Abstract
The choroid plexus (CP), constituting the blood–cerebrospinal fluid barrier, has the capacity to remove beta-amyloid (Aβ) from the cerebrospinal fluid. Our previous work indicates that exposure to lead (Pb) results in Aβ accumulation in the CP by decreasing the expression of low density lipoprotein receptor protein-1 (LRP1), a protein involved in the transport and clearance of Aβ. The current study was designed to explore the relationship between Aβ accumulation, protein kinase C (PKC) activity, and LRP1 status in the CP following Pb exposure. Confocal microscopy revealed that LRP1 was primarily localized in the cytosol of the CP in control rats and migrated distinctly towards the apical surface and the microvilli following acute Pb exposure (27 mg Pb/kg, ip, 24 hr). Co-immunostaining revealed a co-localization of both PKC-δ and LRP1 in the cytosol of control rats, with a distinct relocalization of both towards the apical membrane following Pb exposure. Preincubation of the tissues with PKC-δ inhibitor rottlerin (2 µM) prior to Pb exposure in vitro, resulted in abolishing the Pb-induced relocalization of LRP1 to the apical surface. Importantly, a significant elevation in intracellular Aβ levels (p<0.01) was observed in the cytosol of the CP following Pb exposure, which was abolished following preincubation with rottlerin. In addition, rottlerin caused a relocalization of Aβ from the cytosol to the nucleus in both Pb-treated and control CP tissues. Finally, co-immunoprecipitation studies revealed a strong protein-protein interaction between LRP1 and PKC-δ in the CP. These studies suggest that Pb exposure disrupts Aβ homeostasis at the CP, owing partly to a Pb-induced relocalization of LRP1 via PKC-δ.
Keywords: blood–CSF barrier, lead or Pb, Aβ, LRP1, choroid plexus, protein kinase C, PKC-δ, rottlerin
1. Introduction
Alzheimer’s disease (AD) is the most common form of dementia, currently affecting 20 million people worldwide with 4.6 million new cases of dementia every year [1, 2]. According to the United Nations population projection and other statistics, this number may exceed 100 million in the next 50 years [3, 4]. Although AD is a multi-factorial disorder, only 3–5% cases are known to be associated with familial or genetic factors [5] suggesting the contribution of non-genetic, environmental causes of the disease.
Histopathologically, one of the distinguishing hallmarks of AD is the presence of abundant extra-neuronal deposits of beta-amyloid (Aβ). Strong evidence suggests that increases in extracellular concentrations of Aβ peptides result in neuronal alterations such as changes in synaptic plasticity, decreased long term potentiation, and impaired hippocampal function in both humans and animal models [6–8]. Aβ accumulation in brains of AD patients may occur by one or more processes, including overproduction by neurons, inadequate metabolic clearance within the brain, and/or an improper balance of import and export of Aβ at brain barriers [9–11]. There are primarily two brain barriers separating brain cells and cerebral fluids from the blood, namely the blood-brain barrier (BBB) and the blood – cerebrospinal fluid barrier (BCB). The BBB is tightly connected with cerebral capillary endothelia and is present between the blood and cerebral interstitial fluid. Aβ transport across this barrier has been extensively studied [10, 11].
The BCB comprises relatively leaky epithelial cells, separating blood from the cerebrospinal fluid (CSF). The choroid plexus (CP), a major component of the BCB, has been shown to be immunoreactive to antibodies against Aβ and its precursor protein, APP [12, 13] and is involved in clearing Aβ from the CSF [9, 12]. Recent studies from this laboratory have shown that exposure to toxic metal lead (Pb) increases the accumulation of Aβ in the CP, possibly by interferring with the expression of low density lipoprotein receptor protein 1 (LRP1) [14], a major Aβ transporter implicated in AD etiology [11, 15, 16]. Although the use of Pb in paints, ceramic products and pipe solder has been dramatically reduced in recent years due to health concerns, it is still receiving continued public attention due to its indispensable and growing use in several sectors of industry including smelting, battery manufacture, paints, plastic materials and other consumer products [17, 18]. Recent findings indicate that workers occupationally exposed to Pb demonstrate an increase in neurodegeneration and cortical atrophy along with behavioral deficits similar to those seen in AD patients [19–21] with the persistence of its toxicity even 16 years post exposure [22]. Elevated concentrations of Pb were found in diffuse neurofibrillary tangles, a form of pre-senile dementia, in 10 AD cases compared with 9 controls [23]. Animal studies corroborate human findings in that Pb exposure during early development has been associated with alterations in the expression and regulation of amyloid precursor protein (APP) in mice, rats and non-human primates, with increased memory impairments later in life [5, 24–27]. Although Pb toxicity has long been associated with learning deficits in children, its direct deleterious effects on neurodegeneration in adults have only recently been identified [20, 21, 28, 29].
One of the major target regions of the brain for selective Pb accumulation is the CP. This finding was demonstrated as early as 1983 based on a study on brain autopsies of 51 human subjects who had lived in New York City and died from causes other than Pb-induced encephalopathy [30]. These observations were independently confirmed by Manton and his colleagues [31] who reported a 100-fold increase of Pb in the human CP compared with that in the brain cortex. Studies in rodents showed a similar accumulation of Pb in the CP at concentrations 57 and 70 fold greater than the brain cortex and CSF, and were found to be dose- dependent and time related [32, 33].
In addition to being a target for Pb accumulation, the CP, where the BCB resides, has shown to mediate the transport and clearance of Aβ from the CSF [9, 13, 34]. Interestingly, Aβ has also been detected in the CP of AD patients [35]. However, the relationship between Aβ transport/metabolism in response to Pb deserves further exploration. Recent findings from our laboratory reveal that Pb exposure results in a significant accumulation of Aβ in the CP, possibly by decreasing the expression of LRP1 [14], a transmembrane glycoprotein previously implicated in Aβ export from the brain at the BBB [36–39] as well as in receptor-mediated endocytosis and cell signaling [40]. However, the mechanism of Pb-induced effects on Aβ and LRP1 remain unknown. Hence, as an extension of our previous findings, we sought to explore the relationship between the Pb-induced Aβ accumulation in the CP, subcellular distribution of LRP1, and the status of protein kinase C-delta (PKC-δ).
PKC-δ belongs to a “novel” isoform of the PKC family, a class of Ca2+ and phospholipid-dependent protein kinases that catalyze the transfer of the phosphate in ATP to phosphor-acceptor serine or threonine residues in protein and peptide substrates [41,42]. The importance of studying the role of PKC in AD stems from several studies in literature including a cross-sectional human study among Korean workers who were occupationally exposed to Pb. These workers showed decrements in neurobehavioral test scores, mainly in the domains of manual dexterity and psychomotor function with a correlation of neuronal dysfunction with high blood PKC levels [43]. Other studies on human autopsy or animal experiments have also established that a malfunction in PKC or PKC-mediated activities, such as PKC-dependent phosphorylation, neurotransmitter release and neuronal plasticity, may contribute to the etiology of AD [44–48]. More recently, some studies have suggested that PKC-δ is activated in the LRP1-mediated signaling pathway, where it induces the shedding of the extracellular domain of LRP1, thereby releasing the intracellular domain in the cytoplasm [49, 50]. Although PKC has been associated with AD, the role of the PKC-δ isoform in mediating LRP1 function at the brain barriers remains unexplored.
Our rationale for studying the involvement of PKC in Pb- mediated elevations in Aβ stemmed from findings in literature which suggest that low concentrations of Pb modulate PKC activity in brain barrier systems by translocating the kinase from the soluble cytosolic fraction to the membrane-associated particulate component of cells [42, 51–53]. Although several isoforms of PKC have been identified and studied in the CP [42], the PKC-δ isoform has neither been identified nor characterized at this barrier. Since Pb has previously been shown to activate other isoforms of PKC and since PKC-δ has been implicated in LRP1-mediated cell signaling, there was a sound basis to investigate a potential linkage between Pb toxicity, PKC-δ status and LRP1 function, which may ultimately contribute to an intracellular Aβ accumulation in the CP. Thus, the purpose of this study was to investigate (1) whether Pb exposure affected the subcellular location of LRP1, (2) whether the subcellular distribution of LRP1 was mediated by PKC-δ, (3) whether Pb-induced LRP1 malfunction was associated with the effect of Pb on PKC-δ, and finally (4) how the alteration of this signaling pathway affected intracellular Aβ levels in the CP. Since the current U.S. Occupational Safety and Health Administration (OSHA) regulations generally require the removal of workers from Pb exposure when whole-blood Pb concentrations exceed 50 or 60 µg/dL [54, 55], our current study employed a concentration of 27 mg/kg Pb i.p. in rats which corresponded to blood Pb levels of 350 µg/dL [32]. This dose was approximately six times higher than the current occupational exposure guidelines in humans and was in-line with toxicity testing doses which take into consideration animal-to-human uncertainty factors. It should be kept in mind that the purpose of this dose regimen was not to mimic real life exposure, but instead, to produce a condition in which the amount of Pb in the choroid plexus could build up significantly during a fairly short period of time.
