Abstract
The signaling pathway(s) and molecular target(s) for 5,6-dimethylxanthenone-4-acetic acid (DMXAA), a tumor vascular disrupting agent in late stages of clinical development, are still undefined. As an approach toward identifying potential targets for DMXAA, a tritiated azido-analog of DMXAA was used to probe for cellular binding proteins. More than 20 cytosolic proteins from murine splenocytes, RAW 264.7 cells, and the HECPP immortalized endothelial cells were photoaffinity-labeled. Although no protein domain, fold, or binding site for a specific ligand was found to be shared by all the candidate proteins, essentially all were noted to be oxidizable proteins, implicating a role for redox signaling in the action of DMXAA. Consistent with this hypothesis, DMXAA caused an increase in concentrations of reactive oxygen species (ROS) in RAW264.7 cells during the first 2 hours. This increase in ROS was suppressed in the presence of the antioxidant, N-acetyl-l-cysteine, which also suppressed DMXAA-induced cytokine production in the RAW 264.7 cells with no effects on cell viability. Short interfering RNA (siRNA)-mediated knockdown of one of the photoaffinity-labeled proteins, superoxide dismutase 1, an ROS scavenger, resulted in an increase in tumor necrosis factor-α production by RAW 264.7 cells in response to DMXAA compared with negative or positive controls transfected with nontargeting or lamin A/C-targeting siRNA molecules, respectively. The results from these lines of study all suggest that redox signaling plays a central role in cytokine induction by DMXAA.
Introduction
A number of low-molecular tumor vascular-disrupting agents are in late clinical evaluation. Most of these agents, including the combretastatins, taxanes, and vinca alkaloids, have disruption of normal tubulin polymerization in endothelial cells as their primary mode of action [1]. Tubulin does not seem to be a primary target for 5,6-dimethylxanthenone-4-acetic acid (DMXAA), a small molecule that has both vascular-disrupting activity and cytokine modulatory activity. DMXAA was synthesized at the Auckland Cancer Society Research Centre [2] as a derivative of flavone acetic acid (FAA), a flavonoid initially synthesized by Lyonnaise Industrielle Pharmaceutique (LIPHA, Lyon, France) as part of their antiinflammatory program [3].When FAA was tested by the National Cancer Institute, Bethesda, MD, it showed curative properties against a number of transplantable murine tumors that were resistant to current chemotherapies [4]. A hallmark activity of DMXAA and of FAA is the rapid onset of hemorrhagic necrosis of the implanted tumors [5], resulting from vascular collapse [6], caused by the induction of apoptosis selectively in tumor vascular endothelial cells [7]. After the initial direct antivascular effects, a large panel of cytokines are produced [8,9], leading to a cascade of secondary host antitumor responses. Tumor necrosis factor-α (TNF-α), itself a potent vascular-disrupting agent [10], is suggested to amplify and prolong the direct antivascular effects of DMXAA and FAA [11,12], whereas the production of type 1 interferons (IFNs) [13] has been attributed to systemic increases in tumor-specific CD8+ T lymphocytes [14]. More recently, the major influx of neutrophils into tumors after DMXAA treatment was suggested to be linked to the production of chemokines that included IFN-γ-inducible protein 10, RANTES, macrophage inflammatory protein-1(MIP-1), and monocyte chemoattractant protein-1 [8,15].
The molecular mechanism of cytokine induction by DMXAA is not fully understood, although there is strong evidence for the involvement of the nuclear factor (NF) κB pathway [16–18], as well as the TANK-binding kinase 1 (TBK1)-interferon (IFN) regulatory factor 3 (IRF-3) signaling axis [13]. Previous studies from our laboratory using tritiated-DMXAA indicated that the compound diffused rapidly into cells [18], but specific binding to any cellular proteins could not be determined because of the low affinity of binding of the compound. To overcome this problem, photoactivatable azido-analogs of DMXAA were synthesized in an approach to photoaffinity label potential target proteins [19]. Azido substitution at the 5- or 6- position of the xanthenone ring produced analogs capable of inducing NF-κB activation and cytokine production in cultured splenocytes and inducing hemorrhagic necrosis of tumors in mice [19]. Those studies indicated that the azido-analogs had the same profile of activities as DMXAA and were therefore likely to have the same target(s). Covalent bonds formed between the azido-compound and the interacting proteins after photoactivation were predicted to overcome the problems of the reversible low-affinity binding that occur with DMXAA and its target(s). The receptors for a number of drugs including verapamil [20] and paclitaxel [21] were successfully located using a photoaffinity labeling approach. We report here studies using a tritiated azido-XAA analog to photoaffinity label potential DMXAA-binding proteins. More than 20 oxidizable proteins were labeled, leading to the hypothesis that DMXAA may be acting through modulation of redox signaling. Subsequent studies measuring concentrations of reactive oxygen species (ROS) in cells and the effect of the antioxidant N-acetyl-Ll-ysteine (NAC) on DMXAA-induced cytokine production support this hypothesis.
Materials and Methods
Drugs and Reagents
DMXAA was synthesized as the sodium salt at the Auckland Cancer Society Research Centre [2] and dissolved in α-minimal essential medium (α-MEM). 5-Azidoxanthenone-4-acetic acid (5-AzXAA) was also synthesized at the center [19] and was dissolved in acetonitrile. For photoaffinity labeling experiments, 5-AzXAA was custom radiolabeled with tritium by AmBios Labs, Inc (Newington, CT) to display a specific activity of 0.1 Ci/mmol. NAC (Sigma-Aldrich, Inc, St Louis, MO) was dissolved in α-MEM.
Preparation of Cell Lysates
Murine RAW 264.7 macrophage-like cell line was maintained in α-MEM supplemented with 10% (vol./vol.) fetal calf serum (FCS), 100 U/ml penicillin-G, and 100 µg/ml streptomycin sulfate at 37°C in a humidified atmosphere of 5% CO2/air. The murine HECPP endothelial cell line [22] was maintained in M199 medium supplemented with FCS (10%) and antibiotics. Murine splenocytes were obtained from C57BL/6 mice after cervical dislocation. Spleen cells were collected, and red blood cells were removed by osmotic lysis.
All cells were lysed with potassium phosphate buffer (8.02 mM K2HPO4 and 1.98 mM KH2PO4, pH 7.4) in the presence of 0.5% Nonidet-P40 (Roche Product Ltd, Auckland, New Zealand) and protease inhibitor cocktail from Sigma-Aldrich. Protein concentrations in the lysates were determined by the Bradford assay [23]. Aliquots were stored at -80°C until use.
Photoaffinity Labeling and Gel Electrophoresis
Cell lysates (150 µg) were incubated with 1.5 µg of [3H]-5-AzXAA for 30 minutes on ice and UV-irradiated for 10 minutes (Stratlinker 1800; Stratagene, La Jolla, CA). The samples were then precipitated using 2D Clean-up Kit (Amersham Biosciences, Piscataway, NJ) according to the manufacturer's instructions. The resulting protein pellets were resuspended in 125 µl of rehydration buffer (9.8 M urea, 2% wt./vol. CHAPS, 0.5% vol./vol. ampholyte, pH 3–10 NL, 0.002% wt./vol. bromophenol blue, 2.8 mg/ml dithiothreitol [DTT] in H2O) and subjected to two-dimensional PAGE using 7-cm isoelectric focusing (IEF) strips containing an immobilized nonlinear pH gradient ranging from pH 3 to 10 (Immobiline DryStrips pH 3–10 NL; GE Healthcare Bio-Sciences, Uppsala, Sweden). After overnight gel rehydration, IEF was carried out at 20°C with a current limit of 50 µA per strip using the Ettan IPGphor IEF System (Amersham Biosciences, Uppsala, Sweden). The focused IEF strips were equilibrated in 2.5 ml of equilibration buffer (50 mM Tris-HCl, pH 8.8, 6 Murea, 30% wt./vol. glycerol, 2% wt./vol. SDS, and 0.002% wt./vol. bromophenol blue) containing 10 mg/ml DTT, followed by alkylation in 2.5 ml of equilibration buffer containing 25 mg/ml iodoacetamide for 15 minutes each. Equilibrated IEF strips were loaded onto 12% SDS-polyacrylamide gels, and electrophoresis was performed in a Mini PROTEAN 3 cell (Bio-Rad Laboratories, Hercules, CA) for 1.5 hours at 120 V. Two dimensional gels were stained with Coomassie Blue and soaked in Amplify Fluorographic Solution (Amersham Biosciences, Buckinghamshire, England) for 30 minutes before transferring onto 3-mm filter paper, and vacuum-dried. The dried amplify-treated gels were exposed to autoradiographic film (Kodak BioMax XAR Scientific Imaging Film; Carestream Health, Paris, France) at -80°C for 8 weeks. After autoradiography, films were developed and overlaid on the dried Coomassie Blue-stained gels to locate radiolabeled protein spots.
