells (19). We characterize the effects of the botanicals resveratrol and sulforaphane on the Nrf2-mediated antioxidant response in DRG neurons and Schwann cells. In addition, we examine the ability of other DRG neuron-protective agents identified by a compound screen to activate the antioxidant response. We find that resveratrol, sulforaphane, and the PPAR agonist fenofibrate all protect DRG neurons against glucose-induced injury in a time-dependent manner. These treatment strategies stimulate Nrf2 translocation to the nucleus, expression of ARE-driven antioxidant enzymes, and maintenance of cellular antioxidant potential. These data suggest that pharmacologic activation of the antioxidant response as an adjunct to other therapeutic strategies may provide significant protection against diabetic neuropathy.
Materials and Methods
Materials
Falcon-brand tissue-culture supplies were purchased from BD Biosciences, Bedford, MA. Chemicals were purchased from Sigma-Aldrich Corp (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA).
Cell isolation and culture
Dissociated DRG neurons were isolated from E15 Sprague–Dawley rat embryos or the adult dams. Cells were plated on collagen-coated tissue-culture plates or collagen-coated glass coverslips. Embryonic cells were cultured in Neurobasal media supplemented with B27 (without antioxidants), 10 ng/ml 2.5S nerve growth factor (NGF), 30 μM FUDR and penicillin/streptomycin/neomycin (5,000 U/5 mg/10 mg/ml, respectively) and 1.4 mM l-glutamine. After 24 h, cultures were refed with fresh Neurobasal media with all the same supplements except B-27 or l-glutamine. Adult cultures are grown in a 1:1 mix of low-glucose DMEM:F-10 containing 1 × B27 additives, 40 μM fluoro-2-deoxyuridine (FUDR), and 1,000 U/ml penicillin/streptomycin/neomycin. Cultures were used for experiments after 3 days in culture, at which time >95% of the cells are DRG neurons. Glucose, H2O2, or tert-butylhydroquinone (BHQ), an alternative prooxidant, was applied as a single bolus directly to the culture media. Standard Neurobasal media contains 25 mM glucose, and hyperglycemia was simulated by adding 20 mM glucose to the media, producing 45 mM final glucose concentration (78–81, 83). In adult DRG cultures, basal glucose was 5.7 mM; so 20 mM added glucose yields 25.7 mM final glucose concentration. Schwann cells were isolated from sciatic nerves of P3 rat pups. Sciatic nerves were dissected from perineurium in ice-cold L15 and cells dissociated in 1 ml 1% collagenase, and then 1 ml 2.5% trypsin at 37°C for 30 min. Cells were plated on poly-l-lysine-coated plates in DMEM/10% FBS. At confluence, fibroblasts were removed by complement lysis by using thy1.1 antibody and rabbit complement. Schwann cells were maintained in low glucose (1 g/L) DMEM containing 10% heat-inactivated FBS, 2 μM forskolin, 20 μg/ml bovine pituitary extract, and penicillin/streptomycin/neomycin. Before experiments, Schwann cells were switched to defined media for 24–48 h [1:1 mix of low glucose (1 g/L) DMEM and Ham's F12 media containing 10 μg/ml transferrin, 10 μM putrescine, 20 nM progesterone, and 30 nM sodium selenite].
TUNEL analysis
Apoptosis was assessed by counting the number of TUNEL-positive cells, identified by using the ApopTag Peroxidase In Situ Apoptosis Detection Kit as previously described (76, 78, 80, 81).
Western immunoblotting
Western blot analyses were performed as previously described (81). Cell lysates were collected by using modified RIPA buffer containing 20 mM Tris pH 7.4, 150 mM NaCl, 1% sodium deoxycholate, 10 μg/ml leupeptin, 10 μg/ml aprotonin, and 100 μg/ml PMSF. Five micrograms of protein was loaded and subjected to SDS-PAGE. After transfer to nitrocellulose membranes (Hybond; Amersham Biosciences Corp., Piscataway, NJ), membranes were incubated in a blocking solution composed of 5% milk [Carnation, nonfat dry, dissolved in tris-buffered saline (TBS) containing 0.1% tween-20 (TBST) for 2 h at 22°C or for 16 h at 4°C]. Blots were incubated with anti-NQO1 (Abcam, Cambridge, MA), anti-catalase (Rockland, Gilbertsville, PA), or anti-Nrf2 (Santa Cruz Biotechnology Inc., clone 722, Santa Cruz, CA). Primary antibodies were used at 1:1,000 dilution in blocking solution for 2 h at 22°C or for 16 h at 4°C. After extensive washing in TBST, blots were incubated with horseradish per-oxidase conjugated goat–anti-mouse IgG or goat–anti-rabbit IgG secondary antibody (Santa Cruz Biotechnology, Inc.; 1:1,000) for 1 h at room temperature. Blots were developed with the Phototope-HRP Western Blot Detection Kit (Cell Signaling Technology, Inc.) according to the manufacturer's instructions, and exposed to Hyperfilm-ECL film (GE Healthcare Bio-Sciences Corp., Piscataway, NJ). Blots shown are representative of at least three independent experiments performed.
Immunohistochemistry
Immunohistochemistry was performed as previously described (12, 13, 38) by using an anti-Nrf2 antibody (Santa Cruz Biotechnology). Nuclei were stained with bis-benzimide (Sigma Corporation) by following the manufacturer's protocol. Samples were mounted in ProLong antifade mounting media (Invitrogen, Carlsbad, CA) and examined by using an Olympus FluoView 500 confocal microscope with a 60 × 1.2 numerical aperture water-immersion objective at a resolution of 800 × 600 pixels.
Catalase activity
Catalase activity in DRG neuron lysates was determined by assessing the ability of lysates to prevent hydrogen peroxide–induced oxidation of Amplex Red, per the protocol published by the manufacturer (Molecular Probes, Eugene, OR) (79).
GST and reduced glutathione (GSH)
Concentrations of GSH or activity of GST were measured in cell lysates by use of substrate recycling kits according to the manufacturer's protocols (Cayman Chemical, Ann Arbor, MI).
NQO1
The NQO1 activity of cell lysates was determined from the reduction of 2,6-dichloroindophenol with and without dicumarol (10 μM), and the NQO1 activity was taken as the dicumarol-sensitive fraction, as described in (31). Between 1 and 3 μg/ml protein was added to a solution containing final concentrations of 0.04 mM 2,6-dichloroindophenol ± 0.01 mM dicumarol in 25 mM Tris-HCl buffer (pH 7.4). The reaction was initiated by the addition of NAD(P)H (100 μM) and FAD (50 μM) in a final volume of 200 μl. The absorbance at 590 nm was measured at 15-sec intervals over a period of 5 min at room temperature, monitored by using an Ascent Multiskan spectrophotometer (Labsystems, Helsinki, Finland). All reactions were carried out in 96-well plates, and each sample was measured in triplicate. The concentration of oxidized 2,6-dichloroindophenol remaining at each time point was calculated from a molar extinction coefficient of 21 ml/μmol/cm. The reaction rate in nanomoles of 2,6-dichloroindophenol reduced per minute per milligram of total protein was calculated from a plot of A590 against time.
HO-1
The activity of HO-1 was determined by the change in absorption due to oxidation of ferric heme by DRG neuron lysates (46). Reaction mixtures contained DRG neuron lysate (1–3 μg/ml protein), 10 μM hemin, 0.15 mg/ml BSA, 50 μg/ml spinach ferredoxin-NADP, 5 mM ascorbate, 2 mM desferrioxamine in 100 mM HEPES-NaOH buffer, pH 7.2. The reaction was started by adding 0.1 mM final concentration NADPH. Absorbance was read at 750 nm every 60 sec for 10 min. The final hemin absorbance after 5 min indicated the rate of oxidation. Higher absorbance indicates less hemin oxidation and a lower activity of HO-1 in the sample. The values were corrected for the protein concentration.
MitoSOX
MitoSOX (Molecular Probes, Eugene, OR) is a cell-permeable probe that accumulates specifically in mitochondria and becomes fluorescent after oxidation by superoxide. MitoSOX was dissolved in DMSO immediately before use, and then applied to DRG neurons at a final concentration of 4 μM with DMSO diluted to <0.1%. After 15 min, medium was removed and replaced with 100 μL Hepes-buffered saline solution (HBSS: 10 mM HEPES, pH 7.4, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2); then red fluorescence was read at 485 nm excitation and 590 nm emission (Fluoroskan Ascent II plate reader; LabSystems).
Statistical analyses
All quantitative assays were subjected to one-way ANOVA analysis with a Tukey's posttest, performed by using GraphPad Prism 4 software (GraphPad Software, Inc., San Diego, CA). Mean values of at least three independent experiments were included in the analyses. Error bars indicate standard error of the mean (SEM) for all graphs.
Results
Oxidative preconditioning protects against hyperglycemia
DRG neurons exposed to hyperglycemic conditions produce high levels of reactive oxygen species (ROS) and undergo apoptosis (59, 60, 79, 80, 86). One intriguing finding from our previous work was a rapid but transient induction of antioxidant enzymes, including SOD, within 1–3 h of hyperglycemia and before significant development of apoptosis (79). Because oxidative stress is a key component of the mechanisms that cause hyperglycemic DRG injury, we postulated that this reactive induction of antioxidant enzymes in DRG neurons might confer protection against hyperglycemia.