2. Materials and Methods
2.1. Materials
Chemicals and assay kits were purchased from the following sources: FAM-labeled Aβ (catalog # 23514-01) from Anaspec (San Jose, CA), rabbit anti-LRP1 antibody (catalog # ARP32793) from Aviva (San Diego, CA), Alexa-labeled secondary antibody from Molecular Probes (Eugene, OR), mouse anti-PKC-δ (catalog # 41-0030) from Invitrogen (Carlsbad, CA), anti-mouse Texas Red (catalog # sc-2781) from Santacruz (Santacruz, CA), protein A agarose beads (catalog # P-7786), rottlerin (catalog #R5648) and RIPA buffer (catalog # R0278) from Sigma Aldrich (St Louis, MO), and Dulbecco’s modified essential medium (DMEM), fetal bovine serum (FBS), penicillin, streptomycin and gentamycin from Gibco (Grand Island, NY). The polyclonal anti-rabbit LRP1 and anti-mouse monoclonal LRP1 antibodies were a kind gift from Dr. Dudley Strickland, University of Maryland. All reagents were of analytical grade, HPLC grade or the best available pharmaceutical grade.
2.2. Animals and Treatment
Male Sprague-Dawley rats at the time they were used were 8–9 weeks old (250–300g). The animals were housed in a temperature-controlled, 12:12 light/dark room, and were allowed free access to tap water and food. For in vivo studies, rats received an intraperitoneal (i.p) injection of 50 mg/kg Pb acetate (i.e., 27 mg Pb/kg) or an equivalent molar concentration of Na acetate (i.e., 15 mg acetate/kg) as controls. Twenty-four hours post injection the rats were sacrificed using ketamine/xylazine (75:10 mg/mL, 1 mL/kg body weight), the CP was isolated, and immunohistochemistry was performed. For in vitro studies, the CP was isolated and treated with 10 µM Pb in artificial CSF (aCSF) based on cytotoxicity tests and previously published data from this group [14, 56]. All treatments were conducted in a 35-mm dish as described in the section below.
2.3. In Vitro Incubation Studies
Fresh CP tissues were isolated from rats and incubated in aCSF containing Pb (10 µM) at 37°C for 1 h while being bubbled with a 95%- air 5% CO2. For PKC inhibition studies, the tissues were pre-treated with 2 µM rottlerin, a PKC-δ inhibitor, for 20 min, followed by incubation with 10 µM Pb for one hr. The tissues were incubated with FAM-labeled Aβ (5 µM) in aCSF for 1 h and live Aβ uptake was determined using a laser scanning microscope. Immunohistochemistry was performed on another set of treated tissues as described below.
2.4. Immunohistochemistry
Following CP isolation and treatments, the tissues were fixed with 3% paraformaldehyde/0.25% glutaraldehyde in PBS for 10 min. They were permeabilized with 0.5% Triton X-100 for 20 min at room temperature, followed by 5 washes of PBS. After blocking with 1% bovine serum albumin (BSA) in PBS for 1 hr at room temperature, tissues were double immunostained with rabbit anti-LRP1 (1:350) and mouse anti-PKC-δ (1:250) in 1% BSA for 2 hr at 37°C, washed with PBS in 1% BSA, and then incubated with goat-anti rabbit Alexa-488 conjugated secondary antibody (1:5000) and goat anti-mouse Texas Red (1:3500) in 1% BSA at 37°C for 2 hr. After additional washing in PBS with 1% BSA, the tissue was transferred to a 35-mm dish (MatTek, Ashland, MA), a few drops of PBS were added to prevent drying, and observed immediately using an inverted confocal fluorescent microscope as described below. Negative controls were treated similarly except that they were not exposed to any of the primary antibodies.
2.5. Confocal Immunofluorescence Microscopy
To acquire images, the chamber containing the CP specimen was mounted on the stage of an Olympus, FV1000 inverted confocal laser-scanning microscope and viewed through a 40× water-immersion objective (numeric aperture=1.2), with 488-nm (green) and 543-nm (red) laser lines for excitation (Ar-ion laser). The CP was examined under reduced transmitted-light illumination to avoid photo bleaching and an area containing undamaged epithelium with underlying vasculature was selected. Confocal images (512×512×8 bits, 4 frames averaged) were obtained at a rate of one frame per second and care was taken to expose all the tissues for the same period of time. The fluorescence intensity was further quantified using software Image J and reported in arbitrary units (a.u). Data reported, unless otherwise stated, are the results of single experiments representative of three to four replicate experiments.
2.6. Culture of Choroidal Epithelial Z310 Cells
The characteristics of immortalized rat choroidal epithelial Z310 cells have been described in a previous publication [57]. Briefly, cells were maintained in DMEM medium supplemented with 10% FBS, 100 U/mL penicillin, 100 µg/mL streptomycin, and 40µg/mL of gentamycin in a humidified incubator with 95% air–5% CO2 at 37°C and were passaged twice a week.
2.7. Immunoprecipitation Studies
Z310 cells were seeded in 10-cm cell culture plates at a density of 1×106cells/plate and were allowed to grow for 2–3 days until they reached 85–90% confluence. They were washed twice with cold PBS and lysed in 0.5 ml of RIPA buffer. Lysates were centrifuged at 10,000 rpm at 4 °C, and the supernatants were incubated with 200 µL of polyclonal anti-rabbit LRP1 for 1 h on ice, and subsequently with 40 µL of 10% protein A-agarose overnight at 4 °C on an agitator. Agarose beads were collected by centrifugation at 6,000 rpm for 10 mins and washed 4 times with PBS in 0.5% Tween.20. A negative control was prepared by precipitating the beads with an irrelevant antibody (β-actin); cell lysate was used as a positive control. Immunoprecipitates were boiled for 5 min in SDS sample buffer and resolved by SDS-PAGE.
2.8. Immunoblotting
The samples obtained above were transferred to polyvinylidene difluoride membranes (PVDF) using a Bio-Rad blotting apparatus at 30 V overnight at 4 °C. Membranes were blocked for 1 h with 10 mM Tris/Cl, pH 7.4, 150 mM NaCl, 0.2% Tween-20 (TBST) containing 5% dried milk and incubated with 1:200 dilution of monoclonal anti-mouse PKC-δ in TBST with 5% milk for 1 h at room temperature. Blots were washed three times for 15 min with TBST. Membranes were then incubated for 1h with horseradish peroxidase goat anti-mouse (Bio-Rad) diluted 1:7500 in TBST and washed as before. Reactive proteins were visualized by ECL reagent.
2.9. Statistical Analysis
Statistical analyses of the differences between groups were carried out by a one-way ANOVA with post hoc comparisons by the Dunnett’s tests (Kaleidagraph 3.6) Quantification of fluorescent signals were performed using Image J software and the fluorescence was reported in arbitrary units (a.u). All data are expressed as mean + SD. Differences between two means were considered significant when p was equal or less than 0.05.