In-gel Digestion and Mass Spectrometry for Protein Identification
Protein spots that had been radiolabeled were excised from fresh two-dimensional gels. Gel pieces were destained in 0.1 M ammonium bicarbonate/50% acetonitrile (45 minutes at 37°C), dehydrated in 100% acetonitrile, dried in a vacuum centrifuge for 5 minutes, and rehydrated in 50 µl of 20 mM DTT/0.1 M ammonium bicarbonate for 30 minutes at 56°C. After another dehydration step in 100% acetonitrile, gel pieces were incubated with 50 µl of 55 mM iodoacetamide/0.1 M ammonium bicarbonate for 15 minutes at room temperature in the dark. Subsequently, the gel pieces were washed with 0.1 M ammonium bicarbonate, followed by a dehydration step (100% acetonitrile), and another wash with milli-Q water. After a final dehydration step with 100% acetonitrile, the gel pieces were vacuum-dried for 5 minutes. The dried gel pieces were left to absorb 15 µl of trypsin solution (50 ng/µl trypsin [sequencing grade; Promega, Madison, WI], 0.1 M ammonium bicarbonate, 10% acetonitrile) for 10 minutes, after which 30 µl of 0.1 M Tris-HCl (pH 9.2)/10% acetonitrile was added, and left overnight at 37°C. The supernatants were collected the following day, and the peptides were extracted by two incubations in 150 µl of 0.1% trifluoroacetic acid/60% acetonitrile at 37°C for 30 minutes each. The peptide extracts were reduced in volume to 1 to 2 µl by vacuum centrifugation. Fifteen microliters of solvent A (0.1% acetic acid/0.005% heptafluorobutyric acid) was added, and samples were processed using a high-performance liquid chromatography system coupled to an ion trap mass spectrometer (Agilent 1100 Series LC/MSD Trap System; Agilent Technologies, Boeblingen, Germany). A 0.5 x 150-mm Zorbax SB C18 column (Agilent Technologies) was pre-equilibrated with solvent A and kept at a constant temperature of 2°C, onto which 8 µl of peptide samples was injected. Peptides were eluted off the column at a flow rate of 12 µl/min using a linear gradient from 90% solvent A (0.1% acetic acid, 0.005% heptafluorobutyric acid in water) and 10% solvent B (0.1% acetic acid, 0.005% heptafluorobutyric acid, 80% acetonitrile in water) 70% solvent B for 45 minutes. The eluted peptides were directly fed into the electrospray ionize of the mass spectrometer, with a spray voltage of 3.5 kV. The electrospray interface was set in positive mode, the nebulizer gas was set at 12 psi, and the drying gas (nitrogen) was delivered at a flow rate of 4.4 L/min at a temperature of 325°C. Ion mass spectra were collected in the range of 200 to 2000 m/z with a threshold of 15,000. The LC/MSD Trap 5.2 software (DataAnalysis version 3.2; Bruker Daltonik, Agilent Technologies) was used to identify compounds for each ion mass spectrum. The resulting data were entered into the Mascot MS/MS Ion Search Engine [24] and compared with spectra in the SwissProt database (www.expasy.ch/sprot).
Measurement of Intracellular ROS
Intracellular ROS concentrations were determined by oxidation of 2′,7′-dichlorodihydrofluorescein. RAW 264.7 cells (106 cells/well) cultured in 24-well plates were incubated for different periods with DMXAA (10 µg/ml). The cells were washed and incubated in the dark for 20 minutes in PBS containing 0.5% FCS (vol./vol.) and H2DCF diacetate (Sigma-Aldrich). After another wash, the cells were resuspended in saline. The mean fluorescence intensity (excitation, 488 nm; emission, 515–540 nm) was measured using flow cytometry (FACScan Flow Cytometer; BD Bioscience, San Diego, CA).
Effects of NAC on DMXAA Activity
RAW 264.7 cells were seeded in triplicate at 106 cells/well in flat bottomed 96-well plates and preincubated with NAC (1–20 mM) for 1 hour. DMXAA (10 µg/ml final concentration) was then added, and ROS was measured after 2 hours of incubation at 37°C. Culture supernatants were collected 8 hours after the addition of DMXAA and assayed using ELISA cytokine kits (OptEIA murine IL-6 and murine TNF-α; BD Biosciences) or with a multiplex cytokine kit (catalog no. MCYTO-70K; Linco Research, St Charles, MO) and a Luminex 100 instrument (Luminex Corporation, Austin, TX). Viability of the cells was determined using the sulforhodamine assay [25]. Each treatment was assayed in triplicate, and results were expressed as mean ± SEM. Data between two groups were compared using unpaired Student t test or ANOVA if multiple comparisons were made and were considered significant when the P value was ≤.05.
RNA Interference of SOD1
A pool of four predesigned small interfering RNA (siRNA) molecules (“SmartPool”) targeting murine SOD1 were purchased from Dharmacon, Inc (Thermo Fisher Scientific, Inc, Lafayette, CT), together with the positive control siRNA molecules targeting lamin A/C, and the negative control nontargeting siRNA molecule no. 2. SiRNA molecules were introduced into cells at 40 nM using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). RAW264.7 cells (5 x 105/well) were seeded onto the preformed transfection complexes in six-well plates in OPTIMEM medium (Invitrogen) without serum. At 4 hours after transfection, α-MEM supplemented with 20% FCS was added to each well, and the cells were allowed to grow. At 48 hours after transfection, the cells were treated with DMXAA (10 µg/ml) for 4 hours, after which the supernatant was harvested for determination of TNF-α concentrations using ELISA, whereas the cells were washed in ice-cold PBS and their proteins were extracted using RIPA buffer containing 1 x Halt protease cocktail inhibitor (Thermo Scientific, Rockford, IL). The lysates were used for immunoblot analysis to assess the degree of knockdown of the target protein. Samples (25 µg protein/well) were electrophoresed using precast NuPAGE Novex Bis-Tris gel (Invitrogen) and transferred to a nitrocellulose membrane that was blocked in PBS containing 0.5% Tween 20 (PBS-T) and 5% nonfat dried milk powder. Membranes were incubated overnight at 4°C with rabbit anti-SOD1 primary antibodies (Santa Cruz, San Diego, CA) diluted at 1:2500 and then for 1 hour at room temperature with HRS-conjugated secondary antibodies (Santa Cruz) diluted at 1:10,000 in PBS-T containing 5% milk powder. Signals were detected using SuperSignal West Pico Chemiluminescent substrate (Pierce Thermo Scientific, Rockford, IL), and images were captured on a Fujifilm LAS 3000 imaging system (Fujifilm, Tokyo, Japan). The blots were stripped in Restore Western Blot Stripping Buffer (Pierce Thermo Scientific) before reblocking in PBS-T containing 5% nonfat dried milk powder for determination of loading using a mouse monoclonal antibody to actin (Millipore, Billerica, MA).