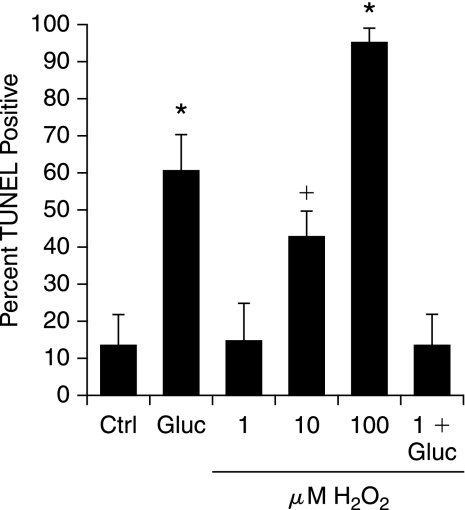
The initial experiment was designed to determine whether mild stress could induce a protective response in DRG neurons. DRG neurons were exposed to 20 mM added glucose per our previous studies (59, 78–81, 83), or a range of doses of H2O2. Cell injury was assessed by using the TUNEL assay (Fig. 1). Although DRG neurons are exquisitely sensitive to high concentrations of H2O2, the 1 μM dose did not induce injury. Therefore, we next exposed DRG neurons to 1 μM H2O2 for 3 h, followed by 20 mM added glucose, and assessed injury after 24 h (last bar, Fig. 1). Pretreatment with 1 μM H2O2 for 3 h before the application of 20 mM glucose completely prevented glucose-induced DRG neuron injury.
FIG. 1.
Mild prooxidant stress prevents dorsal root ganglion (DRG) neuron injury in subsequent exposure to hyperglycemia. DRG neurons were exposed to a range of concentrations of H2O2 (1–100 μM) and then examined for cell injury by TUNEL after 24 h. Hyperglycemia was modeled by adding 20 mM glucose, giving a final concentration of 45 mM glucose (Gluc). In the last bar, DRG neurons were preincubated with the nontoxic concentration of H2O2 (1 μM) for 3 h before hyperglycemia. Values are expressed as mean ± SEM. *p < 0.01 versus control (Ctrl). +p < 0.05 versus Ctrl.
Oxidative preconditioning involves activation of Nrf2
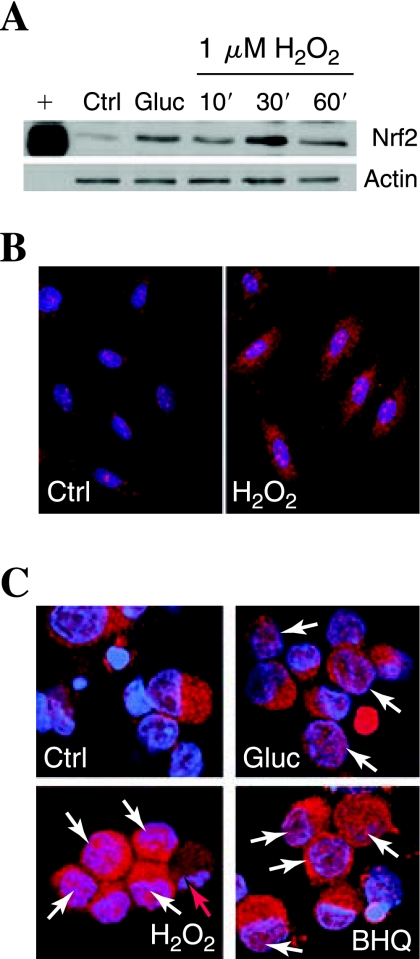
After we established that low-dose oxidative stress induces a protective response in DRG, we tested the hypothesis that the mechanism involved activation of the ARE. We first examined the expression of Nrf2, the transcription factor associated with proteins containing the ARE promoter (44). DRG neurons were treated with mild prooxidant stress (1 μM H2O2) for 30 min to 3 h, and Nrf2 levels were assessed with Western immunoblotting. Any increase in DRG Nrf2 expression after prooxidant treatments was insignificant and inconsistent (data not shown). In Schwann cells, however, which are resistant to 20 mM added glucose (10), a basal expression of Nrf2 rapidly and robustly increased with exposure to either 20 mM added glucose or 1 mM H2O2 (Fig. 2A).
FIG. 2.
Prooxidants induce Nrf2 accumulation in the nucleus. (A) Expression of Nrf2 was assessed in Schwann cells with Western blotting. Actin was used as a loading control (Ctrl). Schwann cells were exposed to 20 mM added glucose (Gluc) or 1 μM H2O2 for 10, 30, or 60 min. A positive control (+) was compared with the primary cell samples. (B, C) The nuclear localization of Nrf2 was assessed by staining red with an antibody for Nrf2 and counterstaining the nuclei blue by using bis-benzimide. In all conditions shown, cells were fixed and stained after 30-min treatment. Colocalization of Nrf2 with nucleus appears pink and is indicated with white arrows. Representative images of Schwann cells are shown in (B), and dorsal root ganglion (DRG) neurons, in (C). (D) Red arrow, A DRG neuron undergoing programmed cell death, with condensed and fragmented chromatin.
We next examined the localization of Nrf2 in Schwann cells and DRG neurons (Fig. 2B, C). In Schwann cells, basal Nrf2 (red) is almost entirely localized in the cytoplasm, but prooxidant treatment led to rapid and marked translocation of a portion of the Nrf2 to the nucleus (pink appearance of red Nrf2 overlaid with blue nuclear stain, Fig. 2B). In untreated control neurons, little Nrf2 was localized in the cytoplasm, but in DRG neurons treated for 1 h with prooxidant BHQ or 1 μM H2O2, a number of cells displayed a portion of Nrf2 localized in the nucleus (white arrows). Low levels of nuclear Nrf2 are occasionally observed in glucose-treated DRG neurons (Glu). We observe a greater increase in red Nrf2 staining in all treatment conditions compared with the staining in the control that is not supported by a large increase in the Western blot. This is most likely due to some epitope masking when Nrf2 is bound in its inactive form in the cytoplasm and not a significant increase in Nrf2 protein (30).
Catalase activity is increased after activation of Nrf2
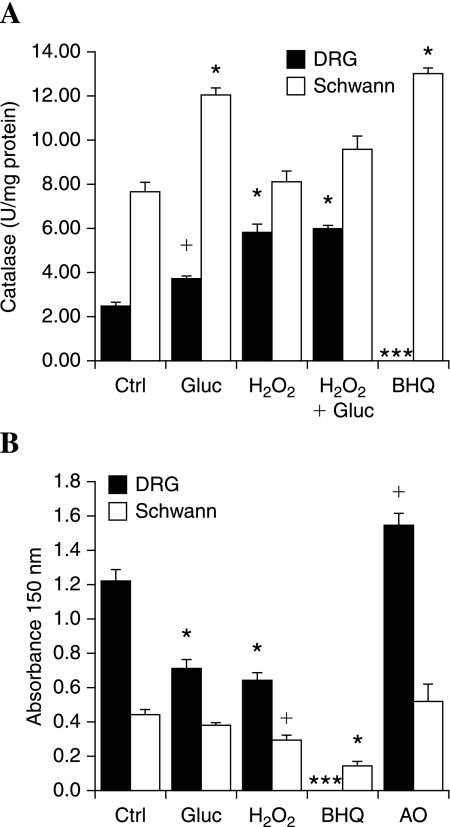
We next determined the activity and expression of Nrf2-regulated enzymes subsequent to Nrf2 translocation to the nucleus after exposure to 1 μM H2O2. First, we examined catalase, an important antioxidant, but not reported to be regulated by Nrf2. Similar to previous studies (79), we found that exposure to 20 mM added glucose for 3 h produced a 1.5-fold increase in catalase activity in DRG neurons and ~1.6-fold in Schwann cells (Fig. 3A). In DRG neurons, a 3-h pretreatment with 1 μM H2O2 produced a threefold increase in catalase activity. Similar results were obtained when a 3-h exposure to 1 μM H2O2 was followed by 20 mM added glucose. Increases in catalase in response to 1 μM H2O2 were more modest in Schwann cells, but basal catalase is already higher in Schwann cells than induced levels in DRG neurons. Exposure to the more potent prooxidant 10 μM BHQ produces a robust induction of catalase in the Schwann cells. This level of prooxidant leads to cellular breakdown in the sensitive DRG neurons, and enzyme activity cannot be measured in these samples.
FIG. 3.
Mild prooxidant stress activates antioxidant enzymes. Dorsal root ganglion (DRG) neurons or Schwann cells were exposed to hyperglycemia (20 mM added glucose), mild prooxidant [1 μM H2O2 or 10 μM tert-butylhydro-quinout (BHQ)], or the antioxidant (AO) α-lipoic acid (100 μM) for 3 h. H2O2 + glucose indicates 3-h H2O2 followed by 3-h glucose. After 3 h, antioxidant enzyme activities were measured: (A) Catalase, (B) HO-1. +p < 0.05 compared with control (Ctrl). *p < 0.01 compared with Ctrl.
Heme oxygenase (HO-1) activity is increased after activation of Nrf2
The enzyme HO-1 is known to be regulated by Nrf2. HO-1 activity can be quantified by measuring hemin levels in cell lysates. The level of hemin remaining after a 5-min reaction with DRG neuron or Schwann cell lysates is plotted in Fig. 3B. Higher levels of hemin represent lower heme oxygenase activity. Basal activity is markedly higher in Schwann cells compared with DRG neurons. Treating DRG neurons with an antioxidant (100 μM α-lipoic acid) for 3 h further decreased basal HO-1 activity. However, exposure to 1 μM H2O2 or 20 mM added glucose for 3 h increased HO-1 compared with control, suggesting that mild prooxidant stress increases the activity of this enzyme. In Schwann cells, exposure to 20 mM glucose tended to increase, but did not significantly alter, HO-1 activity over a 3-h period. Exposure to the prooxidants H2O2 (1 μM) or BHQ (10 μM) significantly induced HO-1 activity.