3. Results
3.1. Exposure to Pb Alters the Subcellular Localization of LRP1 in Rat Choroid Plexus
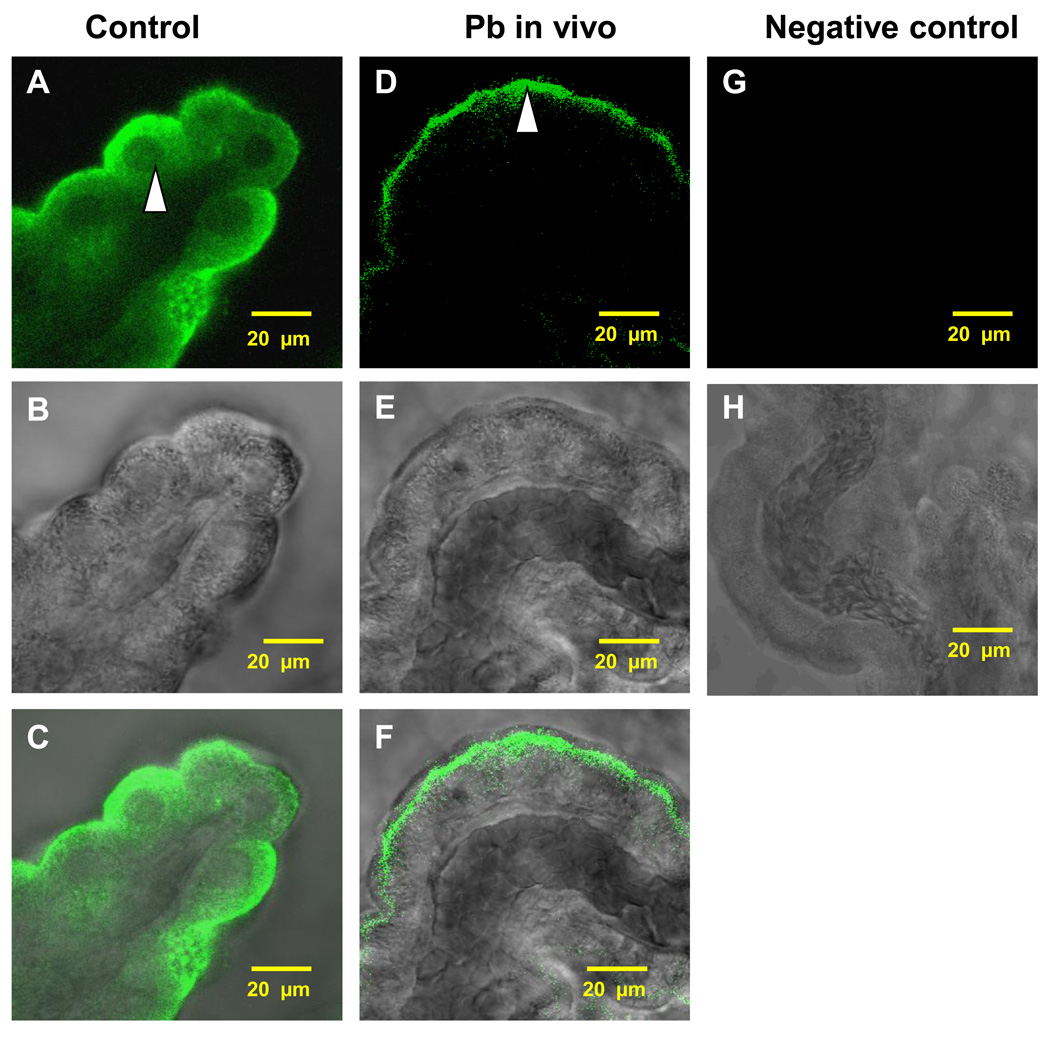
Immunostaining of the normal rat CP tissue revealed a distinct staining of LRP1 in the choroidal epithelia with the LRP1 staining being relatively evenly distributed in the cytosol surrounding the nuclei, yet somewhat toward the apical surface facing the CSF (Fig. 1A, C). When rats were injected with 27 mg Pb/kg ip for 24 h, a visible subcellular relocalization of LRP1 in the choroidal epithelia was observed. Most of the LRP1-associated fluorescent signal was concentrated at the apical surface immediately beneath the brush border of the choroidal epithelial microvilli with much less in the cytosol (Fig. 1D, F). The striking difference in the subcellular distribution of LRP1 between controls and Pb-treated animals existed consistently. Noticeably also, the transmission images (Fig.1B, E) revealed a normal morphology of CP tissues following acute in vivo Pb exposure.
Fig. 1.
Intracellular distribution of LRP1 in rat CP tissues following in vivo acute Pb exposure. Rats received a single ip injection of Pb at a concentration of 27 mg Pb/kg as Pb acetate. The control rats received an equivalent concentration of Na acetate. Twenty-four hours later, the CP tissues were collected. (A–B) Representative CP tissues from a control rat. The arrow indicates a relatively even distribution of LRP1 in the cytosol of choroidal epithelia. (D–E) Representative CP tissues from Pb-exposed rats. The arrow indicates relocalization of LRP1 from the cytoplasm towards the apical pole and in the brush border of the choroidal epithelia. (C, F) Overlays of A & B and B & E, respectively. (G) represents a negative control. The tissue was treated in the same way as in (D) except that it was not exposed to any of the primary antibodies. (B, E, H) Representive corresponding transmission images. The data indicate a normal morphology of the tissues. All images are representative of experiments performed in triplicates.
3.2. PKC-δ is Involved in the Pb-induced Subcellular Relocalization of LRP1
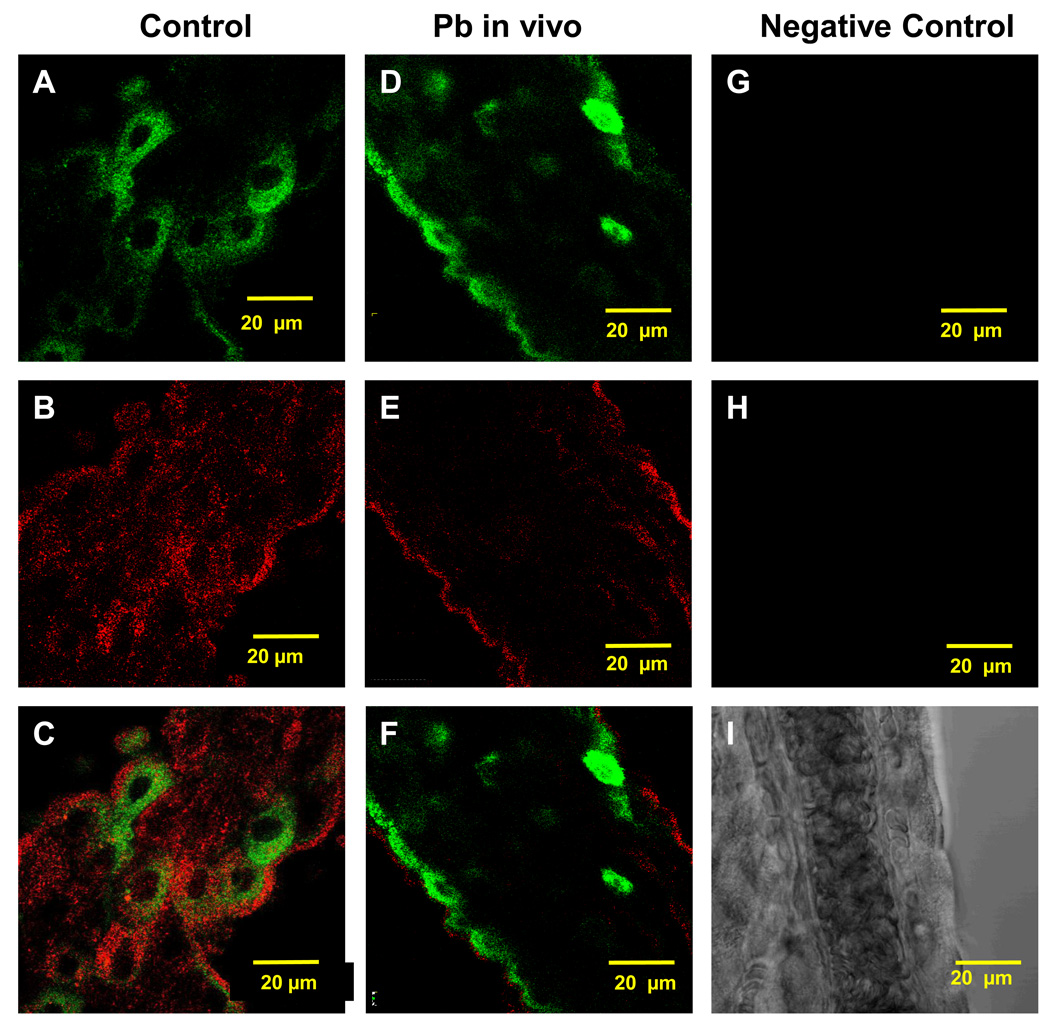
To understand the mechanism by which Pb prompted the relocalization of LRP1, we investigated the participation of the PKC family enzymes, because Pb is known to activate PKC [46–48]. Immunohistochemistry in rat CP tissues revealed a distinct co-localization of LRP1 and PKC-δ in the cytosol of control rats (Fig. 2A–C). An acute single dose of Pb (27 mg Pb/kg ip) not only migrated LRP1 towards the apical surface of the tissues (Fig. 2D), but also prompted PKC-δ signals moving from the cytosol to the apical membrane (Fig. 2E). Both LRP1 and PKC-δ signals apparently overlapped (Fig. 2F), suggesting a possible interaction between the two proteins following Pb exposure.