Results
Specificity of Labeling with 5-AzXAA
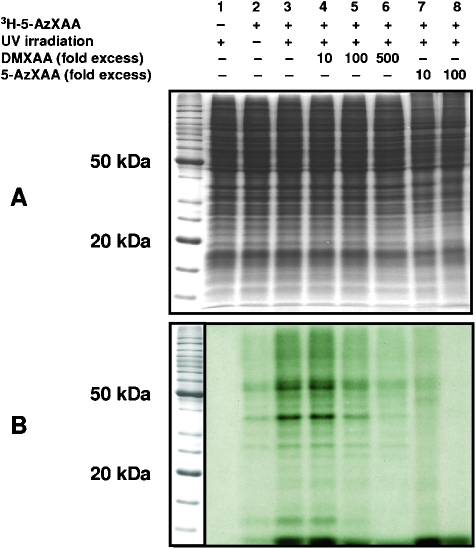
The specificity of the photoaffinity labeling with [3H]-5-AzXAA was examined using competitive binding studies with cold DMXAA or cold 5-AzXAA (Figure 1). Cytosolic protein extracts from RAW 264.7 cells were preincubated with up to 500-fold excess concentrations of cold 5-AzXAA or cold DMXAA before the addition of [3H]-5-AzXAA. The extracts were then exposed to UV irradiation and then analyzed by SDS-PAGE and autoradiography. The intensity of the radiolabeling was markedly decreased in the presence of 100- and 500-fold excess cold DMXAA (Figure 1B, lanes 5 and 6) when compared with the cytosolic protein extracts that were irradiated without competitor (lane 3) and was completely blocked by 10- and 100-fold excess cold 5-AzXAA (lanes 7 and 8).
Figure 1.
(A) SDS-PAGE gel of RAW 264.7 proteins incubated with [3H]-5-AzXAA without UV treatment (lane 2), UV-treated (lane 3), preincubated for 30 minutes with 10, 100, and 500-fold excess cold DMXAA (lanes 4, 5 and 6 respectively), and preincubated with 10- and 100-fold excess cold 5-AzXAA before UV treatment (lanes 7 and 8). (B) Autoradiograph of the gel showing radioactive protein bands (0.45 µg of [3H]-5-AzXAA was used for 45 µg of proteins and 30 µg of protein was loaded per lane).
Photoaffinity Labeling of Cytosolic Proteins
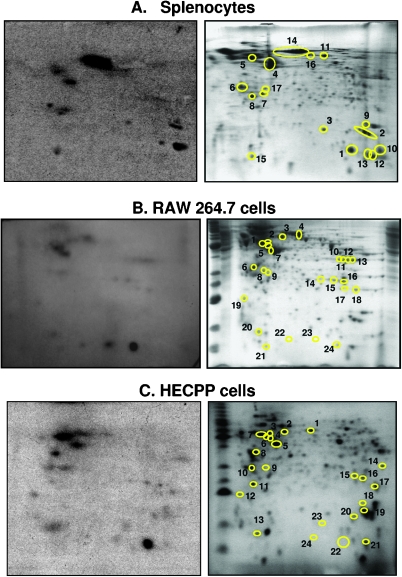
Cytosolic proteins from murine splenocytes and RAW 264.7 macrophage-like cells, used previously for studying cytokine induction [8,13], and the HECPP endothelial cells, used previously for studying apoptosis induction by DMXAA [7], were photoaffinity-labeled with [3H]-5-AzXAA and resolved by two-dimensional PAGE. The twodimensional gels were exposed to radiographic film, after which the radiolabeled protein spots were located by overlaying the autoradiograph onto the respective two-dimensional gel. The autoradiograph and the corresponding Coomassie Blue-stained gel from a representative experiment with murine splenocytes, RAW 264.7 cells, or HECPP cells are shown in Figure 2, A, B, and C, respectively. Each autoradiograph (Figure 2, left) shows a number of darkened spots that could be matched with protein spots on the Coomassie Blue-stained two-dimensional gel (Figure 2, right). Protein spots that were radiolabeled were excised for identification using mass spectrometry and Mascot Search against spectra in SwissProt database. The proteins identified corresponding to each experiment in Figure 2 are listed in Table 1.
Figure 2.
The autoradiograph (left) and the Coomassie Blue-stained two-dimensional gel (right) of lysates from murine splenocytes (A), RAW264.7 cells (B), or HECPP endothelial cells (C), photoreacted with [3H]-5-AzXAA with protein spots that matched the radiolabeled spots from the autoradiograph encircled.
Table 1.
Proteins in Murine Cellular Lysates Photoaffinity Labeled with 5-AzXAA.
| Protein | Spot* | MW (kDa) | pI | Mascot Score† | SwissProt Accession Number |
| (A) Spleen cell lysates | |||||
| Proteins with known role in cytokine production | |||||
| S100A9/Calgranulin B/MRP14 | 1 | 13.0 | 6.73 | 80 | P31725 |
| Cyclophilin A | 2 | 18.0 | 7.88 | 187 | P17742 |
| Superoxide dismutase | 3 | 16.0 | 6.03 | 38 | P08228 |
| Protein disulfide isomerase A3 precursor | 16 | 57.0 | 5.98 | 193 | P11598 |
| Cytoskeletal proteins | |||||
| β-actin | 4 | 42.0 | 5.29 | 155 | Q6ZWM3 |
| β-Tubulin | 5 | 50.1 | 4.78 | 273 | P68372 |
| Tropomyosin a chain | 6 | 32.9 | 4.68 | 206 | P58771 |
| Proteins that alter cytoskeleton dynamics | |||||
| Rho GDP-dissociation inhibitor 1 | 7 | 23.3 | 5.12 | 113 | P31725 |
| Rho GDP-dissociation inhibitor 2 | 8 | 22.8 | 4.97 | 76 | P17742 |
| Cofilin-1 | 9 | 18.6 | 8.26 | 47 | Q99PT1 |
| Profilin-1 | 10 | 15.0 | 8.50 | 142 | P18760 |
| Coronin-1A | 11 | 51.5 | 6.05 | 123 | P62962 |
| Miscellaneous proteins | |||||
| Hemoglobin α-chain | 12 | 15.0 | 8.08 | 41 | P01942 |
| Hemoglobin β-chain | 13 | 15.7 | 7.26 | 95 | P02088 |
| Albumin | 14 | 71.2 | 5.82 | 817 | P07724 |
| SH3 domain-binding glutamic acid-rich-like protein 3 | 15 | 10.5 | 5.02 | 39 | Q91VW3 |
| Chloride intracellular channel protein 1 | 17 | 27.2 | 5.09 | 50 | Q921Q5 |
| (B) RAW 264.7 cell lysates | |||||
| Proteins with known role in cytokine production | |||||
| Macrophage migration inhibitory factor | 22 | 12.7 | 6.90 | 70 | P34884 |
| Thioredoxin 1 | 18 | 12.0 | 4.80 | 79 | P10639 |
| Peroxiredoxin 1 | 16 | 22.4 | 8.26 | 94 | P35700 |
| 60 kDa heat shock protein, mitochondrial | 3 | 61.1 | 5.91 | 207 | P63038 |
| Protein disulfide isomerase A6 precursor | 5 | 48.5 | 5.00 | 237 | Q922R8 |
| Superoxide dismutase [Mn], mitochondrial | 16 | 24.8 | 8.8 | 43 | P09671 |
| Chaperones/stress proteins | |||||
| Stress-70 protein, mitochondrial | 4 | 73.8 | 5.91 | 338 | P38647 |
| Prostaglandin E synthase 3, cytosolic | 17 | 19.0 | 4.36 | 89 | Q9R0Q7 |
| Glycolytic enzymes | |||||
| Glyceraldehyde-3-phosphate dehydrogenase | 110,11,12 | 36.1 | 8.44 | 60 | P16858 |
| Aldose reductase | 7 | 36.1 | 6.71 | 120 | P45376 |
| Triosephosphate isomerase | 13,14 | 27.0 | 6.90 | 55 | P17751 |
| l-Lactate dehydrogenase A chain | 12 | 36.1 | 8.44 | 98 | P06151 |
| Cytoskeletal proteins | |||||
| β-Actin | 6 | 42.1 | 5.29 | 87 | P60710 |
| α-Tubulin | 2 | 50.6 | 4.96 | 224 | P68373 |
| β-Tubulin | 1 | 50.2 | 4.79 | 287 | P68372 |
| Vimentin | 2 | 53.7 | 5.06 | 234 | P20151 |
| Miscellaneous proteins | |||||
| Inositol monophosphatase | 8 | 30.8 | 5.08 | 63 | O55023 |