Oxidative preconditioning promotes the ability of DRG neurons to generate GSH
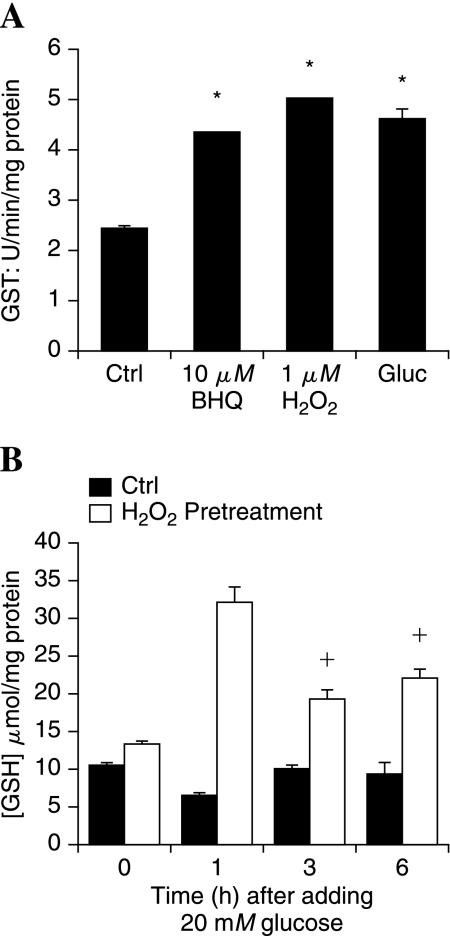
The ability to regenerate GSH is important for neurons to defend against oxidative stress. Treatment with the prooxidants 10 μM BHQ, 1 μM H2O2, or 20 mM added glucose significantly increased the activity of GST over a 3-h period compared with untreated control DRG neurons (Fig. 4A). The impact of increased GST on cellular antioxidant status during oxidative stress was assessed by measuring GSH under different treatment paradigms. DRG neurons were exposed to 1 μM H2O2 for 3 h, and then to 20 mM added glucose for 1, 3, or 6 h. DRG neurons (no pretreatment) also were exposed to 20 mM added glucose over the same time course. Treatment with 1 μM H2O2 alone for 3 h did not markedly alter the cellular levels of GSH (compare bars at 0 h). Exposure to glucose alone produced a modest decrease in GSH after 1 h that returned to control levels by 3 h. In DRG neurons pretreated with prooxidant (1 μM H2O2) before glucose exposure, the levels of GSH were significantly increased at every time point, with the largest peak at 1 h. These levels of GSH reflect the increased activity of GST in prooxidant-treated DRG neurons (Fig. 4A).
FIG. 4.
Oxidant stress preconditions DRG neurons to maintain cellular GSH in the presence of hyperglycemia. (A) DRG neurons were treated with mild prooxidants or 20 mM added glucose (Gluc), and then the activity of GST was measured in cell lysates after 3 h. *p < 0.01 compared with untreated control (Ctrl). (B) DRG neurons were pretreated for 3 h with H2O2 (1 μM) and then exposed to 20 mM added glucose for 0, 1, 3, or 6 h. DRG neurons were lysed, and the concentration of reduced glutathione (GSH) was measured. +p < 0.05 compared with no pretreatment (Ctrl). *p < 0.01 compared with no pretreatment.
Resveratrol and sulforaphane activate the antioxidant response
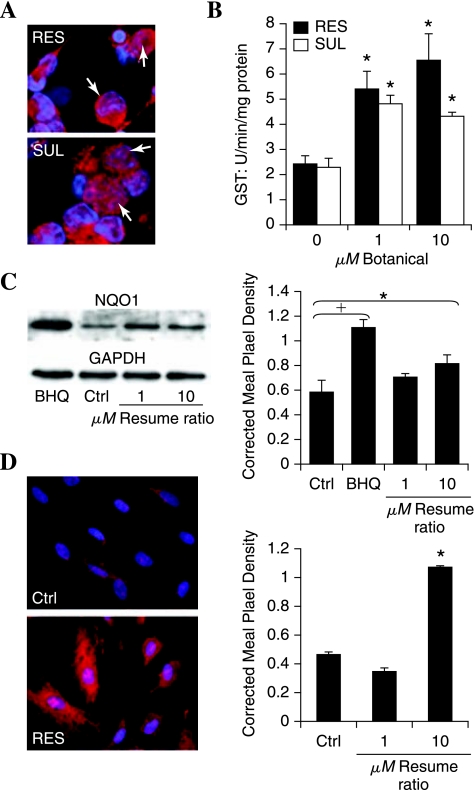
Prooxidants such as H2O2 or BHQ are not viable treatment options in the peripheral nervous system in vivo. Therefore, we examined other compounds known to activate the antioxidant response in other cell types. The botanicals resveratrol and sulforaphane are recognized for their ability to induce Nrf2 activity (29, 40, 44), although this effect has not been tested in DRG neurons. We therefore assessed the ability of resveratrol or sulforaphane to induce an antioxidant response in DRG. Nuclear localization of Nrf2 was assessed after 1-h exposure to 1–25 μM resveratrol or sulforaphane. Similar to the prooxidant treatments, nuclear Nrf2 was observed in 30–50% of DRG neurons after resveratrol or sulforaphane treatment (Fig. 5A).
FIG. 5.
Botanical compounds activate Nrf2 translocation and the antioxidant response. DRG neurons were exposed to 1 or 10 μM resveratrol (RES) or sulforaphane (SUL), and then the antioxidant response was assessed. (A) 1 h after exposure to 10 μM compound, the neurons were fixed and labeled for Nrf2 (red) or nuclei (bis-benzimide, blue). Nuclear Nrf2 is indicated with white arrows. (B) The activity of GST was determined after 3 h. *p < 0.05 compared with control (0 μM). (C) The expression of NQO1 protein was determined with Western blotting after 3-h RES. The prooxidant BHQ (10 μM) was included for comparison. Pixel density of the NQO1 band in each condition was normalized to the corresponding GAPDH band. (D) Schwann cell antioxidant response to resveratrol (10 μM) was assessed. Nrf2 localization after 1 h (left) and NQO1 expression after 3 h (right). Corrected mean pixel density represents the mean ± SEM for each condition from three replicate blots. †p < 0.01 and *p < 0.05 versus untreated control (C).
The effect of the botanical compounds on DRG neuron antioxidant enzyme expression was confirmed by measuring the activity of GST and NQO1. A dose-dependent increase in both GST activity (Fig. 5B) and NQO1 expression (Fig. 5C) was observed 3 h after exposure to resveratrol or sulforaphane. In Fig. 5C, data for resveratrol are shown, and similar results were obtained by using sulforaphane (data not shown).
Similar to findings of other glial studies (10, 18), 10 μM resveratrol induced a robust antioxidant response in the Schwann cells as evidenced by Nrf2 translocation to the nucleus and by a greater than twofold increase in NQO1 activity (Fig. 5D).
Activation of the antioxidant response by botanicals protects against hyperglycemia
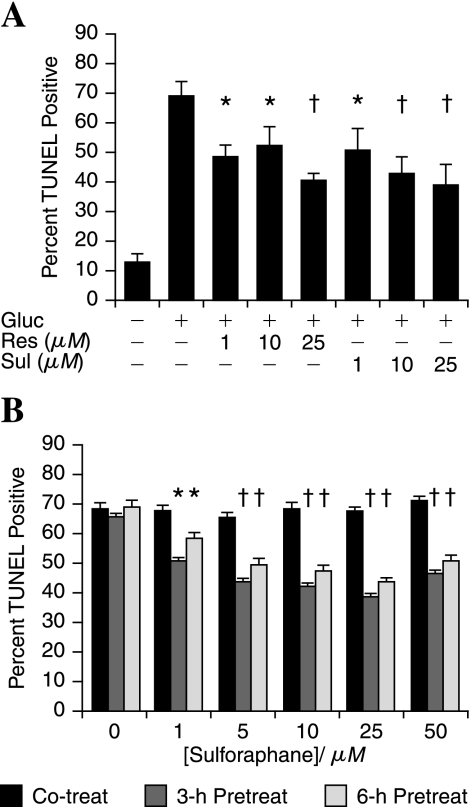
We next determined whether the botanical activators of Nrf2 could confer protection against DRG injury in the presence of hyperglycemia. Note, as stated earlier, that Schwann cells are resistant to hyperglycemic injury. DRG neurons were exposed to increasing concentrations of either resveratrol or sulforaphane for 3 h, followed by 20 mM added glucose. After 24-h glucose treatment, DRG neurons were assessed for cell death by the TUNEL assay (Fig. 6A). Botanical doses as low as 1 μM significantly decreased subsequent glucose-induced DRG neuron death. Resveratrol (1 μM) decreased death from ~70% in glucose-treated neurons to 50%. Increasing the concentration of resveratrol to 25 μM further decreased DRG neuron death to ~42%. Similar results were observed with the same concentrations of sulforaphane (Fig. 6A).
FIG. 6.
Resveratrol and sulforaphane pretreatment can prevent hyperglycemic injury in DRG neurons. (A) Increasing concentrations of resveratrol (Res) or sulforaphane (Sul) were applied to DRG neurons 3 h before the application of 20 mM glucose (Gluc). (B) Increasing concentrations of sulforaphane were applied to DRG neurons either at the same time (Co-treat), 3 h before, or 6 h before 20 mM added glucose. (A, B) Cells were fixed after 24 h and TUNEL stained. The bars represent the mean percentage TUNEL positive for three independent experiments ± SEM. †p < 0.01 and *p < 0.05 versus glucose only (A) or no pretreatment (B).