Fig. 2.
Co-localization of LRP1 and PKC-δ in the rat CP tissues following acute Pb exposure in vivo. (A–C) Confocal image of LRP1 and PKC-δ in the cytosol of a representative CP tissue from a control rat. (D–F) Confocal image of LRP1 and PKC-δ in a representative CP tissue from a Pb exposed rat. LRP1 is stained in green (A, D) and PKC-δ in red (B, E). (G–I) represents a negative control which was treated similarly except that it was not exposed to any of the primary antibodies. (C, F) represents an overlay of LRP1 and PKC-δ in controls and Pb-treated rats, respectively. Please see the legend in Fig. 1 for Pb-dose regimen. The data are representative of experiments performed in triplicates.
3.3. Inhibition of PKC-δ Activity by Rottlerin Abolishes the Pb-Induced Relocalization of LRP1 In Vitro
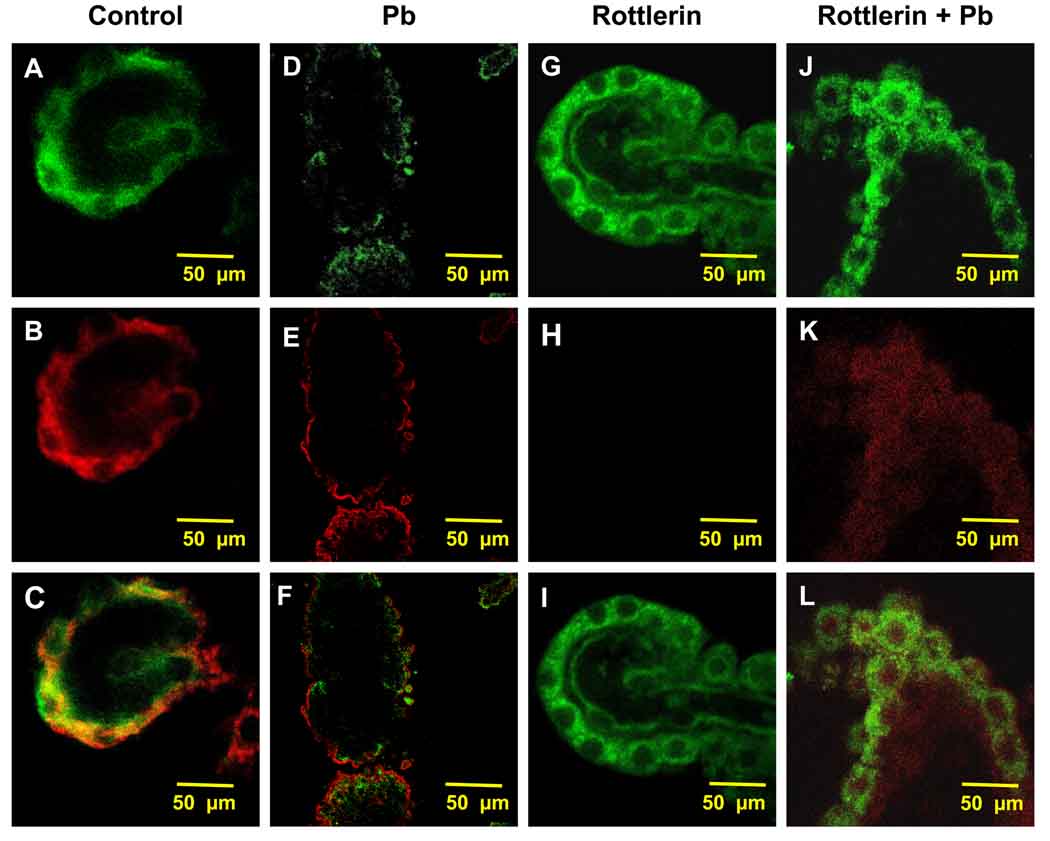
To confirm the involvement of PKC-δ in Pb-induced relocalization of LRP1, freshly isolated CP tissues were pre-incubated with rottlerin (2 µM, 20 min), a PKC-δ inhibitor, followed by Pb treatment (10 µM, 1 h). Immunohistochemical studies revealed a cytosolic distribution of LRP1 and PKC-δ (Fig. 3A,B) with a distinct co-localization of both (Fig. 3C). Following 1-h Pb exposure, the fluorescent signals of LRP1 (Fig. 3D) and PKC-δ (Fig. 3E) migrated towards the apical membrane; a significant overlap of both was evident (Fig. 3F). When the tissues were incubated with 2 µM rottlerin in the absence of Pb, there was no evident alteration in the localization of either LRP1 or PKC-δ (Fig. 3G,H,I). However, when the tissues were pre-incubated with rottlerin followed by Pb exposure, the shift in LRP1 and PKC-δ to the apical side of the choroidal epithelia was completely abolished (Fig. 3J,K,L), suggesting again, the involvement of PKC-δ in the Pb induced relocalization of LRP1.
Fig. 3.
Inhibition of Pb-induced migration of LRP1 by rottlerin. Rat CP tissues were isolated and pre-treated with rottlerin (2 µM, 20 min) alone or followed by Pb (10 µM, 1 h) exposure in vitro. (A–C) Confocal image of LRP1 and PKC-δ in the cytosol of a representative CP tissue from a control rat. (D–F) Confocal image of LRP1 and PKC-δ in a representative CP tissue from a Pb-exposed tissue in absence of rottlerin pre-treatment. (G–I) Confocal image of a representative CP tissue pre-treated with rottlerin in the absence of Pb. (J–L) Representative image of a CP tissue pre-treated with rottlerin followed by Pb exposure. LRP1 is stained in green (A, D, G, J), and PKC-δ in red (B, E, H, K). The data are representative of experiments performed in triplicate.
3.4. Interaction between PKC-δ and LRP1 in the Choroid Plexus
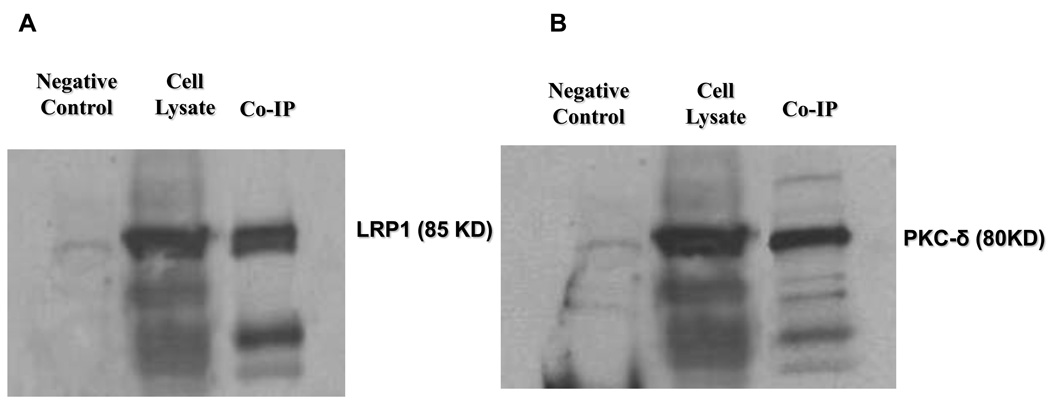
To provide further evidence to support the involvement of PKC-δ in the subcellular relocalization of LRP1, we examined the potential binding of PKC-δ and LRP1 in choroidal epithelial cells using a co-immunoprecipitation approach. After the cell lysates were treated with anti-LRP1 antibody and the precipitated complexes were separated and using SDS-PAGE, not only did we find LRP1 protein in cell precipitates (Fig. 4A), but more interestingly, we found a distinct PKC-δ band in the precipitate (Fig. 4B). These data suggest a physical binding of PKC-δ to LRP1 in the CP. Non-specific binding was ruled out by the absence of a band in the negative control group which was precipitated by an irrelevant antibody (β-actin).