| 26S protease regulatory subunit 6B | 5 | 47.4 | 5.18 | 55 | P54775 |
| Chloride intracellular channel protein 1 | 9 | 27.3 | 5.09 | 95 | Q9Z1Q5 |
| (C) HECPP cell lysates | |||||
| Proteins with known role in cytokine production | |||||
| Cyclophilin A | 19 | 18.0 | 7.88 | 163 | P17742 |
| Thioredoxin 1 | 13 | 12.0 | 4.80 | 82 | P10639 |
| Peroxiredoxin 1 | 17 | 22.4 | 8.26 | 94 | P35700 |
| 60 kDa heat shock protein, mitochondrial | 2 | 61.1 | 5.91 | 297 | P63038 |
| Protein disulfide isomerase A1 precursor | 4 | 57.5 | 4.79 | 356 | P09103 |
| Macrophage migration inhibitory factor | 21 | 12.7 | 6.9 | 70 | P34884 |
| Chaperones/stress proteins | |||||
| Prostaglandin E synthase 3, cytosolic | 12 | 19.0 | 4.36 | 89 | Q9R0Q7 |
| Calreticulin | 7 | 48.1 | 4.33 | 96 | P14211 |
| Glycolytic enzymes | |||||
| Glyceraldehyde-3-phosphate dehydrogenase | 14 | 36.1 | 8.44 | 76 | P16858 |
| Triosephosphate isomerase | 15,16 | 27.0 | 6.90 | 48 | P17751 |
| Cytoskeletal proteins | |||||
| α-Tubulin | 3 | 50.6 | 4.96 | 359 | P68373 |
| β-Tubulin | 6 | 50.1 | 4.78 | 527 | P68372 |
| β-Actin | 5 | 42.0 | 5.29 | 198 | Q6ZWM3 |
| Vimentin | 3 | 53.7 | 5.06 | 69 | P20151 |
| Proteins that alter cytoskeleton dynamics | |||||
| Rho GDP-dissociation inhibitor 1 | 9 | 23.3 | 5.12 | 109 | P31725 |
| Miscellaneous proteins | |||||
| Albumin 1 | 71.2 | 5.82 | 64 | P07724 | |
| ATP synthase β chain | 6 | 56.2 | 5.15 | 134 | P56480 |
| Nucleophosmin | 11 | 32.7 | 4.62 | 138 | Q61937 |
| Proteasome subunit α6 | 10 | 27.8 | 6.34 | 234 | Q9QUM9 |
| SH3 domain-binding glutamic acid-rich-like protein 3 | 13 | 10.5 | 5.02 | 45 | Q91VW3 |
Spots as in Figure 2A (splenocytes), Figure 2B (RAW 264.7 cells), or Figure 2C (HECPP cells); spots 15, 19, 20, and 21 (Figure 2B; RAW 264.7 cells) and spots 8, 18, 20, 22, 23, and 24 (Figure 2C; HECPP cells) could not be identified.
Mascot score of 30 or greater indicates identity or extensive homology (P < .05).
The separation of proteins was not influenced by incubation with [3H]-5-AzXAA or by UV irradiation. Coomassie Blue-stained control two-dimensional gels of protein samples that had not been exposed to [3H]-5-AzXAA or irradiation (Figure W1A), treated with UV-light only (Figure W1B), or incubated with [3H]-5-AzXAA without photoactivation (Figure W1C) all showed a similar pattern of distribution of protein spots.
A minimum of three independent experiments was carried out with each cell type. A list of the identified proteins compiled from all the experiments for each cell type is presented in Table 2. Spots 12 and 13 from Figure 2A, identified as hemoglobin a and hemoglobin β, respectively, were not included in the final list because they most likely represent contaminants from red blood cells in the original spleen suspension and were not consistently detected in repeat experiments. A total of 24, 18, and 30 labeled proteins were identified for RAW 264.7 cells, splenocytes, and HECPP cells, respectively (Table 2). Of these, eight proteins (thioredoxin 1, stress-70 protein, SH3 domain-binding glutamic acid-rich-like protein-3, protein disulfide isomerase A3/6, Hsc70-interaction protein, albumin, β-actin, and α-tubulin) were detected from lysates from all three cell types (Table 2), although albumin is likely a contaminant from tissue culture. Almost all of the photoaffinity-labeled proteins have been reported to be oxidizable, either by glutathionylation and/or by forming disulfide bonds at one of their cysteine residues in response to oxidative stress [26–36].
Table 2.
Oxidizable Proteins Photoaffinity-Labeled with 5-AzXAA.
| Protein | Murine RAW 264.7 | Murine Splenocytes | Murine HECPP | Oxidative Thiol Modifications* |
| α-Tubulin | • | • | • | D |
| β-Tubulin | • | • | G, D | |
| β-Actin | • | • | • | G, D |
| 40S Ribosomal protein 5A | • | - | ||
| 60 kDa heat shock protein, mitochondrial | • | • | G | |
| Heat shock cognate 71 kDa protein | • | G | ||
| Albumin | • | • | • | G, D |
| Alcohol dehydrogenase [NADP+] | • | - | ||
| Aldose reductase | • | • | G | |
| Annexin A1/2 | • | G | ||
| ATP synthase α chain | • | - | ||
| Calreticulin | • | D | ||
| Cathepsin B | • | G | ||
| Chloride intracellular channel protein 1 | • | • | D | |
| Cofilin-1A | • | G | ||
| Coronin-1A | • | G or D | ||
| Cyclophilin A | • | • | G | |
| Elongation factor-1 | • | G | ||
| Glyceraldehyde-3-phosphate dehydrogenase | • | • | G, D | |
| Hsc70-interaction protein | • | • | • | - |
| Inorganic pyrophosphatase | • | • | G | |
| Inositol monophosphatase | • | - | ||
| l-Lactate dehydrogenase A chain | • | • | G, D | |
| Macrophage migration inhibitory factor | • | G | ||
| Nucleophosmin | • | G | ||
| Peroxiredoxin-1 | • | • | G, D | |
| Phosphoglycerate mutase 1 | • | G or D | ||
| Profilin-1A | • | G | ||
| Prostaglandin E synthase 3 | • | • | - | |
| Proteasome subunit α | • | • | - | |
| Protein disulfide isomerase A6 | • | • | • | G |
| Rho GDP-dissociation inhibitor 1/2 | • | • | G | |
| S100A9 | • | G | ||
| SH3 domain-binding glutamic acid-rich-like protein 3 | • | • | • | - |
| Stress-70 protein, mitochondrial | • | • | • | G |
| Superoxide dismutase [Cu/Zn] | • | D | ||
| Superoxide dismutase [Mn] | • | D | ||
| Thioredoxin 1 | • | • | • | G |
| Triosephosphate isomerase | • | • | G, D | |
| Tropomyosin α chain | • | • | G, D | |
| Vimentin | • | • | G |
Denotes sensitivity to oxidation as determined by oxidative modification by glutathionylation (G) or protein disulfide bond formation (D) of cysteine residues. 760 Oxidizable Proteins and Redox Signaling in DMXAA Brauer et al.