To determine whether the effects of resveratrol and sulforaphane result from a direct effect on the glucose-induced injury mechanism or a change in protein expression or modification, increasing concentrations of sulforaphane were applied to DRG neurons either 2 min before (Co-treat), or 3 or 6 h before the application of 20 mM glucose (Fig. 6B). In the co-treatment group, no difference was noted in the degree of DRG neuron death between the glucose alone and any dose of sulforaphane. Instead, the greatest protection was observed after 3-h pretreatment. The protection afforded by either the 6-h pretreatment or the 3-h pretreatment was similar. The data suggest that sufficient time for a change in protein expression exists, and the duration of the protective response suggests that new protein expression is involved.
Multiple mechanisms of antioxidant protection via different bioactive compounds
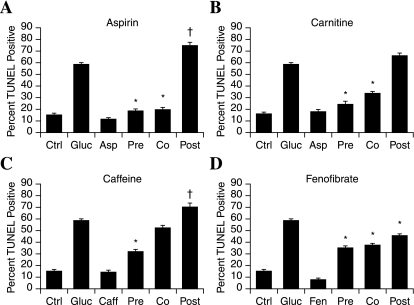
We recently completed a screen of 1,040 compounds for the ability to prevent glucose-induced mitochondrial superoxide during hyperglycemia (78). We identified 25 compounds that prevented both mitochondrial superoxide and DRG neuron injury. In the present study, we selected four compounds of interest and explored their protective mechanisms in adult DRG neurons. First, we repeated the pre-, co-, and posttreatment paradigms with respect to addition of 20 mM glucose (Fig. 7). All of the compounds were used at the screening concentration of 1 μM. All four of the drugs prevented glucose-induced DRG neuron injury per the screening paradigm (78), but the experiment demonstrated differences among the compounds.
FIG. 7.
Compounds that prevent glucose-induced oxidative injury operate via different mechanisms. Four lead compounds that prevent DRG neuron mitochondrial oxidative stress and injury in hyperglycemia were assessed in different treatment paradigms in adult rat DRG neurons. The 1 μM compound was added at the same time as (Co), 3 h before (Pre), or 1 h after (Post) 20 mM glucose. The bars represent the mean percentage of TUNEL positive for two independent experiments ± SEM. *p < 0.01 compared with glucose only. +p < 0.05 compared with glucose only.
Aspirin
Aspirin significantly decreased glucose-induced DRG neuron injury when applied 3 h before, or at the same time as the 20 mM glucose insult (Fig. 7A). However, when 20 mM glucose was applied first and the aspirin added after 1 h, DRG neuron injury significantly increased compared with glucose alone.
Carnitine
Carnitine significantly decreased glucose-induced DRG neuron injury when applied 3 h before, or at the same time as the 20 mM glucose insult (Fig. 7B). When carnitine was applied 1 h after the glucose insult, the protection was lost, and DRG neuron survival was similar to that with glucose treatment alone.
Caffeine
Caffeine was highly protective in the pretreatment paradigm, but protection was lost when the compound was added at the same time as the glucose (Fig. 7C). When caffeine was applied 1 h subsequent to glucose, DRG neuron death was significantly increased compared with that with glucose alone.
Fenofibrate
Fenofibrate significantly promotes DRG neuron survival when applied in any of the treatment paradigms (Fig. 7D). Even when applied 1 h after the glucose load, significant protection was afforded, although the degree of protection was less than the co- and pretreatment paradigms.
Caffeine induces mitochondrial superoxide, and protection appears to be mediated via the antioxidant response
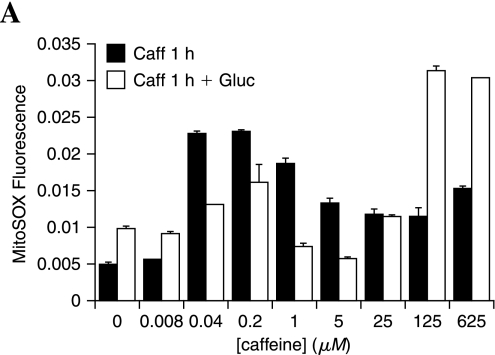
Further to explore the different mechanisms of action of these compounds, we examined their ability to alter mitochondrial superoxide generation. Aspirin and carnitine are known antioxidants, so these were not further assessed in this system. Increasing concentrations (0.008–625 μM) of caffeine were applied to adult DRG neurons in the presence or absence of 20 mM added glucose, and mitochondrial superoxide was assessed after 1 h (Fig. 8). Only the 0.008 μM dose did not alter DRG neuron mitochondrial superoxide in the presence or absence of glucose. Caffeine produced a dose-dependent increase in mitochondrial superoxide up to 1 μM that declined and leveled off at higher concentrations of caffeine. In the presence of glucose, an intermediate level of mitochondrial superoxide generation was lower than caffeine alone, but higher than glucose alone. At high concentrations of caffeine (125 and 625 μM), application of 20 mM glucose led to a synergistic increase in mitochondrial superoxide to 12-fold higher than control levels.
FIG. 8.
Caffeine promotes mitochondrial oxidative stress. Adult DRG neurons were exposed to increasing concentrations of caffeine alone for 1 h or caffeine for 1 h followed by 20 mM added glucose for 1 h. DRG neurons were loaded with MitoSOX and read at 485 nm ex, 590 em on a plate reader. Bars indicate the mean of three replicates + SEM.
Fenofibrate both is a direct antioxidant and activates the antioxidant response
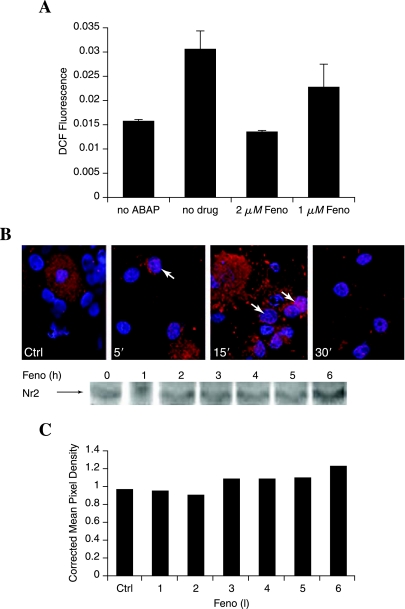
Fenofibrate decreases glucose-induced DRG neuron injury even when applied 1 h subsequent to the glucose (Fig. 7D), suggesting that it operates as a direct antioxidant. To test this possibility, DCFDA oxidation in the presence of ABAP was used as a cell-free prooxidant system to test the ability of fenofibrate to block oxidation (Fig. 9A). A control sample containing 2 μM BSA (no drug) was used as a comparison. The 2 μM fenofibrate completely blocked DCFDA oxidation to fluorescent DCF by ABAP. Fenofibrate antioxidant capacity was dose dependent, with 1 μM fenofibrate decreasing ABAP-induced DCFDA oxidation by ~50%. Because DRG neuron protection against hyperglycemia is greater with earlier (co-treatment or 3-h pretreatment) paradigms, this suggests that direct antioxidant capacity may not be the only mechanism of action. We examined activation of Nrf2 by fenofibrate to assess its effect on the antioxidant response (Fig. 9B). Up to 50% of DRG neurons demonstrated some nuclear localization (pink overlay of red Nrf2 and blue nucleus) of Nrf2 within 5–15 min after application of fenofibrate. By 30 min, most DRG neuron nuclei had no evidence of nuclear Nrf2.
FIG. 9.
Fenofibrate is an antioxidant that also activates Nrf2. (A) A cell-free assay was performed by mixing fenofibrate with DCFDA (10 μM) in HBSS solution, and then adding 50 mM oxidizing agent 2,2′-azobis (amidinopropane) dihydrochloride (ABAP). The oxidation of DCFDA to DCF was determined by measuring DCF fluorescence after 2 min in a plate reader. A control containing BSA instead of fenofibrate (no drug) was included for comparison. (B) The localization of Nrf2 was assessed with immunohistochemistry in adult DRG neuron cultures after exposure to 10 μM fenofibrate. Nrf2 was stained red, and the nuclei were counterstained blue by using bis-benzimide. Colocalization appears pink (white arrows). (C) Changes in Nrf2 expression were assessed with Western blotting after 1- to 6-h exposure to 1 μM fenofibrate. The representative blot with corresponding densitometry (normalized against GAPDH) suggests a modest increase in Nrf2 expression after 3 h, with a further increase by 6 h.
We assessed Nrf2 expression in the fenofibrate-treated DRG neurons (Fig. 9C). Similar to the earlier studies described for Fig. 2, changes in Nrf2 expression in DRG neurons were small and inconsistent. In the single representative blot (Fig, 9C), a modest increase in Nrf2 expression increased further by 6 h.
Compounds that prevent mitochondrial superoxide have variable effects on antioxidant enzyme expression
To assess the effects of our four lead compounds on the antioxidant response, we measured the expression of NQO1. Lysates were prepared from adult DRG neurons 3 h after application of 1 μM drug or 1 μM H2O2 (Fig. 10A) and immunoblotted for NQO1 protein. Aspirin did not increase NQO1 expression but tended to decrease it. Both fenofibrate and carnitine modestly increased NQO1 expression. The increase was statistically significant (p < 0.05). Caffeine markedly increased NQO1 expression to a level higher than that with H2O2 treatment.