Fig. 4.
Co-immunoprecipitation of LRP1 and PKC-δ for protein-protein interaction. Lysates from control Z310 cells were captured with anti-LRP1 in the presence of protein-A agarose beads and the complex was run on SDS-PAGE. (A) immunoblot with anti-LRP1. (B) immunoblot with anti- PKC-δ. The data are representative of experiments performed in triplicate.
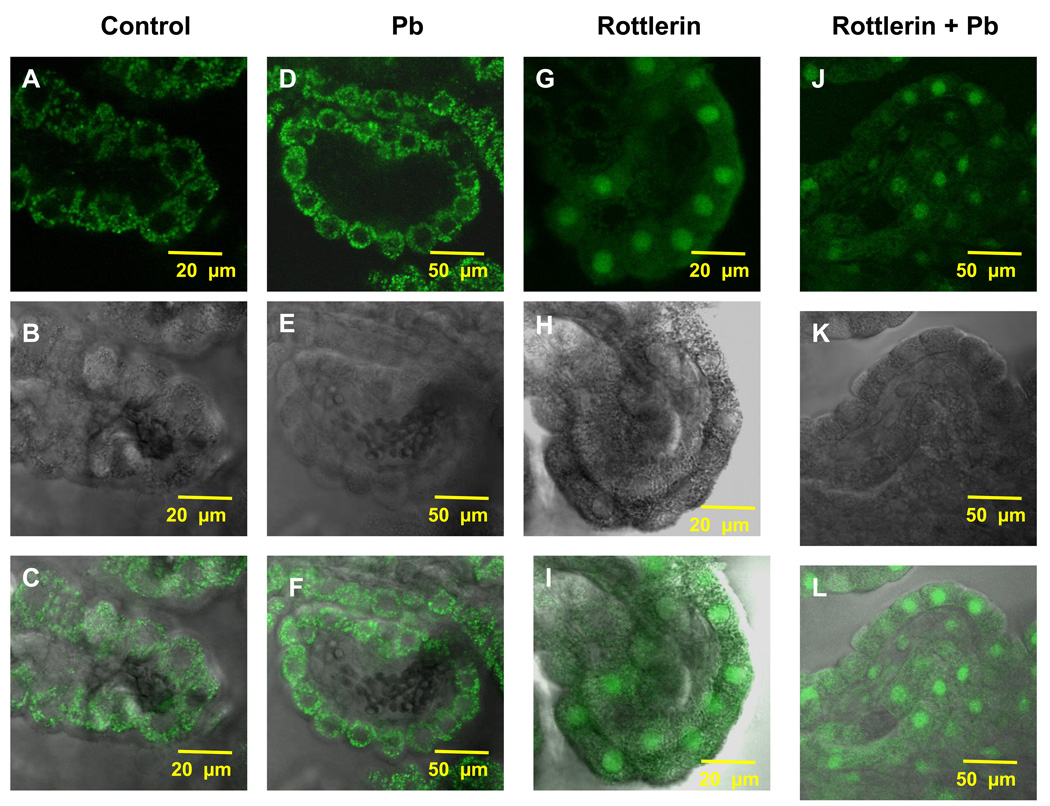
3.5. Rottlerin Abolishes the Pb-Induced Intracellular Aβ Accumulation and Potentiates Aβ Accumulation in Nuclei
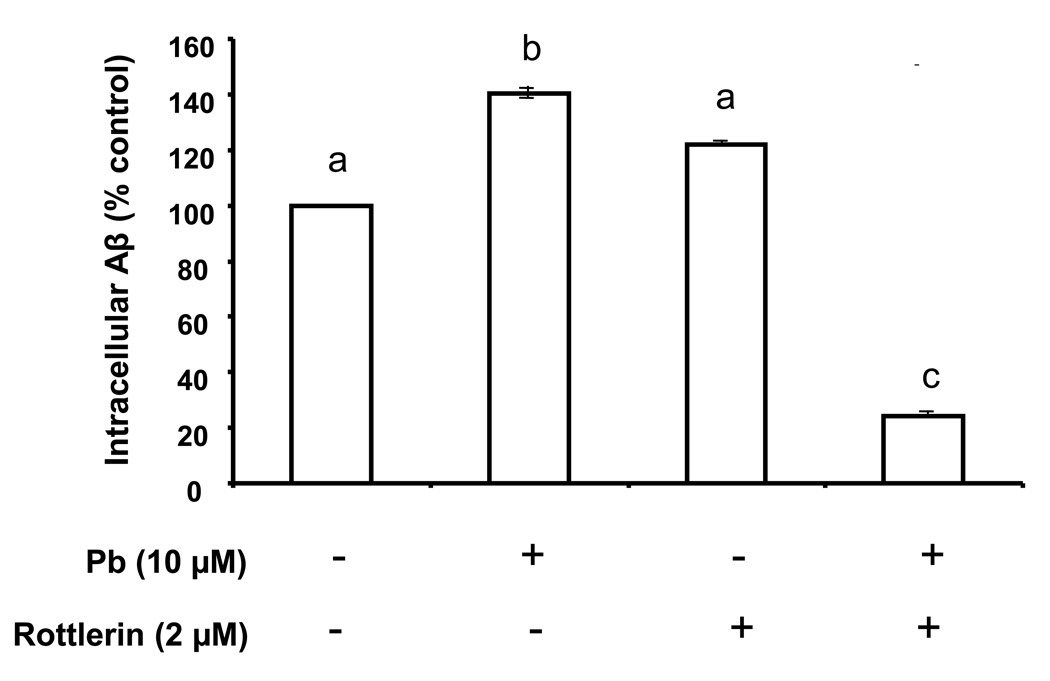
Since rottlerin apparently blocked the Pb-induced LRP1 relocalization, an interesting question raised was whether rottlerin also prevents against Pb-initiated cellular accumulation of Aβ in the CP. To test this hypothesis, we used a similar immunohistochemical in vitro approach to study Aβ accumulation in CP tissue. The CP tissues when exposed with Pb (10 µM, 1 h) showed a distinct increase in cytosolic intracellular Aβ levels (Fig. 5D–F) compared with untreated controls (Fig. 5A–C). Pre-incubation with rottlerin (2 µM, 20 min) alone did not seem to reduce the Aβ in CP tissues (Fig. 6); interestingly, it caused an apparent concentration of Aβ from the cytosol to the nucleus (Fig. 5G–I). When the CP tissues were pre-incubated with rottlerin followed by Pb treatment, there was a visible and significant reduction in Aβ in the CP (Fig. 6). However, it remains unclear as to why rottlerin did not simply abolish the Pb-induced increase in cellular Aβ, but instead, actively reduced Aβ concentrations below levels seen in untreated controls (p<0.01).
Fig. 5.
Rottlerin attenuated the Pb-induced increase in Aβ accumulation. (A,C) Confocal image from a representative CP tissue of a control rat demonstrating the accumulation of Aβ primarily in the cytosol with minimal staining in the nuclei. (D, F). Confocal image from a representative CP tissue in a Pb-exposed rat demonstrating an increase in Aβ signal. (G, I) Confocal image from a representative CP tissue pre-treated with rottlerin in absence of Pb. (J, L) Confocal image from a representative CP tissue pre-treated with rottlerin in presence of Pb. (B, E, H, K) represent transmission images revealing normal morphology of the tissues. Please see the legend to Fig.3 for Pb-dose regimen. The data are representative of experiments from four replicates.
Fig. 6.
Quantification of the fluorescent signals using Laser Scanning Cytometry. The fluorescent signals in Fig. 5 were quantified using software Image J. The bar designated Pb(−)/rottlerin (−) represents untreated controls, Pb(+)/rottlerin(−) represents treatment with Pb in absence of rottlerin, Pb(−)/rottlerin (+) represents incubation with rottlerin in absence of Pb, and Pb (+)/rottlerin(+) represents tissues pre-treated with rottlerin followed by Pb. Data represent mean ± SD, n=16 (a total of 16 cells per group taken from 4 tissue samples with fluorescence averaged from 4 cells per sample). Bars with different superscripts labeled as a,b,c indicate significant difference from one another.