Modulation of Cellular ROS Concentrations by DMXAA and Its Effects on Cytokine Production
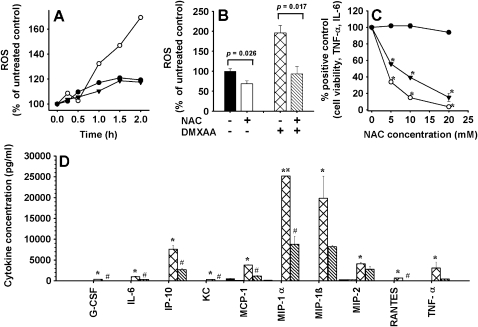
The observation that oxidizable proteins were preferentially labeled using 5-AzXAA led us to investigate whether modulation of redox signaling was involved in DMXAA-mediated cytokine production. We measured DMXAA-induced changes in intracellular concentrations of ROS in RAW 264.7 cells. Intracellular concentrations of ROS increased during the first 2 hours after the addition of DMXAA in three independent experiments (Figure 3A). Preincubation with the antioxidant NAC decreased background concentrations of ROS and reduced DMXAA-induced ROS concentrations (Figure 3B). We next tested the ability of NAC to modulate DMXAA-induced TNF-α and IL-6 production in RAW264.7 cells. At the concentrations tested, NAC had no effects on cell viability but reduced the production of both TNF-α and IL-6 induced with DMXAA in a dose-dependent manner (Figure 3C). Using a 32-plex cytokine assay, 10 cytokines (G-CSF, IL-6, IFN-γ-inducible protein 10, keratinocyte chemoattractant, monocyte chemoattractant protein-1, MIP-1α, MIP-1β, MIP-2, RANTES, and TNF-α) from the panel were found to be induced by DMXAA in the RAW 264.7 cells. Supernatants from cultures preincubated with NAC before the addition of DMXAA had lower concentrations of all 10 cytokines (Figure 3D). NAC alone did not induce cytokines (Figure 3D). Concentration of cytokines in the entire panel assayed is presented in Table 3.
Figure 3.
(A) Accumulation of ROS after the addition of DMXAA (10 µg/ml) in cultures of RAW 264.7 cells in three independent experiments. (B) Effect of 1 hour of preincubation with NAC (20 mM) on ROS concentrations in RAW 264.7 cells untreated or after treatment with DMXAA (10 µg/ml) for 2 hours. Mean ± SEM of triplicate cultures. (C) Effect of 1 hour of pretreatment with various concentrations of NAC on cell viability (•), TNF-α (○), and IL-6 production (▾) in cultures of RAW 264.7 cells stimulated 8 hours with DMXAA (10 µg/ml). Mean ± SEM of triplicate cultures. *Statistical significance compared with cultures treated with DMXAA only and no NAC (P < .05, one way ANOVA). (D) Cytokine levels in RAW 264.7 cell cultures without treatment (black bar), preincubated with NAC (1 hour, 20 mM) (open bars), or cultured 8 hours with DMXAA (10 µg/ml) without preincubation with NAC (hatched bars) or with preincubation with NAC (striped bars). Mean ± SEM of triplicate cultures. *Statistical significance compared with untreated cultures. #Statistical difference compared with cultures treated with DMXAA but without NAC preincubation. •Value above maximum detection limit.
Table 3.
Cytokine Production in RAW264.7 Cells Untreated, NAC Only, or DMXAA-Treated with or without Previous Incubation with NAC.
| Cytokine Concentration (pg/ml)* | ||||
| Untreated | NAC | DMXAA | DMXAA + NAC | |
| Eotaxin | 6 ± 0 | 6 ± 0 | 9 ± 1 | 6 ± 0 |
| G-CSF | 7 ± 1 | 6 ± 0 | 392 ± 79 | 40 ± 24 |
| GM-CSF | 6 ± 0 | 6 ± 0 | 8 ± 1 | 8 ± 1 |
| IFN-γ | 6 ± 0 | 6 ± 0 | 9 ± 0 | 6 ± 0 |
| IL-10 | 6 ± 0 | 6 ± 0 | 6 ± 0 | 6 ± 0 |
| IL-12(p40) | 6 ± 0 | 6 ± 0 | 6 ± 0 | 6 ± 0 |
| IL-12(p70) | 6 ± 0 | 6 ± 0 | 19 ± 1 | 10 ± 1 |
| IL-13 | 6 ± 0 | 6 ± 0 | 6 ± 0 | 6 ± 0 |
| IL-15 | 6 ± 0 | 6 ± 0 | 6 ± 0 | 6 ± 0 |
| IL-17 | 6 ± 0 | 6 ± 0 | 26 ± 3 | 14 ± 3 |
| IL-1α | 6 ± 0 | 6 ± 0 | 27 ± 8 | 7 ± 0 |
| IL-1β | 19 ± 2 | 44 ± 4 | 27 ± 5 | 51 ± 6 |
| IL-2 | 7 ± 1 | 7 ± 1 | 12 ± 0 | 8 ± 1 |
| IL-3 | 6 ± 0 | 6 ± 0 | 6 ± 0 | 6 ± 0 |
| IL-4 | 6 ± 0 | 6 ± 0 | 6 ± 0 | 6 ± 0 |
| IL-5 | 6 ± 0 | 6 ± 0 | 27 ± 3 | 11 ± 1 |
| IL-6 | 6 ± 0 | 6 ± 0 | 1002 ± 129 | 309 ± 117 |
| IL-7 | 6 ± 0 | 6 ± 0 | 6 ± 0 | 6 ± 0 |
| IL-9 | 126 ± 19 | 70 ± 16 | 146 ± 2 | 92 ± 11 |
| IP-10 | 17 ± 1 | 6 ± 0 | 7609 ± 904 | 2636 ± 402 |
| KC | 6 ± 0 | 6 ± 0 | 311 ± 23 | 20 ± 4 |
| LIF | 6 ± 0 | 6 ± 0 | 11 ± 1 | 8 ± 1 |
| LIX | 6 ± 0 | 6 ± 0 | 287 ± 43 | 121 ± 44 |
| M-CSF | 6 ± 0 | 6 ± 0 | 10 ± 1 | 8 ± 2 |
| MCP-1 | 546 ± 42 | 141 ± 11 | 3781 ± 145 | 1149 ± 152 |
| MIG | 6 ± 0 | 6 ± 0 | 11 ± 4 | 6 ± 0 |
| MIP-1α | 161 ± 7 | 113 ± 3 | 25,171 ± 0 | 8766 ± 1897 |
| MIP-1β | 75 ± 5 | 29 ± 7 | 19,834 ± 5337 | 8183 ± 418 |
| MIP-2 | 300 ± 16 | 315 ± 26 | 4078 ± 347 | 2779 ± 651 |
| RANTES | 8 ± 2 | 6 ± 0 | 698 ± 13 | 39 ± 3 |
| TNF-α | 8 ± 1 | 6 ± 0 | 3052 ± 1494 | 431 ± 98 |
| VEGF | 313 ± 19 | 6 ± 0 | 355 ± 25 | 6 ± 0 |
Mean ± SEM of three cultures per treatment group.
GM-CSF indicates granulocyte/macrophage colony-stimulating factor; MIG, monokine induced by IFN-γ; VEGF, vascular endothelial growth factor.
SiRNA Knockdown of SOD1
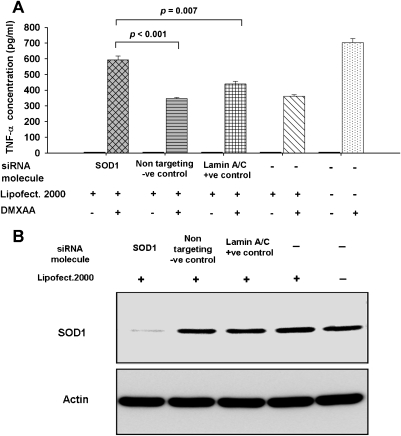
RNA interference was used to knock down the expression of SOD1, a protein with antioxidant functions that was photoaffinitylabeled in both RAW 264.7 cell and spleen cell extracts (Table 2), to examine the effect of reducing its expression on TNF-α induction by DMXAA. Because SOD1 is a scavenger of ROS, we hypothesized that knockdown of SOD1 would attenuate ROS scavenging activity in the cells, resulting in higher ROS concentrations and increased TNF-α production. Consistent with the hypothesis, in four independent experiments, DMXAA-induced TNF-α production in cultures of SOD1 knockdown cells was significantly higher than that of the control cultures of cells transfected with the nontargeting negative control siRNA molecules (P = .003, P < .001, P < .001, and P = .003) or cells transfected with the lamin A/C-positive control molecules (P = .007, P = .007, P = .002, and P = .003). In addition, in all experiments, RAW 264.7 cells transfected with the negative nontargeting control siRNA molecule or the positive control siRNA molecule targeting lamin A/C showed similar levels of TNF-α production as those treated with Lipofectamine 2000 alone, and each was lower than that of untransfected cells. TNF-α levels from a representative experiment are shown in Figure 4A, together with the Western blot of SOD1 in the protein extracts from the various treatment groups (Figure 4B).