FIG. 10.
Differential ability of compounds of interest to activate the antioxidant response in DRG neurons. (A) Adult DRG neurons were exposed to 1 μM compounds or H2O2 for 3 h, and then Western blotted for NQO1. The bar graph illustrates the mean and SEM for three replicates normalized to actin, and below is a representative blot. *p < 0.05 compared with untreated control. The compounds are fenofibrate (Feno), caffeine (Caff), carnitine (Carn), and aspirin (Asp). (B, C) Adult DRG neurons were exposed to 1 μM fenofibrate for 1–6 h, and then Western blotted for NQO1 (B) or assayed for GST activity (C). *p < 0.05 compared with untreated control.
Because fenofibrate appears to have a mild, positive effect on the antioxidant response, we further characterized the time course and extent of this response. We measured the expression of NQO1 (Fig. 10B) and activity of GST (Fig. 10C) up to 6 h after the application of 1 μM fenofibrate. Increases in NQO1 activity remained modest. The representative blot indicates a mild increase in NQO1 expression by 3 h that was sustained up to 5 h. GST activity significantly increased by 3 h and increased further by 5 h.
Antioxidant response–mediated DRG neuron protection involves protein synthesis
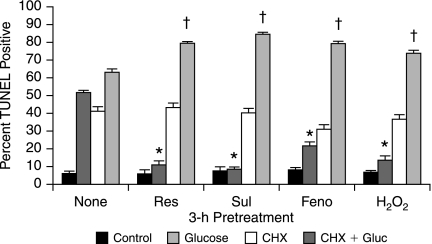
Finally, because our data demonstrate that DRG neuron protection after mild prooxidant or our panel of drugs involves transcription factor (Nrf2) activation, we assessed the role of protein synthesis. Adult DRG neurons were pre-treated for 3 h with 1 μM H2O2 or 1 μM antioxidant response–activating drug in the presence or absence of 20 μg/ml cycloheximide (CHX), then exposed to 20 mM added glucose for 24 h (Fig. 11). CHX alone produced a significant increase in DRG neuron injury. Despite the toxicity of CHX, the data clearly demonstrate that the DRG neuron protection of resveratrol, sulforaphane, fenofibrate, and 1 μM H2O2 all depend on protein synthesis to prevent glucose-induced injury.
FIG. 11.
DRG neuron protection via Nrf2-activating drugs requires protein synthesis. Adult DRG neurons were exposed to 1 μM resveratrol (Res), sulforaphane (Sul), fenofibrate (Feno), or H2O2 for 3 h in the presence or absence of 20 μg/ml CHX. Glucose (20 mM) was then added, and cells were fixed after 24 h and TUNEL stained. *Drugs decreased DRG neuron injury compared with glucose only, p < 0.01. †CHX increased glucose-induced injury in the presence of the drugs, p < 0.05.
Discussion
The central role of excessive free radical generation in the development of diabetes complications is a well-established pathogenic mechanism (5, 9, 21, 35, 66, 74). As the field of oxidative stress research has advanced, so has the accumulation of evidence of free radical damage in complication-prone tissues (82, 83). In particular, we previously characterized glucose-induced injury in DRG neurons (79, 80). Early during the course of hyperglycemic injury, we observed an increase in SOD and catalase activities and proposed that this was evidence of an antioxidant response (79). However, in acute hyperglycemia, this response was too little and too late to prevent DRG neuron injury. The current study was designed to explore the potential significance of the antioxidant response in DRG neurons. We compared the antioxidant response of DRG neurons with Schwann cells that are not injured by 20 mM added glucose (19) and appear resistant to oxidative injury.
Hyperglycemia rapidly leads to ROS formation in DRG neurons that activates the antioxidant response. We initially replicated this response by using exposure to a prooxidant. Concentrations of H2O2 of 10 μM or more injured the DRG neurons. Similarly, 10 μM BHQ destroyed the DRG neurons. This concentration is relatively low compared with concentrations that injure other cell types in culture. In bone marrow (45) or epithelial (23) cells, at least 100–1,000 μM H2O2 must be applied to produce a cytotoxic response. This 10- to 100-fold difference in susceptibility to prooxidants may underlie the marked sensitivity of DRG neurons to hyperglycemia-induced injury (80). A lower concentration, 1 μM, H2O2 did not injure the DRG neurons, but activated a protective mechanism (Fig. 1). The concept that low concentrations of free radicals act as second messengers with positive effects on cell survival is not new (16). This mechanism underlies the well-established phenomenon known as ischemic/hypoxic preconditioning that significantly decreases the effects of a stroke (41). However, this concept has not been considered in diabetes, in which oxidative stress is a significant contributor to the development of complications (21, 25).
We hypothesized that H2O2-induced DRG neuron protection was mediated via activation of Nrf2. By using immunohistochemistry, we demonstrated that 1 μM H2O2 led to rapid Nrf2 translocation from the cytoplasm to the nucleus in DRG neurons. Schwann cells displayed basal expression of Nrf2 that was both increased and translocated to the nuclei after exposure to 20 mM glucose or 1 μM H2O2. Nrf2 plays a role in antioxidant gene expression in the brain, but has not been demonstrated in other areas of the nervous system. Our finding in Schwann cells was anticipated, because brain glial cells such as astrocytes respond to oxidative stress by translocating Nrf2 to the nucleus and expressing >200 genes (65).
In our previous study, SOD and catalase activities increased in DRG neurons in response to hyperglycemia (79). This response peaked after 3 h of hyperglycemia, but significant oxidative stress occurred within 1 h and was sufficient to lead to loss of mitochondrial function and cell death. Now we demonstrate that sublethal oxidative stress prevents subsequent injury by increasing the ability to detoxify free radicals. Increased DRG neuron protection by prooxidant conditioning was associated with increased expression and activity of the antioxidant enzymes catalase, HO-1, and GST. Preactivation of an antioxidant response prevents cell injury in many paradigms. The best-characterized examples of this response are in the hypoxic/ischemic preconditioning that provides cardiac protection during coronary artery occlusion or decreases cerebral infarct areas after a stroke (17, 33, 53, 56, 57). Insults that lead to mild oxidative stress produce a gene expression–dependent resistance to a subsequent greater stress that would normally cause cell or tissue injury.
In Schwann cells, the basal activity of these enzymes was similar to the maximum stimulated activities in the DRG neurons. Addition of prooxidants further increased these enzyme activities in Schwann cells. Our data demonstrate a strong antioxidant capacity in Schwann cells that may underlie their capacity to survive fluctuations in glucose concentration (19). These data do not suggest that Schwann cells are unaffected by hyperglycemia. We demonstrate that Schwann cell gene expression changes, and others suggest that proliferation is altered (4), which may contribute to diabetic neuropathy.
Next, we sought an alternative approach to activation of the antioxidant response that will be more readily translated to in vivo models. Botanical compounds are therapeutic for a wide variety of diseases through mechanisms that include activation of phase II enzymes. In particular, resveratrol and sulforaphane, isolated from red grapes and cruciferous vegetables, respectively, have entered clinical trials for cancer and Alzheimer's disease (26, 36, 42, 62, 89). Both these compounds led to Nrf2 nuclear localization within 1 h in DRG neurons and Schwann cells (Fig. 5).
After botanical-induced Nrf2 translocation to the nucleus, we observed increases in the antioxidant enzymes GST and NQO1 after 3 h. NQO1 normally detoxifies ROS within cells of the nervous system (3) and is one of the most highly induced enzymes in response to resveratrol in other systems (39). GST is essential for the generation of glutathione, the major cellular antioxidant in DRG neurons (55). Thus, application of botanicals induced the activities of key DRG neuron defense mechanisms against oxidative stress.
The activation of the antioxidant response by resveratrol or sulforaphane in DRG neurons led to their ability to withstand a hyperglycemic insult. The time dependence of the protection conferred by these compounds supports our conclusion that protection is mediated via activation of the anti-oxidant response. If sufficient time does not exist between botanical treatment and exposure to stress, the protection is lost (Fig. 6), suggesting that new gene expression is required. We also note that increasing the pretreatment period to 6 h rather than the optimum 3 h tended to decrease the protective effect, although the difference was small. Further studies are needed to assess the transience of the antioxidant response in DRG neurons and whether continual reconditioning of the DRG neurons with botanicals is necessary and possible.
The finding that increasing DRG neuron antioxidant potential prevents glucose-mediated injury is supported by our previous studies with exogenous antioxidants (79, 84). However, manipulation of the endogenous antioxidant response is an attractive alternative target for DRG neuron protection for many reasons. Bioavailabilty of oral antioxidants in specific cells may be low (74). Moreover, long-term use of single antioxidants such as vitamin E appears to cause an imbalance in the antioxidant pool that decreases efficacy (85).
Both resveratrol and sulforaphane increase a spectrum of antioxidant enzymes and small-molecule antioxidants in many cell types (10, 20, 40). Sulforaphane has an additional effect of promoting the cell-mediated immune response by augmenting the proliferative effects of mitogens and the secretion of cytokines (70, 87). Most notably, resveratrol effectively protects endothelial cells against apoptotic damage from oxidized low-density lipoproteins (15, 51). These oxidized lipoproteins are particularly prevalent in patients with type 2 diabetes, and thus resveratrol may provide added benefit against complications. Recent intervention studies in diabetic rodents demonstrated in vivo that resveratrol may decrease microvascular complications (63, 64). Studies using botanical compounds are ongoing, and we are continuing to seek additional adjunct therapies to improve the management of diabetic complications.