4. Discussion
The data from the current study confirm our previous findings that Pb exposure increases Aβ accumulation in the CP [14]. Furthermore, we demonstrated that Pb exposure, both in vivo and in vitro, prompted the relocalization of LRP1 to the apical side of the choroidal epithelial membrane; this effect appeared to be associated with Pb-induced alteration in PKC-δ in the CP.
The finding that the Pb- mediated translocation of LRP1 appears to involve PKC-δ is supported by several critical pieces of experimental evidence: (1) when the CP tissues were pre-incubated with PKC-δ inhibitor rottlerin, the relocalization of LRP1 initiated by Pb exposure was completely blocked, suggesting the involvement of the PKC-δ signal transduction in intracellular LRP1 migration. (2) In cell fractions that were immunoprecipitated by anti-LRP1 antibody, there was an evident presence of PKC-δ proteins; this provides direct evidence of a protein-protein interaction between LRP1 and PKC-δ. (3) Inhibition of PKC-δ activity by rottlerin ultimately abolished the Pb-induced cellular accumulation of Aβ. Since LRP1 is responsible for expelling Aβ from the cells, it appears that Pb exposure may affect LRP1 function via the action of Pb on PKC-δ, subsequently affecting Aβ transport/clearance at the BCB.
The above findings raise several important questions with respect to Aβ homeostasis at the BCB and its dysregulation following Pb exposure. First, what is the role of PKC-δ in Pb-induced translocation of LRP1? Although the exact mechanism whereby Pb acts on PKC remains unknown, it has long been shown that Pb can activate PKC and translocate it from the cytosol to the membrane in various tissues [57,58,63], including the CP [42]. The current study supports findings in literature that the action of Pb does not appear to be due to competition with Ca2+, but instead, may occur on its catalytic domain [59]. This is because PKC-δ, a novel isoform of PKC, does not have a Ca2+ binding domain and responds only to diacylglycerol (DG), in contrast to the conventional PKC isozymes which are recruited to membranes by two modules, a C1-DG domain, and a C2 domain comprising a Ca2+ - triggered phospholipid-binding module [36]. The activated PKC-δ could then potentially phosphorylate LRP1 and relocalize the complex to the membrane [44]. Further studies using subcellular fractionation and the role of PKC- δ activation are warranted in order to accurately determine the cellular compartments for the shift in LRP1 protein following Pb exposure.
It may be noteworthy that due to a lack of substantial quantity of fresh choroid plexus tissues, our immunoprecipitation studies were conducted on Z310 cells. Although this cell line has been well characterized, it may be of future interest to assess protein-protein interaction between LRP1 and PKC-δ in the CP tissues using a different technique.
Second, is the PKC-δ-mediated relocalization of LRP1 transient or relatively long-lasting? Previous studies from this laboratory have shown that while Pb translocates PKC in plexus epithelial cells in vitro, there was no evidence of a relocalization of PKC in CP tissues following chronic Pb exposure in vivo [37]. It should be pointed out that our previous study assessed a chronic, low level of Pb exposure as compared to the current study which employed an acute, much higher concentration of Pb. Hence, it is possible that PKC activation may be seen as an initial response of the cells to Pb in the CP following acute exposure while other brain regions such as the hippocampus may be affected in a delayed pattern following chronic Pb exposure. In fact, studies in literature have shown a distinct translocation of PKC from the cytosol to the membrane in the hippocampus of a chronic Pb exposed animal model which correlates with memory deficits [55]. However, further studies to verify the effects of chronic Pb exposure on PKC-δ activation in the CP should be performed in order to explore the transient versus long-term effects of Pb-induced activation of PKC.
Finally, what are the implications of preventing PKC-δ activation and attenuating Pb-induced increase in Aβ levels in the CP? Studies have shown that activation of PKC-δ results in the generation of oxidative stress and cell death [56, 57], whereas its inhibition by rottlerin can exert a neuroprotective effect and prevent cell death in dopaminergic neurons and MPTP animal models of Parkinson’s disease [58–61]. Our observation that rottlerin prevents the Pb-induced relocalization of LRP1 and reduces Pb-initiated cellular accumulation of Aβ appears in agreement with the above reports. As seen in Fig. 3J, treatment with rottlerin appears to abolish the Pb-induced subcellular relocalization (Fig. 3D). It is interesting to note that the signals for PKC-δ are relatively lost with rottlerin treatment alone (Fig. 3H). Although the exact mechanism for this finding remains unknown, we speculate that this could be due to a down-regulation of PKC-δ by rottlerin treatment or due to a possible rottlerin-induced alteration in PKC-δ conformation. By attenuating the Pb-induced relocalization of Aβ transporter LRP1 to the membrane, more LRP1 would be retained in the cytoplasm for it to bind to and clear Aβ. Hence, rottlerin may elicit a “protective” effect against Aβ toxicity and the subsequent development of AD. Interestingly, while rottlerin treatment reduces the overall amounts of Aβ in CP tissues, it concentrates Aβ in the nucleus (Fig. 5G). The implication of this observation remains unknown. Clearly, future studies are required to explore the molecular mechanisms whereby PKC mediates the phosphorylation of LRP1 and the implications for rottlerin to induce relocalization of Aβ to the nucleus.
5. Conclusions
In summary, this study confirms that the intracellular distribution of LRP1 is mediated by PKC-δ. Pb exposure prompts the relocalization of LRP1 from the cytosol to apical member of the choroidal epithelial cells and this effect is possibly due to Pb activation of PKC-δ. The combined effect of a reduction in LRP1 protein expression and loss-of- function in mobilizing intracellular Aβ following Pb exposure may underlie the Pb-induced increase of Aβ levels in the BCB; a damaged clearance of Aβ from the brain may ultimately impair brain homeostasis of Aβ.
Acknowledgements
This work was supported in part by NIH Grants R21-ES017055 and RO1-ES008146.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that they have no competing interests.