Figure 4.
(A) TNF-α in culture supernatants untreated and DMXAA-treated (300 µg/ml) in RAW 264.7 cells that had been transfected using Lipofectamine 2000 with siRNA molecules to SOD1, negative control nontargeting molecules, positive control lamin A/C targeting molecules, or cells treated with Lipofectamine 2000 only or untransfected cells. (B) Immunoblots showing SOD1 protein levels in the various siRNA treatment groups.
Discussion
The present study sought to identify the cellular target protein(s) of DMXAA, a vascular-disrupting agent that is currently undergoing phase 3 clinical evaluation, but whose mode of action is still only partly understood. To this end, a photoaffinity labeling approach was taken using tritiated 5-AzXAA as the photoactive ligand. The specificity of this method was confirmed in competitive binding experiments with splenocyte extracts [19] and with RAW 264.7 cellular lysates (Figure 1).
A total of 24, 18, and 30 proteins were successfully identified in this study as being photoaffinity-labeled with [3H]-5-AzXAA in cytosolic extracts from RAW 264.7 cells, murine splenocytes, and HECPP cells, respectively. In terms of their broad physiological function, the labeled cytosolic proteins included those with a known role in cytokine production, cytoskeletal proteins, proteins that alter cytoskeleton dynamics, chaperones, glycolytic enzymes, and proteins with miscellaneous functions (Table 1). This large number of potential DMXAA target proteins was unexpected, particularly because the two-dimensional gel system used was capable of resolving only the more abundant cellular proteins. Essentially all the labeled proteins shared a common feature, namely, oxidizable thiols. This conclusion was derived from literature reports showing that those proteins could undergo thiol specific oxidative modification through glutathionylation and/or disulfide bridge formation, on exposure of the cell to oxidative stress [26–36], and led us to consider that DMXAA may interact with target proteins through their accessible and oxidizable thiol groups, for example, cysteine residues. Determination of whether the photoaffinity label is indeed linked to peptide fragments containing cysteine residues is planned. Of note, the cytoskeletal proteins actin and tubulin were among the eight proteins that were photoaffinity-labeled in all cell types, and treatment of endothelial cells with DMXAA has been shown to cause partial dissolution of the actin cytoskeleton, which may be part of its antivascular action [37].
Although the results here suggest that 5-AzXAA with UV irradiation, covalently binds to cellular proteins in vitro, it is not yet known whether such adducts are formed with this class of compounds under physiological conditions in vivo. Adduct formation between proteins and xenobiotics, including taxol [21], 1,4-benzoquinone, and 1,4-naphthoquinone [38] or endogenous compounds, such as dopamine or its metabolite dihydroxyphenylacetic acid [39], is widely reported in the literature. Covalent binding of DMXAA to target proteins conceivably may also occur. In this regard, DMXAA and its predecessor FAA have been proposed to undergo intramolecular protonation to form pyrylium-type cationic salts, which would be expected to display a high affinity for electrons and be capable of electron transfer [40]. Furthermore, oxidation of FAA generates carbon radical species after decarboxylation [41], which could also lead to covalent bond formation with proteins. DMXAA also decarboxylates in solution when exposed to sunlight [42]. Agents capable of electron transfer are able to transmit an electron to oxygen and generate a wide range of ROS. The formation of ROS in RAW 264.7 cells in response to DMXAA (Figure 3B) supports the idea that DMXAA might indeed be capable of electron transfer. The acetic acid group at positions 4 and 8, respectively, is essential for the formation of DMXAA- and FAA-derived pyrylium salts [40] and for the generation of radicals after a decarboxylation pathway [41]. Interestingly, structure-activity studies of xanthone and flavone analogs showed an absolute requirement for the acetic acid group at those positions for analogs with antitumor activity [2,3]. It is not known at this point whether oxidation of DMXAA can occur spontaneously under physiological conditions or is an enzymatically catalyzed process.
The discovery that oxidizable proteins were preferentially labeled in all three cell types (Table 2) suggests that DMXAA might act through the modulation of redox signaling. Many key effects of DMXAA are inducible by redox signaling: reorganization of the actin cytoskeleton and stimulation of apoptosis in endothelial cells [43], activation of NF-κB [44], and TNF-α production [45]. DMXAA induced an increase in the generation of ROS in RAW 264.7 cells, and preincubation with the antioxidant NAC reversed both ROS generation and induction of a number of cytokines, including TNF-α by DMXAA (Figure 3), consistent with the hypothesis that ROS play a central role in its molecular action. SiRNA knockdown of SOD1, a direct scavenger of ROS, increased TNF-α production in response to DMXAA (Figure 4), again consistent with the involvement of ROS in DMXAA-induced cytokine production.
The suggestion that DMXAA induces cytokine production through modulation of redox signaling does not rule out that activation of classic signaling pathway(s) after binding to yet unidentified receptor(s) also occurs. In many inflammatory responses, cytokine production can occur through both ROS-dependent and ROS-independent pathways, and lipopolysaccharide (LPS)-mediated TNF-α production would be a prime example [45]. The ROS-dependent pathway of NF-κB-mediated transcriptional activation of the TNF gene after exposure to LPS in RAW 264.7 cells was shown to be responsible for approximately 50% of the TNF-α produced [45].
Synergistic TNF-α production by human peripheral blood leukocytes and murine splenocytes in culture in response to DMXAA has been shown with the addition of a costimulator [46,47]. Agents that were effective as costimulators with DMXAA for TNF-α production included LPS, IL-1, phorbol myristate acetate, and okadaic acid at suboptimal concentrations that did not, by themselves, induce the cytokine [46,47]. Interestingly, all these compounds have been reported to promote the production of ROS [44,48,49]. Approaches that increase cellular ROS concentrations might provide beneficial strategies for improving the activity of DMXAA. Of note, the phase 3 evaluation of DMXAA is in combination with carboplatin and paclitaxel (ATTRACT-1 and ATTRACT-2; Novartis, Basel, Switzerland). Both carboplatin [50] and paclitaxel have been demonstrated to induce the generation of ROS, with the accumulation of hydrogen peroxide being crucial for paclitaxel-induced cancer cell death in vivo [51].
In summary, the studies here support a role of redox signaling in the action of DMXAA. Whether the observed increase in ROS is a direct or indirect effect of the compound and the identification of the enzymes and mechanisms involved in generating a radical species from DMXAA under physiological conditions require further investigation.
Supplementary Material
Acknowledgments
The authors thank Aron B. Fisher and Steven M. Albelda, University of Pennsylvania, and Tobias P. Dick, German Cancer Research Centre, for their valuable discussion and critical assessment of the article.
Abbreviations
- DMXAA
5,6-dimethylxanthenone-4-acetic acid
- FCS
fetal calf serum
- LPS
lipopolysaccharide
- NAC
N-acetyl-l-cysteine
- 5-AzXAA
5-azidoxanthenone-4-acetic acid
- DTT
dithiothreitol
- ROS
reactive oxygen species
- IEF
isoelectric focusing
Footnotes
The work was funded by a Health Research Council of New Zealand grant to L.-M.C. and a National Institutes of Health grant (AI-18797) to S.N.V.