We identified compounds that prevent mitochondrial oxidative stress in DRG neurons exposed to hyperglycemia (78, 80, 81). Improved therapies for diabetic neuropathy may be obtained by using combinations of compounds that operate at different targets on the same pathway. Therefore, we further explored the mechanisms of action of four lead compounds. The major finding was that some compounds, notably caffeine, activate the antioxidant response by producing oxidative stress (Figs. 7C and 8). This would suggest that the compound would not be useful for promoting the antioxidant response in vivo because a cell that is already subject to oxidative stress is likely to be injured by exposure to a prooxidant. This conclusion is supported by our post-treatment experiments in which the compound was applied 1 h subsequent to the glucose insult, and DRG neuron injury was increased. A broad literature suggests that caffeine may provide therapeutic benefit in neurodegenerative diseases (47, 58). However, the data are inconsistent, and our demonstration of the prooxidant activity suggests why these data conflict. Experimental results will depend on whether the caffeine generates oxidative stress in a cell or tissue of interest when additional stress is injurious.
Our most promising compound from these studies is fenofibrate. In contrast to caffeine, fenofibrate decreased DRG neuron oxidative stress both by operating as a direct antioxidant and by activating the antioxidant response. Fenofibrate is used in type 2 diabetic patients as an adjunct to lipid-lowering therapy aimed at decreasing cardiovascular disease (72). Recent clinical trials demonstrated that fenofibrate also may provide therapeutic protection against retinopathy (1). In type 2 diabetic rats, fenofibrate decreased evidence of nephropathy (11). Taken with our present study, fenofibrate appears to be a strong candidate for the prevention of macro- and microvascular complications in diabetes. The ACCORD trial has many patients taking fenofibrate and should provide valuable insight into the clinical benefits of fenofibrate therapy (67). Fenofibrate, like resveratrol and sulforaphane, requires new protein synthesis to confer DRG neuron protection against glucose (Fig. 11). The protein synthesis–inhibitor study further supports the role of new protein expression, which we contend is mediated via Nrf2 activation of the antioxidant response.
In summary, we report that activation of the antioxidant response is a promising therapeutic option for the protection of DRG neurons against glucose-mediated injury. This protection involves the induction of ARE-containing enzymes such as NQO1 and GST in an Nrf2-dependent manner. The mechanisms by which compounds activate the antioxidant response will be important. Specifically, the mechanism of fenofibrate that activates the response without oxidative stress should be explored. This study supports the concept that increasing the antioxidant potential within neurons will promote resistance to hyperglycemia and confirms the importance of further exploration of botanical and other compounds for therapeutic strategies against diabetic neuropathy.
Acknowledgments
This work was supported by the Program for Neurology Research and Discovery, the Juvenile Diabetes Research Foundation Center for the Study of Complications in Diabetes, and grants from the National Institutes of Health (NIH RO1 NS36778, and NIH RO1 NS38849). This work used the Morphology and Image Analysis Core of the Michigan Diabetes Research and Training Center funded by NIH5P60 DK20572 from the National Institute of Diabetes & Digestive & Kidney Diseases.
Abbreviations
ABAP, 2,2′-azobis(amidinopropane) dihydrochloride; ARE, antioxidant response element; BHQ, tert-butylhydroquinone; CHX, cycloheximide; DCFDA, 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate; DRG, dorsal root ganglia; GST, glutathione S-transferase; HO-1, heme oxygenase-1; Nrf2, nuclear factor-E2–related factor-2; NGF, nerve growth factor; NQO1, NAD(P)H:quinone oxidoreductase-1; ROS, reactive oxygen species; SOD, superoxide dismutase; TBS, tris-buffered saline solution; TUNEL, terminal deoxy-UTP nick-end labeling;
Disclosure Statement
No competing financial interests exist.
References
- 1.Reducing ocular damage in type 2 diabetes: the FIELD study shows fenofibrate benefits. Cardiovasc J Afr. 2007;18:400. [PubMed] [Google Scholar]
- 2.Afaq F. Mukhtar H. Botanical antioxidants in the prevention of photocarcinogenesis and photoaging. Exp Dermatol. 2006;15:678–684. doi: 10.1111/j.1600-0625.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- 3.Ahlgren-Beckendorf JA. Reising AM. Schander MA. Herdler JW. Johnson JA. Coordinate regulation of NAD(P)H:quinone oxidoreductase and glutathione-S-transferases in primary cultures of rat neurons and glia: role of the antioxidant/electrophile responsive element. Glia. 1999;25:131–142. [PubMed] [Google Scholar]
- 4.Almhanna K. Wilkins PL. Bavis JR. Harwalkar S. Berti-Mattera LN. Hyperglycemia triggers abnormal signaling and proliferative responses in Schwann cells. Neurochem Res. 2002;27:1341–1347. doi: 10.1023/a:1021671615939. [DOI] [PubMed] [Google Scholar]
- 5.Bonnard C. Durand A. Peyrol S. Chanseaume E. Chauvin MA. Morio B. Vidal H. Rieusset J. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest. 2008;118:789–800. doi: 10.1172/JCI32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulton AJ. Vileikyte L. Ragnarson-Tennvall G. Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719–1724. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 7.Boulton AJ. Vinik AI. Arezzo JC. Bril V. Feldman EL. Freeman R. Malik RA. Maser RE. Sosenko JM. Ziegler D. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 8.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 9.Cameron NE. Eaton SE. Cotter MA. Tesfaye S. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia. 2001;44:1973–1988. doi: 10.1007/s001250100001. [DOI] [PubMed] [Google Scholar]
- 10.Candelario-Jalil E. de Oliveira AC. Graf S. Bhatia HS. Hull M. Munoz E. Fiebich BL. Resveratrol potently reduces prostaglandin E2 production and free radical formation in lipopolysaccharide-activated primary rat microglia. J Neuroinflammation. 2007;4:25. doi: 10.1186/1742-2094-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen YJ. Quilley J. Fenofibrate treatment of diabetic rats reduces nitrosative stress, renal cyclooxygenase-2 expression, and enhanced renal prostaglandin release. J Pharmacol Exp Ther. 2008;324:658–663. doi: 10.1124/jpet.107.129197. [DOI] [PubMed] [Google Scholar]
- 12.Cheng HL. Randolph A. Yee D. Delafontaine P. Tennekoon G. Feldman EL. Characterization of insulin-like growth factor-I (IGF-I), IGF-I receptor and binding proteins in transected nerves and cultured Schwann cells. J Neurochem. 1996;66:525–536. doi: 10.1046/j.1471-4159.1996.66020525.x. [DOI] [PubMed] [Google Scholar]
- 13.Cheng HL. Sullivan KA. Feldman EL. Immunohisto-chemical localization of insulin-like growth factor binding protein-5 in the developing rat nervous system. Brain Res Dev Brain Res. 1996;92:211–218. doi: 10.1016/0165-3806(96)00016-8. [DOI] [PubMed] [Google Scholar]
- 14.Chilton FH. Rudel LL. Parks JS. Arm JP. Seeds MC. Mechanisms by which botanical lipids affect inflammatory disorders. Am J Clin Nutr. 2008;87:498S–503S. doi: 10.1093/ajcn/87.2.498S. [DOI] [PubMed] [Google Scholar]
- 15.Chow SE. Hshu YC. Wang JS. Chen JK. Resveratrol attenuates oxLDL-stimulated NADPH oxidase activity and protects endothelial cells from oxidative functional damages. J Appl Physiol. 2007;102:1520–1527. doi: 10.1152/japplphysiol.00881.2006. [DOI] [PubMed] [Google Scholar]
- 16.Cristiano F. de Haan JB. Iannello RC. Kola I. Changes in the levels of enzymes which modulate the antioxidant balance occur during aging and correlate with cellular damage. Mech Ageing Dev. 1995;80:93–105. doi: 10.1016/0047-6374(94)01561-y. [DOI] [PubMed] [Google Scholar]
- 17.Das DK. Engelman RM. Kimura Y. Molecular adaptation of cellular defences following preconditioning of the heart by repeated ischaemia. Cardiovasc Res. 1993;27:578–584. doi: 10.1093/cvr/27.4.578. [DOI] [PubMed] [Google Scholar]
- 18.de Almeida LM. Pineiro CC. Leite MC. Brolese G. Tramontina F. Feoli AM. Gottfried C. Goncalves CA. Resveratrol increases glutamate uptake, glutathione content, and S100B secretion in cortical astrocyte cultures. Cell Mol Neurobiol. 2007;27:661–668. doi: 10.1007/s10571-007-9152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delaney CL. Russell JW. Cheng HL. Feldman EL. Insulin-like growth factor-I and over-expression of Bcl-xL prevent glucose-mediated apoptosis in Schwann cells. J Neuropathol Exp Neurol. 2001;60:147–160. doi: 10.1093/jnen/60.2.147. [DOI] [PubMed] [Google Scholar]