References
- 1.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M. Alzheimer's Disease International Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberson ED, Mucke L. 100 years and counting: prospects for defeating Alzheimer's disease. Science. 2006;3:781–784. doi: 10.1126/science.1132813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu G, Huang W, Moir RD, Vanderburg CR, Lai B, Peng Z, Tanzi RE, Rogers JT, Huang X. Metal exposure and Alzheimer's pathogenesis. J Struct Biol. 2006;155:45–51. doi: 10.1016/j.jsb.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Suh YH, Checler F. Amyloid precursor protein, presenilins, and alpha-synuclein: Molecular pathogenesis and pharmacological applications in Alzheimer's disease. Pharmacol Rev. 2002;54:469–525. doi: 10.1124/pr.54.3.469. [DOI] [PubMed] [Google Scholar]
- 5.Wu J, Basha MR, Zawia NH. The environment, epigenetics and amyloidogenesis. J Mol Neurosci. 2008a;34:1–7. doi: 10.1007/s12031-007-0009-4. [DOI] [PubMed] [Google Scholar]
- 6.Brody DL, Magnoni S, Schwetye KE, Spinner ML, Esparza TJ, Stocchetti N, Zipfel GJ, Holtzman DM. Amyloid-beta dynamics correlate with neurological status in the injured human brain. Science. 2008;321:1221–1224. doi: 10.1126/science.1161591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat. Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 9.Crossgrove JS, Li GJ, Zheng W. The choroid plexus removes betaamyloid from the cerebrospinal fluid. Exp Biol Med. 2005;230:771–776. doi: 10.1177/153537020523001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deane R, Du YS, Submamaryan R, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt A, Armstrong D, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 11.Deane R, Sagare A, Zlokovic BV. The role of the cell surface LRP and soluble LRP in blood-brain barrier a clearance in Alzheimer's Disease. Curr Pharm Des. 2008;14:1601–1605. doi: 10.2174/138161208784705487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crossgrove JS, Smith EL, Zheng W. Macromolecules involved in production and metabolism of beta-amyloid at the brain barriers. Brain Res. 2007;138:187–195. doi: 10.1016/j.brainres.2006.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki A, Iijima M, Yokoo H, Shoji M, Nakazato Y. Human choroid plexus is an uniquely involved area of the brain in amyloidosis: a histochemical, immunohistochemical and ultrastructural study. Brain Res. 1997;755:93–201. doi: 10.1016/s0006-8993(97)00097-8. [DOI] [PubMed] [Google Scholar]
- 14.Behl M, Zhang Y, Monnot AD, Jiang W, Zheng W. Increased beta-amyloid levels in the choroid plexus following lead exposure and the involvement of low-density lipoprotein receptor protein-1. Toxicol Appl Pharmacol. 2009a;240:245–254. doi: 10.1016/j.taap.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donahue JE, Flaherty SL, Johanson CE, Duncan JA, Silverberg GD, Miller MC, Tavares R, Yang W, Wu Q, Sabo E, Hovanesian V, Stopa EG. RAGE, LRP-1, and amyloid-beta protein in Alzheimer's disease Acta. Neuropathol. 2006;112:405–415. doi: 10.1007/s00401-006-0115-3. [DOI] [PubMed] [Google Scholar]
- 16.Johanson C, Flaherty S, Messier A, Duncan J, III, Silverberg G. Expression of the beta-amyloid transporter, LRP-1, in aging choroid plexus: implications for the CSF-brain system in NPH and Alzheimer's disease, Oral Presentation. CSF Res. 2006;3:S29. [Google Scholar]
- 17.Schwartz BS, Stewart WF. Lead and cognitive function in adults: a questions and answers approach to a review of the evidence for cause, treatment, and prevention. Int. Rev. Psychiatry. 2007;19:671–692. doi: 10.1080/09540260701797936. [DOI] [PubMed] [Google Scholar]
- 18.Staudinger KC, Roth VS. Occupational Lead Poisoning. Am Fam Phy. 1998;57:1–15. [PubMed] [Google Scholar]
- 19.Shih RA, Hu H, Weisskopf MG, Schwartz BS. Cumulative Lead Dose and Cognitive Function in Adults: A Review of Studies That Measured Both Blood Lead and Bone Lead. Environ Health Perspect. 2007;115:483–492. doi: 10.1289/ehp.9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart WF, Schwartz BS, Simon D, Kelsey K, Todd AC. ApoE genotype, past adult lead exposure, and neurobehavioral function. Environ Health Perspect. 2002;110:501–505. doi: 10.1289/ehp.02110501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart WF, Schwartz BS, Davatzikos C, Shen D, Liu D, Wu X, Todd AC, Shi W, Bassett S, Youssem D. Past adult lead exposure is linked to neurodegeneration measured by brain MRI. Neurology. 2006;66:1476–1484. doi: 10.1212/01.wnl.0000216138.69777.15. [DOI] [PubMed] [Google Scholar]
- 22.Jiang YM, Long LL, Zhu XY, Zheng H, Fu X, Ou SY, Wei DL, Zhou HL, Zheng W. Evidence for altered hippocampal volume and metabolites in workers occupationally exposed to lead: A study by magnetic resonance imaging and 1H magnetic resonance spectroscopy. Toxicol Lett. 2008;181:118–125. doi: 10.1016/j.toxlet.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haraguchi T, Ishizu H, Takehisa Y, Kawai K, Yokota O, Terada S, Tsuchiya K, Ikeda K, Morita K, Horike T, Kira S, Kuroda S. Lead content of brain tissue in diffuse neurofibrillary tangles with calcification (DNTC): the possibility of lead neurotoxicity. Neuroreport. 2002;13(1) doi: 10.1097/00001756-200112210-00006. inside back cover. [DOI] [PubMed] [Google Scholar]
- 24.Basha MR, Wei W, Bakheet SA, Benitez N, Siddiqi HK, Ge YW, Lahiri DK, Zawia NH. The fetal basis of amyloidogenesis: exposure to lead and latent overexpression of amyloid precursor protein and beta-amyloid in the aging brain. J Neurosci. 2005a;25:823–829. doi: 10.1523/JNEUROSCI.4335-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basha MR, Murali M, Siddiqi HK, Ghosal K, Siddiqi OK, Lashuel HA, Ge YW, Lahiri DK, Zawia NH. Lead (Pb) exposure and its effect on APP proteolysis and Aβ aggregation. FASEB J. 2005b;10:1–16. doi: 10.1096/fj.05-4375fje. [DOI] [PubMed] [Google Scholar]
- 26.Kuhlmann AC, McGlothan JL, Guilarte TR. Developmental lead exposure causes spatial learning deficits in adult rats. Neurosc Lett. 1997;233:101–104. doi: 10.1016/s0304-3940(97)00633-2. [DOI] [PubMed] [Google Scholar]
- 27.Prince M. Is chronic low-level lead exposure in early life an etiologic factor in Alzheimer's disease? Epidemiology. 1998;9:618–621. [PubMed] [Google Scholar]
- 28.Counter SA, Buchanan LH, Ortega F. Neurocognitive impairment in lead-exposed children of Andean lead glazing workers. J Occup Environ Med. 2005;47:306–312. doi: 10.1097/01.jom.0000155717.45594.65. [DOI] [PubMed] [Google Scholar]
- 29.White LD, Cory-Slechta DA, Gilbert ME, Tiffany-Castiglioni E, Zawia NH, Virgolini M, Rossi-George A, Lasley SM, Qian YC, Basha MR. New and evolving concepts in the neurotoxicology of lead. Toxicol Appl Pharmacol. 2007;225:1–27. doi: 10.1016/j.taap.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Friedheim E, Corvi C, Graziano J, Donnelli T, Breslin D. Choroid Plexus as a Protective Sink for Heavy Metals? Lancet. 1983;8331:981–982. doi: 10.1016/s0140-6736(83)92099-8. [DOI] [PubMed] [Google Scholar]
- 31.Manton WI, Kirkpatrick JB, Cook JD. Does the choroid plexus really protect the brain from lead? Lancet. 1984;8398:351. doi: 10.1016/s0140-6736(84)92719-3. ii. [DOI] [PubMed] [Google Scholar]
- 32.Zheng W, Perry DF, Nelson DL, Aposhian HV. Protection of cerebrospinal fluid against toxic metals by the choroid plexus. FASEB J. 1991;5:2188–2193. doi: 10.1096/fasebj.5.8.1850706. [DOI] [PubMed] [Google Scholar]
- 33.Zheng W, Shen H, Blaner WS, Zhao Q, Ren X, Graziano JH. Chronic Lead Exposure Alters Transthyretin Concentration in Rat Cerebrospinal Fluid: The Role of the Choroid Plexus. Toxicol Appl Pharmacol. 1996;139:445–450. doi: 10.1006/taap.1996.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monro OR, Mackic JB, Yamada S, Segal MB, Ghiso J, Maurer C, Calero M, Frangione B, Zlokovic BV. Substitution at codon 22 reduces clearance of Alzheimer's amyloid-beta peptide from the cerebrospinal fluid and prevents its transport from the central nervous system into blood. Neurobiol Aging. 2002;23:405–412. doi: 10.1016/s0197-4580(01)00317-7. [DOI] [PubMed] [Google Scholar]