This article refers to a supplementary material, which is designated by Figure W1 and is available online at www.neoplasia.com.
References
- 1.Lippert JW. Vascular disrupting agents. Bioorg Med Chem. 2006;15:605–615. doi: 10.1016/j.bmc.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 2.Rewcastle GW, Atwell GJ, Li ZA, Baguley BC, Denny WA. Potential antitumor agents, 61: Structure-activity relationships for in vivo colon 38 activity among disubstituted 9-oxo-9H-xanthene-4-acetic acids. J Med Chem. 1991;34:217–222. doi: 10.1021/jm00105a034. [DOI] [PubMed] [Google Scholar]
- 3.Attasi G, Briet P, Berthelon J-J, Collinges F. Synthesis and anti-tumor activity of some 8-substituted-4-oxo-4H-1-benzopyrans. Eur J Med Chem. 1985;20:393–402. [Google Scholar]
- 4.Corbet TH, Bissery MC, Wozniak A, Plowman J, Polin L, Tapzoglou E, Diekman J, Valeriote F. Activity of flavone acetic acid (NSC-347512) against solid tumors of mice. Invest New Drugs. 1986;4:207–220. doi: 10.1007/BF00179586. [DOI] [PubMed] [Google Scholar]
- 5.Baguley BC, Calveley SB, Crowe KK, Fray LM, O'Rourke SA, Smith GP. Comparison of the effects of flavone acetic acid, fostriecin, homoharringtonine and tumor necrosis factor alpha on colon 38 tumors in mice. Eur J Cancer Clin Oncol. 1989;25:263–269. doi: 10.1016/0277-5379(89)90018-7. [DOI] [PubMed] [Google Scholar]
- 6.Zwi LJ, Baguley BC, Gavin JB, Wilson WR. Blood flow failure as a major determinant in the antitumor action of flavones acetic acid (NSC 34512) J Natl Cancer Inst. 1989;81:1005–1013. doi: 10.1093/jnci/81.13.1005. [DOI] [PubMed] [Google Scholar]
- 7.Ching L-M, Cao Z, Kieda C, Zwain S, Jameson MB, Baguley BC. Induction of endothelial cell apoptosis by the antivascular agent 5,6-dimethylxanthenone-4-acetic acid. Br J Cancer. 2002;86:1937–1942. doi: 10.1038/sj.bjc.6600368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L-CS, Thomsen L, Sutherland R, Reddy CB, Tijono SM, Chen C-JJ, Angel CE, Dunbar PR, Ching L-M. Neutrophil influx and chemokine production during the early phases of the antitumor response to the vascular disrupting agent DMXAA (ASA404) Neoplasia. 2009;11:793–803. doi: 10.1593/neo.09506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jassar AS, Suzuki E, Kapoor V, Sun J, Silverberg MB, Cheung L, Burdick MD, Strieter RM, Ching L-M, Kaiser LR, et al. Activation of tumor-associated macrophages by the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid induces an effective CD8+ T-cell-mediated antitumor immune response in murine models of lung cancer and mesothelioma. Cancer Res. 2005;65:11752–11761. doi: 10.1158/0008-5472.CAN-05-1658. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe N, Niitsi Y, Umeno H, Kuriyama H, Neda H, Yamauchi N, Maeda M, Urushizaki I. Toxic effect of tumor necrosis factor on tumor vasculature in mice. Cancer Res. 1988;48:2179–2183. [PubMed] [Google Scholar]
- 11.Mahadevan V, Malik STA, Meager A, Fiers W, Lewis GP, Hart IR. Role of tumor necrosis factor in flavone acetic acid-induced tumor vasculature shutdown. Cancer Res. 1990;50:5537–5542. [PubMed] [Google Scholar]
- 12.Zhao J, Ching L-M, Kestell P, Baguley BC. The antitumor activity of 5,6-dimethylxanthenone-4-acetic acid (DMXAA) in TNF receptor-1 knockout mice. Br J Cancer. 2002;87:465–470. doi: 10.1038/sj.bjc.6600479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts ZJ, Goutagny N, Perera P-Y, Kato H, Kumar H, Kawai T, Akira S, Savan R, van Echo D, Fitzgerald KA, et al. The chemotherapeutic agent DMXAA potently and specifically activates the TBK1-IRF-3 signaling axis. J Exp Med. 2007;204:1559–1569. doi: 10.1084/jem.20061845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallace A, LaRosa DF, Kapoor V, Jing S, Cheng G, Jassar A, Blouin A, Ching L-M, Albelda SM. The vascular disrupting agent, DMXAA, directly activates dendritic cells through a MyD88-independent mechanism and generates antitumor cytotoxic T lymphocytes. Cancer Res. 2007;67:7011–7019. doi: 10.1158/0008-5472.CAN-06-3757. [DOI] [PubMed] [Google Scholar]
- 15.Roberts ZJ, Ching L-M, Vogel SN. IFN-β-dependent inhibition of tumor growth by the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid (DMXAA) J Interferon Cytokine Res. 2008;28:133–139. doi: 10.1089/jir.2007.0992. [DOI] [PubMed] [Google Scholar]
- 16.Wang L-CS, Woon S-T, Baguley BC, Ching L-M. Inhibition of DMXAA-induced tumor necrosis factor production in murine splenocyte cultures by NF-κB inhibitors. Oncol Res. 2006;16:1–14. doi: 10.3727/000000006783981288. [DOI] [PubMed] [Google Scholar]
- 17.Woon S-T, Zwain S, Schooltink MA, Newth AL, Baguley BC, Ching L-M. NF-κB activation in vivo in both host and tumor cells by the antivascular agent 5,6-dimethylxanthenone-4-acetic acid (DMXAA) Eur J Cancer. 2003;39:1176–1183. doi: 10.1016/s0959-8049(03)00196-5. [DOI] [PubMed] [Google Scholar]
- 18.Woon S-T, Baguley BC, Palmer BC, Fraser JD, Ching L-M. Uptake of the antivascular agent 5,6-dimethylxanthenone-4-acetic acid (DMXAA) and activation of NF-κB in human tumor cell lines. Oncol Res. 2002;13:95–101. [PubMed] [Google Scholar]
- 19.Palmer BD, Henare K, Woon S-T, Sutherland R, Reddy C, Wang L-CS, Kieda C, Ching L-M. Synthesis and biological activity of azido analogues of 5,6-dimethylxanthenone-4-acetic acid for use in photoaffinity labeling. J Med Chem. 2007;50:3757–3764. doi: 10.1021/jm0702175. [DOI] [PubMed] [Google Scholar]
- 20.Safa AR. Photoaffinity labeling of the multidrug-resistance-related P-glycoprotein with photoactive analogs of verapamil. Proc Natl Acad Sci USA. 1988;85:7187–7191. doi: 10.1073/pnas.85.19.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhat N, Perera P-Y, Carboni JM, Blanco J, Golenbock DT, Mayadas TN, Vogel SN. Use of a photoactivatable taxol analogue to identify unique cellular targets in murine macrophages: identification of murine CD18 as a major taxol-binding protein and a role for Mac-1 in taxol-induced gene expression. J Immunol. 1999;162:7335–7342. [PubMed] [Google Scholar]
- 22.Bizouarne N, Mitterand M, Monsigny M, Kieda C. Characterization of membrane sugar-specific receptors in cultured high endothelial cells from mouse peripheral lymph nodes. Biol Cell. 1993;79:27–35. doi: 10.1016/0248-4900(93)90259-h. [DOI] [PubMed] [Google Scholar]
- 23.Bradford MM. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 24.Perkins DN, Papin DJC, Creasy DM, Cotrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 25.Skehan P, Storeng R, Scudiero D, Monks A, McMahon M, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 26.Ghezzi P, Bonetto V, Frattelli M. Thiol-disulfide balance: from the concept of oxidative stress to that of redox regulation. Antioxid Redox Signal. 2005;7:964–972. doi: 10.1089/ars.2005.7.964. [DOI] [PubMed] [Google Scholar]