- 20.Dinkova-Kostova AT. Fahey JW. Wade KL. Jenkins SN. Shapiro TA. Fuchs EJ. Kerns ML. Talalay P. Induction of the phase 2 response in mouse and human skin by sulforaphane-containing broccoli sprout extracts. Cancer Epidemiol Biomarkers Prev. 2007;16:847–851. doi: 10.1158/1055-9965.EPI-06-0934. [DOI] [PubMed] [Google Scholar]
- 21.Du XL. Edelstein D. Rossetti L. Fantus IG. Goldberg H. Ziyadeh F. Wu J. Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldman EL. Oxidative stress and diabetic neuropathy: a new understanding of an old problem. J Clin Invest. 2003;111:431–433. doi: 10.1172/JCI17862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Godley BF. Jin GF. Guo YS. Hurst JS. Bcl-2 overexpression increases survival in human retinal pigment epithelial cells exposed to H(2)O(2) Exp Eye Res. 2002;74:663–669. doi: 10.1006/exer.2001.1146. [DOI] [PubMed] [Google Scholar]
- 24.Gong P. Stewart D. Hu B. Li N. Cook J. Nel A. Alam J. Activation of the mouse heme oxygenase-1 gene by 15-deoxy-delta(12,14)-prostaglandin J(2) is mediated by the stress response elements and transcription factor Nrf2. Antioxid. Redox Signal. 2002;4:249–257. doi: 10.1089/152308602753666307. [DOI] [PubMed] [Google Scholar]
- 25.Greene DA. Stevens MJ. Obrosova I. Feldman EL. Glucose-induced oxidative stress and programmed cell death in diabetic neuropathy. Eur J Pharmacol. 1999;375:217–223. doi: 10.1016/s0014-2999(99)00356-8. [DOI] [PubMed] [Google Scholar]
- 26.Harikumar KB. Aggarwal BB. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7:1020–1035. doi: 10.4161/cc.7.8.5740. [DOI] [PubMed] [Google Scholar]
- 27.He X. Chen MG. Lin GX. Ma Q. Arsenic induces NAD(P)H-quinone oxidoreductase I by disrupting the Nrf2 × Keap1 × Cul3 complex and recruiting Nrf2 × Maf to the antioxidant response element enhancer. J Biol Chem. 2006;281:23620–23631. doi: 10.1074/jbc.M604120200. [DOI] [PubMed] [Google Scholar]
- 28.Heber D. Seeram NP. Wyatt H. Henning SM. Zhang Y. Ogden LG. Dreher M. Hill JO. Safety and antioxidant activity of a pomegranate ellagitannin-enriched polyphenol dietary supplement in overweight individuals with increased waist size. J Agric Food Chem. 2007;55:10050–10054. doi: 10.1021/jf071689v. [DOI] [PubMed] [Google Scholar]
- 29.Hsieh TC. Lu X. Wang Z. Wu JM. Induction of quinone reductase NQO1 by resveratrol in human K562 cells involves the antioxidant response element ARE and is accompanied by nuclear translocation of transcription factor Nrf2. Med Chem. 2006;2:275–285. doi: 10.2174/157340606776930709. [DOI] [PubMed] [Google Scholar]
- 30.Jain AK. Bloom DA. Jaiswal AK. Nuclear import and export signals in control of Nrf2. J Biol Chem. 2005;280:29158–29168. doi: 10.1074/jbc.M502083200. [DOI] [PubMed] [Google Scholar]
- 31.Jaiswal AK. Characterization and partial purification of microsomal NAD(P)H:quinone oxidoreductases. Arch Biochem Biophys. 2005;375:62–68. doi: 10.1006/abbi.1999.1650. [DOI] [PubMed] [Google Scholar]
- 32.Jeyapaul J. Jaiswal AK. Nrf2 and c-Jun regulation of anti-oxidant response element (ARE)-mediated expression and induction of gamma-glutamylcysteine synthetase heavy subunit gene. Biochem Pharmacol. 2000;59:1433–1439. doi: 10.1016/s0006-2952(00)00256-2. [DOI] [PubMed] [Google Scholar]
- 33.Kaeffer N. Richard V. Thuillez C. Delayed coronary endothelial protection 24 h after preconditioning: role of free radicals. Circulation. 1997;96:2311–2316. doi: 10.1161/01.cir.96.7.2311. [DOI] [PubMed] [Google Scholar]
- 34.Kang KW. Lee SJ. Kim SG. Molecular mechanism of nrf2 activation by oxidative stress. Antioxid. Redox Signal. 2005;7:1664–1673. doi: 10.1089/ars.2005.7.1664. [DOI] [PubMed] [Google Scholar]
- 35.Kellogg AP. Cheng HT. Pop-Busui R. Cyclooxygenase-2 pathway as a potential therapeutic target in diabetic peripheral neuropathy. Curr Drug Targets. 2008;9:68–76. doi: 10.2174/138945008783431691. [DOI] [PubMed] [Google Scholar]
- 36.Kim D. Nguyen MD. Dobbin MM. Fischer A. Sananbenesi F. Rodgers JT. Delalle I. Baur JA. Sui G. Armour SM. Puigserver P. Sinclair DA. Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JM. Hanson JM. Chu WA. Johnson JA. Phosphatidylinositol 3-kinase, not extracellular signal-regulated kinase, regulates activation of the antioxidant-responsive element in IMR-32 human neuroblastoma cells. J Biol Chem. 2001;276:20011–20016. doi: 10.1074/jbc.M100734200. [DOI] [PubMed] [Google Scholar]
- 38.Leventhal PS. Feldman EL. The tyrosine kinase inhibitor methyl 2,5-dihydroxycinnimate disrupts changes in the actin cytoskeleton required for neurite formation. Brain Res Mol Brain Res. 1996;43:338–340. doi: 10.1016/s0169-328x(96)00221-5. [DOI] [PubMed] [Google Scholar]
- 39.Li Y. Cao Z. Zhu H. Upregulation of endogenous antioxidants and phase 2 enzymes by the red wine polyphenol, resveratrol in cultured aortic smooth muscle cells leads to cytoprotection against oxidative and electrophilic stress. Pharmacol Res. 2006;53:6–15. doi: 10.1016/j.phrs.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Li Y. Cao Z. Zhu H. Upregulation of endogenous antioxidants and phase 2 enzymes by the red wine polyphenol, resveratrol, in cultured aortic smooth muscle cells leads to cytoprotection against oxidative and electrophilic stress. Pharmacol Res. 2006;53:6–15. doi: 10.1016/j.phrs.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Lin AM. Dung SW. Chen CF. Chen WH. Ho LT. Hypoxic preconditioning prevents cortical infarction by transient focal ischemia-reperfusion. Ann N Y Acad Sci. 2003;993:168–178. doi: 10.1111/j.1749-6632.2003.tb07527.x. discussion 195–196. [DOI] [PubMed] [Google Scholar]
- 42.Liu BL. Zhang X. Zhang W. Zhen HN. New enlightenment of French paradox: resveratrol's potential for cancer chemoprevention and anti-cancer therapy. Cancer Biol Ther. 2007;6:1833–1836. doi: 10.4161/cbt.6.12.5161. [DOI] [PubMed] [Google Scholar]
- 43.Martin D. Rojo AI. Salinas M. Diaz R. Gallardo G. Alam J. Ruiz De Galarreta CM. Cuadrado A. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J Biol Chem. 2004;279:8919–8929. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- 44.McMahon M. Itoh K. Yamamoto M. Chanas SA. Henderson CJ. McLellan LI. Wolf CR. Cavin C. Hayes JD. The Cap'n’Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001;61:3299–3307. [PubMed] [Google Scholar]
- 45.Mody N. Parhami F. Sarafian TA. Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001;31:509–519. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 46.Motterlini R. Hidalgo A. Sammut I. Shah KA. Mohammed S. Srai K. Green CJ. A precursor of the nitric oxide donor SIN-1 modulates the stress protein heme oxygenase-1 in rat liver. Biochem Biophys Res Commun. 1996;225:167–172. doi: 10.1006/bbrc.1996.1148. [DOI] [PubMed] [Google Scholar]
- 47.Nakaso K. Ito S. Nakashima K. Caffeine activates the PI3K/Akt pathway and prevents apoptotic cell death in a Parkinson's disease model of SH-SY5Y cells. Neurosci Lett. 2008;432:146–150. doi: 10.1016/j.neulet.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen T. Sherratt PJ. Nioi P. Yang CS. Pickett CB. Nrf2 controls constitutive and inducible expression of ARE-driven genes through a dynamic pathway involving nucleocytoplasmic shuttling by Keap1. J Biol Chem. 2006;280:32485–32492. doi: 10.1074/jbc.M503074200. [DOI] [PubMed] [Google Scholar]
- 49.Nguyen T. Sherratt PJ. Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- 50.Nioi P. Hayes JD. Contribution of NAD(P)H:quinone oxidoreductase 1 to protection against carcinogenesis, and regulation of its gene by the Nrf2 basic-region leucine zipper and the arylhydrocarbon receptor basic helix-loop-helix transcription factors. Mutat Res. 2004;555:149–171. doi: 10.1016/j.mrfmmm.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 51.Ou HC. Chou FP. Sheen HM. Lin TM. Yang CH. Huey-Herng Sheu W. Resveratrol, a polyphenolic compound in red wine, protects against oxidized LDL-induced cytotoxicity in endothelial cells. Clin Chim Acta. 2006;364:196–204. doi: 10.1016/j.cccn.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 52.Pennathur S. Heinecke JW. Mechanisms of oxidative stress in diabetes: implications for the pathogenesis of vascular disease and antioxidant therapy. Front Biosci. 2004;9:565–574. doi: 10.2741/1257. [DOI] [PubMed] [Google Scholar]