- 35.Miklossy J, Taddei K, Martins R, Escher G, Kraftsik R, Pillevuit O, Lepori D, Campiche M. Alzheimer Disease: Curly fibers and tangles in organs other than brain. J Neuropathol Exp Neurol. 1999;58:803–814. doi: 10.1097/00005072-199908000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Goto JJ, Tanzi RE. The role of the low-density lipoprotein receptor-related protein (LRP1) in Alzheimer's Abeta generation: development of a cell-based model system. J. Mol. Neurosci. 2002;19:37–41. doi: 10.1007/s12031-002-0008-4. [DOI] [PubMed] [Google Scholar]
- 37.Knauer MF, Orlando RA, Glabe CG. Cell surface APP751 forms complexes with protease nexin 2 ligands and is internalized via the low density lipoprotein receptor-related protein (LRP) Brain Res. 1996;740:6–14. doi: 10.1016/s0006-8993(96)00711-1. [DOI] [PubMed] [Google Scholar]
- 38.Kounnas MZ, Moir RD, Rebeck GW, Nush AI, Argraves WS, Tanzi RE, Hyman BT, Strickland DK. LDL receptor-related protein, a multifunctional ApoE receptor, binds secreted β-amyloid precursor protein and mediates its degradation. Cell. 1995;82:331–340. doi: 10.1016/0092-8674(95)90320-8. [DOI] [PubMed] [Google Scholar]
- 39.Moir RD, Tanzi RE. LRP-mediated clearance of Abeta is inhibited by KPI containing isoforms of APP. Curr. Alzheimer Res. 2005;2:269–273. doi: 10.2174/1567205053585918. [DOI] [PubMed] [Google Scholar]
- 40.Hyman BT, Strickland D, Rebeck GW. Role of the low-density lipoprotein receptor-related protein in β-amyloid metabolism and Alzheimer disease. Arch. Neurol. 2000;57:646–665. doi: 10.1001/archneur.57.5.646. [DOI] [PubMed] [Google Scholar]
- 41.Giorgione JR, Lin JH, McCammon JA, Newton AC. Increased membrane affinity of the C1 domain of protein kinase C delta compensates for the lack of involvement of its C2 domain in membrane recruitment. J. Biol Chem. 2006;281:1660–1669. doi: 10.1074/jbc.M510251200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao Q, Slavkovich V, Zheng W. Lead exposure promotes translocation of protein kinase C activities in rat choroid plexus in vitro, but not in vivo. Toxicol Appl Pharmacol. 1998;149:99–106. doi: 10.1006/taap.1997.8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hwang KY, Lee BK, Bressler JP, Bolla KI, Stewart WF, Schwartz BS. Protein Kinase C Activity and the Relations between Blood Lead and Neurobehavioral Function in Lead Workers. Environ Health Perspect. 2002;110:133–138. doi: 10.1289/ehp.02110133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akers RF, Lovinger DM, Colley D, Linden D, Routenberg A. Translocation of protein kinase C activity after LTP may mediate hippocampal synaptic plasticity. Science. 1986;231:587–589. doi: 10.1126/science.3003904. [DOI] [PubMed] [Google Scholar]
- 45.Huynh TV, Cole G, Katzman R, Huang KP, Saitoh T. Reduced Protein Kinase C Immunoreactivity and Altered Protein Phosphorylation in Alzheimer's disease Fibroblasts. Arch Neurol. 1989;46:1195–1199. doi: 10.1001/archneur.1989.00520470049026. [DOI] [PubMed] [Google Scholar]
- 46.Pascale A, Govoni S, Battaini F. Age-related alteration of PKC, a key enzyme in memory processes: physiological and pathological examples. Mol Neurobiol. 1998;16:49–62. doi: 10.1007/BF02740602. [DOI] [PubMed] [Google Scholar]
- 47.Wehner JM, Sleight S, Upchurch M. Hippocampal protein kinase C activity is reduced in poor spatial learners. Brain Res. 1990;523:181–187. doi: 10.1016/0006-8993(90)91485-y. [DOI] [PubMed] [Google Scholar]
- 48.Weiss S, Ellis J, Hendly DD, Lenox RH. Translocation and activation of protein kinase C in striatal neurons in primary culture: relationship to phorphol dibutyrate actions on the inositol phosphate generating system and neurotransmitter release. J Neurochem. 1989;52:530–536. doi: 10.1111/j.1471-4159.1989.tb09152.x. [DOI] [PubMed] [Google Scholar]
- 49.Hayashi H, Campenot RB, Vance DE, Vance JE. Apolipoprotein E-containing lipoproteins protect neurons from apoptosis via a signaling pathway involving low-density lipoprotein receptor-related protein-1. J Neurosci. 2007;27:1933–1941. doi: 10.1523/JNEUROSCI.5471-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zurhove K, Nakajima C, Herz J, Bock HH, May P. Gamma-secretase limits the inflammatory response through the processing of LRP1. Sci Signal. 2008;1:1–25. doi: 10.1126/scisignal.1164263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang K-P, Huang FL. How is protein kinase C activated in CNS? Biochem. Int. 1993;22:417–433. doi: 10.1016/0197-0186(93)90037-6. [DOI] [PubMed] [Google Scholar]
- 52.Markovac J, Goldstein GW. Lead activates protein kinase C in immature rat brain microvessels. Toxicol. Appl. Pharmacol. 1988a;96:14–23. doi: 10.1016/0041-008x(88)90242-6. [DOI] [PubMed] [Google Scholar]
- 53.Markovac J, Goldstein GW. Picomolar concentrations of lead stimulate brain protein kinase C. Nature. 1988b;334:71–73. doi: 10.1038/334071a0. [DOI] [PubMed] [Google Scholar]
- 54.OSHA. U.S. Occupational Safety and Health Administration. Safety and Health Topics: Lead. Compliance. 2002 Available: http://www.osha.gov/sltc/lead/compliance.html.
- 55.OSHA. U.S. Occupational Safety and Health Administration. Blood Lead Laboratories Program Description and Background. 2005 Available: http://www.osha.gov/SLTC/bloodlead/program.html.
- 56.Shi LZ, Zheng W. Early lead exposure increases the leakage of the blood-cerebrospinal fluid barrier, in vitro. Hum Exp Toxicol. 2007;26:159–167. doi: 10.1177/0960327107070560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng W, Zhao Q. Establishment and characterization of an immortalized Z310 choroidal epithelial cell line from murine choroid plexus. Brain Res. 2002;958:371–380. doi: 10.1016/s0006-8993(02)03683-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laterra J, Bressler JP, Indurti RR, Belloni-Olivi L, Goldstein GW. Inhibition of astroglia-induced endothelial differentiation by inorganic lead: A role for protein kinase C. Proc. Natl. Acad. Sci. 1992;89:10748–10752. doi: 10.1073/pnas.89.22.10748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murakami K, Feng G, Chen SG. Inhibition of brain protein kinase C subtypes by lead. J. Pharmacol. Exp. Ther. 1993;264:757–761. [PubMed] [Google Scholar]
- 60.Chen HH, Ma T, Paul IA, Spencer JL, Ho IK. Developmental Lead Exposure and Two-way Active Avoidance Training Alter the Distribution of Protein Kinase C Activity in the Rat Hippocampus. Neurochem Res. 1997;22:1119–1125. doi: 10.1023/a:1027365202328. [DOI] [PubMed] [Google Scholar]
- 61.Anantharam V, Kitazawa M, Wagner J, Kaul S, Kanthasamy AG. Caspase-3-dependent proteolytic cleavage of protein kinase C delta is essential for oxidative stress-mediated dopaminergic cell death after exposure to methylcyclopentadienyl manganese tricarbonyl. J Neurosci. 2002;22:1738–1751. doi: 10.1523/JNEUROSCI.22-05-01738.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanthasamy AG, Kitazawa M, Kanthasamy A, Anantharam V. Role of proteolytic activation of protein kinase C delta in oxidative stress-induced apoptosis. Antioxid Redox Signal. 2003;5:609–620. doi: 10.1089/152308603770310275. [DOI] [PubMed] [Google Scholar]
- 63.Kaul S, Kanthasamy A, Kitazawa M, Anantharam V, Kanthasamy AG. Caspase-3 dependent proteolytic activation of protein kinase C delta mediates and regulates 1-methyl-4-phenylpyridinium (MPP)-induced apoptotic cell death in dopaminergic cells: relevance to oxidative stress in dopaminergic degeneration. Eur J Neurosci. 2003;18:1387–1401. doi: 10.1046/j.1460-9568.2003.02864.x. [DOI] [PubMed] [Google Scholar]
- 64.Kitazawa M, Anantharam V, Kanthasamy AG. Dieldrin induces apoptosis by promoting caspase-3-dependent proteolytic cleavage of protein kinase C delta in dopaminergic cells: relevance to oxidative stress and dopaminergic degeneration. Neuroscience. 2003;119:945–964. doi: 10.1016/s0306-4522(03)00226-4. [DOI] [PubMed] [Google Scholar]
- 65.Yang Y, Kaul S, Zhang D, Anantharam V, Kanthasamy AG. Suppression of caspase-3-dependent proteolytic activation of protein kinase C delta by small interfering RNA prevents MPP- induced dopaminergic degeneration. Mol Cell Neurosci. 2004;25:406–421. doi: 10.1016/j.mcn.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 66.Zhang D, Anantharam V, Kanthasamy A, Kanthasamy AG. Neuroprotective effect of protein kinase C delta inhibitor rottlerin in cell culture and animal models of Parkinson's disease. J Pharmacol Exp Ther. 2007;322:913–922. doi: 10.1124/jpet.107.124669. [DOI] [PubMed] [Google Scholar]