- 27.Fratelli M, Demol H, Puype M, Casagrande S, Villa P, Eberini I, Vandekerckhove J, Gianazza E, Ghezzi P. Identification of proteins undergoing glutathioylation in oxidatively stressed hepatocytes and hepatoma cells. Proteomics. 2003;3:1154–1161. doi: 10.1002/pmic.200300436. [DOI] [PubMed] [Google Scholar]
- 28.Littler DR, Harrop SJ, Fairlie WD, Brown LJ, Pankhurst GJ, Pankhurst S, DeMaere MZ, Campbell TJ, Bauskin AR, Tonini R, et al. The intracellular chloride ion channel protein CL1C1 undergoes a redox-controlled structural transition. J Biol Chem. 2004;279:9298–9305. doi: 10.1074/jbc.M308444200. [DOI] [PubMed] [Google Scholar]
- 29.Brennan JP, Wait R, Begum S, Bell JR, Dunn MJ, Eaton P. Detection and mapping of widespread intermolecular protein disulfide formation during cardiac oxidative stress using proteomics with diagonal electrophoresis. J Biol Chem. 2004;279:41352–41360. doi: 10.1074/jbc.M403827200. [DOI] [PubMed] [Google Scholar]
- 30.Lind C, Gerdes R, Hamnell Y, Schuppe-Koistinen I, von Lowenhielm HB, Holmgren A, Cotgreave IA. Identification of S-glutathionylated cellular protein during oxidative stress and constitutive metabolism by affinity purification and proteomic analysis. Arch Biochem Biophys. 2002;406:229–240. doi: 10.1016/s0003-9861(02)00468-x. [DOI] [PubMed] [Google Scholar]
- 31.Fratelli M, Demol H, Puype M, Casagrande S, Eberini I, Salmona M, Bonetto V, Mengozzi M, Duffieux F, Miclet E, et al. Identification by redox proteomics of glutathionylated proteins in oxidatively stressed human T lymphocytes. Proc Natl Acad Sci USA. 2002;99:3505–3510. doi: 10.1073/pnas.052592699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson LO. The heterogeneity of bovine serum albumin. Biochem Biophys Acta. 1996;117:115–133. doi: 10.1016/0304-4165(66)90159-0. [DOI] [PubMed] [Google Scholar]
- 33.Baty JW, Hampton MB, Winterbourn CC. Proteomic detection of hydrogen peroxide-senstive thiol proteins in Jurkat cells. Biochem J. 2005;389:785–795. doi: 10.1042/BJ20050337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamnell-Pamment Y, Lind C, Palmberg C, Bergman T, Cotgreave IA. Determination of site-specificity of S-glutathionylated cellular proteins. Biochem Biophys Res Comm. 2005;332:362–369. doi: 10.1016/j.bbrc.2005.04.130. [DOI] [PubMed] [Google Scholar]
- 35.Zhukova L, Zhukov I, Bai W, Wyslouch-Ciesznaska A. Redox modifications of the C-terminal cysteine residue cause structural changes in S100A1 and S100B proteins. Biochim Biophys Acta. 2004;1742:191–201. doi: 10.1016/j.bbamcr.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Townsend DM, Findlay VJ, Fazilev F, Ogle M, Fraser J, Saavedra JE, Ji X, Keefer LK, Tew KD. A glutathione S-transferase pi-activated prodrug causes kinase activation concurrent with S-glutathionylation of proteins. Mol Pharmacol. 2006;69:501–508. doi: 10.1124/mol.105.018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer. 2005;5:423–435. doi: 10.1038/nrc1628. [DOI] [PubMed] [Google Scholar]
- 38.Lamé MW, Jones AD, Wilson DW, Segall HJ. Protein targets of 1,4-benzoquinone and naphthoquinone in human bronchial epithelial cells. Proteomics. 2003;3:479–495. doi: 10.1002/pmic.200390062. [DOI] [PubMed] [Google Scholar]
- 39.Hastings TG, Zigmond MJ. Identification of catechol-protein conjugates in neostriatal slices incubated with [3H]dopamine: impact of ascorbic acid and glutathione. J Neurochem. 1994;63:1126–1132. doi: 10.1046/j.1471-4159.1994.63031126.x. [DOI] [PubMed] [Google Scholar]
- 40.Kovacic P. Fundamental, electron transfer mechanism by pyrylium-type ions for the anticancer drugs 5,6-dimethylxanthenone-4-acetic acid (DMXAA) and flavones-8-acetic acid. Curr Med Chem Anticancer Agents. 2005;5:501–506. doi: 10.2174/1568011054866919. [DOI] [PubMed] [Google Scholar]
- 41.Candeias LP, Everett SA, Wardman P. Free radical intermediates in the oxidation of flavone-8-acetic acid: possible involvement in its antitumor activity. Free Radic Biol Med. 1993;15:385–394. doi: 10.1016/0891-5849(93)90038-v. [DOI] [PubMed] [Google Scholar]
- 42.Rewcastle GW, Kestell P, Baguley BC, Denny WA. Light-induced breakdown of flavones acetic acid and xanthenone analogues in solution. J Natl Can Inst. 1990;82:528–529. doi: 10.1093/jnci/82.6.528. [DOI] [PubMed] [Google Scholar]
- 43.Cai H. Hydrogen peroxide regulation of endothelial functions: origins, mechanisms, and consequences. Cardiovascular Res. 2005;68:26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 44.Gloire G, Legrands-Poels S, Piette J. NF-κB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Sanlioglu S, Williams CM, Samavati L, Butler NS, Wang G, McCray PB, Ritchie TC, Hunninghake GW, Zandi E, Engelhart JF. Lipopolysaccharide induces Rac1-dependent reactive oxygen species formation and coordinates tumor necrosis factor-α secretion through IKK regulation of NF-κB. J Biol Chem. 2001;32:30188–30198. doi: 10.1074/jbc.M102061200. [DOI] [PubMed] [Google Scholar]
- 46.Philpott M, Ching L-M, Baguley BC. The antitumor agent 5,6-dimethylxanthenone-4-acetic acid acts in vitro on human mononuclear cells as a co-stimulator with other inducers of tumor necrosis factor. Eur J Cancer. 2001;37:1930–1937. doi: 10.1016/s0959-8049(01)00210-6. [DOI] [PubMed] [Google Scholar]
- 47.Wang L-CS, Reddy CB, Baguley BC, Kestell P, Sutherland R, Ching L-M. Induction of tumor necrosis factor and interferon-γ in cultured murine splenocytes by the antivascular agent DMXAA and its metabolites. Biochem Pharmacol. 2004;67:937–945. doi: 10.1016/j.bcp.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 48.Boudreau RTM, Conrad DM, Hoskin DW. Differential involvement of reactive oxygen species in apoptosis caused by the inhibition of protein phosphatase 2A in Jurkat and CCRF-CEM human T-leukemia cells. Exp Mol Pathool. 2007;83:347–356. doi: 10.1016/j.yexmp.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Drieschmanns HR, Beck W, Kerl R, Schneider HW, Brunner H, Deppisch R. Inhibition of PMA-induced reactive oxygen species (ROS) by antioxidants. Int J Artificial Organs. 2006;29:518–618. [Google Scholar]
- 50.Husain K, Whitworth C, Somani SM, Rybak LP. Carboplatin-induced oxidative stress in rat cochlea. Hear Res. 2001;159:14–22. doi: 10.1016/s0378-5955(01)00306-9. [DOI] [PubMed] [Google Scholar]
- 51.Alexandre J, Batteux F, Nicco C, Chereau C, Laurent A, Guillevin L, Weill B, Goldwasser F. Accumulation of hydrogen peroxide is an early and crucial step for paclitaxel-induced cancer cell death both in vitro and in vivo. Int J Cancer. 2006;119:41–48. doi: 10.1002/ijc.21685. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.