- 53.Pespeni M. Hodnett M. Pittet JF. In vivo stress preconditioning. Methods. 2005;35:158–164. doi: 10.1016/j.ymeth.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 54.Petzer JP. Navamal M. Johnson JK. Kwak MK. Kensler TW. Fishbein JC. Phase 2 enzyme induction by the major metabolite of oltipraz. Chem Res Toxicol. 2003;16:1463–1469. doi: 10.1021/tx034154e. [DOI] [PubMed] [Google Scholar]
- 55.Philbert MA. Beiswanger CM. Manson MM. Green JA. Novak RF. Primiano T. Reuhl KR. Lowndes HE. Glutathione S-transferases and gamma-glutamyl transpeptidase in the rat nervous systems: a basis for differential susceptibility to neurotoxicants. Neurotoxicology. 1995;16:349–362. [PubMed] [Google Scholar]
- 56.Richard V. Kaeffer N. Tron C. Thuillez C. Ischemic preconditioning protects against coronary endothelial dysfunction induced by ischemia and reperfusion. Circulation. 1994;89:1254–1261. doi: 10.1161/01.cir.89.3.1254. [DOI] [PubMed] [Google Scholar]
- 57.Rodrigo R. Castillo R. Cereceda M. Asenjo R. Zamorano J. Araya J. Non-hypoxic preconditioning of myocardium against postoperative atrial fibrillation: mechanism based on enhancement of the antioxidant defense system. Med Hypotheses. 2007;69:1242–1248. doi: 10.1016/j.mehy.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 58.Ross GW. Petrovitch H. Current evidence for neuro-protective effects of nicotine and caffeine against Parkinson's disease. Drugs Aging. 2001;18:797–806. doi: 10.2165/00002512-200118110-00001. [DOI] [PubMed] [Google Scholar]
- 59.Russell JW. Golovoy D. Vincent AM. Mahendru P. Olzmann JA. Mentzer A. Feldman EL. High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J. 2002;16:1738–1748. doi: 10.1096/fj.01-1027com. [DOI] [PubMed] [Google Scholar]
- 60.Russell JW. Sullivan KA. Windebank AJ. Herrmann DN. Feldman EL. Neurons undergo apoptosis in animal and cell culture models of diabetes. Neurobiol Dis. 1999;6:347–363. doi: 10.1006/nbdi.1999.0254. [DOI] [PubMed] [Google Scholar]
- 61.Schwedhelm E. Maas R. Troost R. Boger RH. Clinical pharmacokinetics of antioxidants and their impact on systemic oxidative stress. Clin Pharmacokinet. 2003;42:437–459. doi: 10.2165/00003088-200342050-00003. [DOI] [PubMed] [Google Scholar]
- 62.Shapiro TA. Fahey JW. Dinkova-Kostova AT. Holtzclaw WD. Stephenson KK. Wade KL. Ye L. Talalay P. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: a clinical phase I study. Nutr Cancer. 2006;55:53–62. doi: 10.1207/s15327914nc5501_7. [DOI] [PubMed] [Google Scholar]
- 63.Sharma S. Anjaneyulu M. Kulkarni SK. Chopra K. Resveratrol, a polyphenolic phytoalexin, attenuates diabetic nephropathy in rats. Pharmacology. 2006;76:69–75. doi: 10.1159/000089720. [DOI] [PubMed] [Google Scholar]
- 64.Sharma S. Kulkarni SK. Chopra K. Resveratrol, a polyphenolic phytoalexin attenuates thermal hyperalgesia and cold allodynia in STZ-induced diabetic rats. Indian J Exp Biol. 2006;44:566–569. [PubMed] [Google Scholar]
- 65.Shih AY. Johnson DA. Wong G. Kraft AD. Jiang L. Erb H. Johnson JA. Murphy TH. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci. 2003;23:3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Srinivasan S. Hatley ME. Bolick DT. Palmer LA. Edelstein D. Brownlee M. Hedrick CC. Hyperglycaemia-induced superoxide production decreases eNOS expression via AP-1 activation in aortic endothelial cells. Diabetologia. 2004;47:1727–1734. doi: 10.1007/s00125-004-1525-1. [DOI] [PubMed] [Google Scholar]
- 67.Steinmetz A. Lipid-lowering therapy in patients with type 2 diabetes: the case for early intervention. Diabetes Metab Res Rev. 2008;24:286–293. doi: 10.1002/dmrr.806. [DOI] [PubMed] [Google Scholar]
- 68.Stevens MJ. Obrosova I. Pop-Busui R. Greene DA. Feldman EL. Pathogenesis of diabetic neuropathy. In: Porte D Jr, editor; Sherwin RS, editor; Baron A, editor. Ellenberg and Rifkin's Diabetes Mellitus. 6th. New York: McGraw Hill; 2002. pp. 747–770. [Google Scholar]
- 69.Syed DN. Afaq F. Mukhtar H. Pomegranate derived products for cancer chemoprevention. Semin Cancer Biol. 2007;17:377–385. doi: 10.1016/j.semcancer.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 70.Thejass P. Kuttan G. Immunomodulatory activity of sulforaphane, a naturally occurring isothiocyanate from broccoli (Brassica oleracea) Phytomedicine. 2007;14:538–545. doi: 10.1016/j.phymed.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 71.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002;287:2563–2569. doi: 10.1001/jama.287.19.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vakkilainen J. Steiner G. Ansquer JC. Perttunen-Nio H. Taskinen MR. Fenofibrate lowers plasma triglycerides and increases LDL particle diameter in subjects with type 2 diabetes. Diabetes Care. 2002;25:627–628. doi: 10.2337/diacare.25.3.627. [DOI] [PubMed] [Google Scholar]
- 73.Venugopal R. Jaiswal AK. Nrf2 and Nrf1 in association with Jun proteins regulate antioxidant response element-mediated expression and coordinated induction of genes encoding detoxifying enzymes. Oncogene. 1998;17:3145–3156. doi: 10.1038/sj.onc.1202237. [DOI] [PubMed] [Google Scholar]
- 74.Vincent AM. Brownlee M. Russell JW. Oxidative stress and programmed cell death in diabetic neuropathy. Ann N Y Acad Sci. 2002;959:368–383. doi: 10.1111/j.1749-6632.2002.tb02108.x. [DOI] [PubMed] [Google Scholar]
- 75.Vincent AM. Edwards JL. Sadidi M. Feldman EL. The antioxidant response as a drug target in diabetic neuropathy. Curr Drug Targets. 2008;9:94–100. doi: 10.2174/138945008783431754. [DOI] [PubMed] [Google Scholar]
- 76.Vincent AM. Feldman EL. Control of cell survival by IGF signaling pathways. Growth Horm IGF Res. 2002;12:193–197. doi: 10.1016/s1096-6374(02)00017-5. [DOI] [PubMed] [Google Scholar]
- 77.Vincent AM. Feldman EL. New insights into the mechanisms of diabetic neuropathy. Rev Endocr Metab Disord. 2004;5:227–236. doi: 10.1023/B:REMD.0000032411.11422.e0. [DOI] [PubMed] [Google Scholar]
- 78.Vincent AM. Feldman EL. Can drug screening lead to candidate therapies for testing in diabetic neuropathy? Antioxid Redox Signal. 2008;10:387–393. doi: 10.1089/ars.2007.1815. [DOI] [PubMed] [Google Scholar]
- 79.Vincent AM. McLean LL. Backus C. Feldman EL. Short-term hyperglycemia produces oxidative damage and apoptosis in neurons. FASEB J. 2005;19:638–640. doi: 10.1096/fj.04-2513fje. [DOI] [PubMed] [Google Scholar]
- 80.Vincent AM. Olzmann JA. Brownlee M. Sivitz WI. Russell JW. Uncoupling proteins prevent glucose-induced neuronal oxidative stress and programmed cell death. Diabetes. 2004;53:726–734. doi: 10.2337/diabetes.53.3.726. [DOI] [PubMed] [Google Scholar]
- 81.Vincent AM. Perrone L. Sullivan KA. Backus C. Sastry AM. Lastoskie C. Feldman EL. Receptor for advanced glycation end products activation injures primary sensory neurons via oxidative stress. Endocrinology. 2007;148:548–558. doi: 10.1210/en.2006-0073. [DOI] [PubMed] [Google Scholar]
- 82.Vincent AM. Russell JW. Low P. Feldman EL. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev. 2004;25:612–628. doi: 10.1210/er.2003-0019. [DOI] [PubMed] [Google Scholar]
- 83.Vincent AM. Russell JW. Sullivan KA. Backus C. Hayes JM. McLean LL. Feldman EL. SOD2 protects neurons from injury in cell culture and animal models of diabetic neuropathy. Exp Neurol. 2007;208:216–227. doi: 10.1016/j.expneurol.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vincent AM. Stevens MJ. Backus C. McLean LL. Feldman EL. Cell culture modeling to test therapies against hyperglycemia-mediated oxidative stress and injury. Antioxid Redox Signal. 2005;7:1494–1506. doi: 10.1089/ars.2005.7.1494. [DOI] [PubMed] [Google Scholar]
- 85.Warnholtz A. Munzel T. Why do antioxidants fail to provide clinical benefit? Curr Control Trials Cardiovasc Med. 2000;1:38–40. doi: 10.1186/cvm-1-1-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Windebank AJ. Feldman EL. Aminoff MJ. Diabetes and the nervous system. Churchill Livingstone., editor. Neurology and General Medicine. 2001;3:341–364. [Google Scholar]
- 87.Woo KJ. Kwon TK. Sulforaphane suppresses lipopolysaccharide-induced cyclooxygenase-2 (COX-2) expression through the modulation of multiple targets in COX-2 gene promoter. Int Immunopharmacol. 2007;7:1776–1783. doi: 10.1016/j.intimp.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 88.Zelko IN. Mariani TJ. Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33:337–349. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- 89.Zykova TA. Zhu F. Zhai X. Ma WY. Ermakova SP. Lee KW. Bode AM. Dong Z. Resveratrol directly targets COX-2 to inhibit carcinogenesis. Mol Carcinog. 2008;47:797–805. doi: 10.1002/mc.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]