Abstract
We previously showed that in a two-alternative choice (2AC) task, olfactory bulb (OB) gamma oscillations (∼70 Hz in rats) were enhanced during discrimination of structurally similar odorants (fine discrimination) versus discrimination of dissimilar odorants (coarse discrimination). In other studies (mostly employing go/no-go tasks) in multiple labs, beta oscillations (15–35 Hz) dominate the local field potential (LFP) signal in olfactory areas during odor sampling. Here we analyzed the beta frequency band power and pairwise coherence in the 2AC task. We show that in a task dominated by gamma in the OB, beta oscillations are also present in three interconnected olfactory areas (OB and anterior and posterior pyriform cortex). Only the beta band showed consistently elevated coherence during odor sniffing across all odor pairs, classes (alcohols and ketones), and discrimination types (fine and coarse), with stronger effects in first than in final criterion sessions (>70% correct). In the first sessions for fine discrimination odor pairs, beta power for incorrect trials was the same as that for correct trials for the other odor in the pair. This pattern was not repeated in coarse discrimination, in which beta power was elevated for correct relative to incorrect trials. This difference between fine and coarse odor discriminations may relate to different behavioral strategies for learning to differentiate similar versus dissimilar odors. Phase analysis showed that the OB led both pyriform areas in the beta frequency band during odor sniffing. We conclude that the beta band may be the means by which information is transmitted from the OB to higher order areas, even though task specifics modify dominance of one frequency band over another within the OB.
INTRODUCTION
Distributed neural systems associated with sensory and cognitive processing show cooperative activity at many scales, from single units (Grossman et al. 2008) to local field activity (Zhang et al. 2008) to large scale slow temporal processes such as the blood-oxygen-level-dependent (BOLD) signal (Plailly et al. 2008). Of these methods, the mesoscopic scale of the local field potential (LFP) measure has some advantages over single unit and functional magnetic resonance imaging (fMRI) studies for examining systemwide coupling, because the LFP is often a good measure of what one brain area receives from another and has good spatial and temporal resolution (Freeman 1975; Goense and Logothetis 2008; Xing et al. 2009).
Sensory and perceptual olfactory LFPs are characterized by oscillations (Kay et al. 2009). Inhalation-coupled gamma oscillations (40–100 Hz) are associated with sensory processing; increases in gamma oscillatory activity in the olfactory bulb (OB) are correlated with increased discrimination of highly overlapping glomerular input patterns (Beshel et al. 2007; Nusser et al. 2001), and decreases in an insect analog of this oscillation are correlated with decreases in ability to discriminate highly overlapping input patterns (Stopfer et al. 1997). Gamma oscillations are tightly linked to local precision in mitral cell firing patterns, such that the oscillations represent the probability of cells firing at a given time (Eeckman and Freeman 1990).
Beta oscillations (15–35 Hz) are less well understood but have been associated with criterion performance in appetitive odor discrimination and with sensitization-like activity in response to unreinforced highly volatile odorants (Lowry and Kay 2007; Martin et al. 2004b, 2007). These oscillations are distinct from gamma oscillations in more than their frequency band. OB beta oscillations show high coherence with activity in the pyriform cortex (PC) (Lowry and Kay 2007; Poo and Isaacson 2009) and require intact feedback to the OB from the rest of the brain, showing a sharp decrease when this feedback is eliminated (Martin et al. 2006; Neville and Haberly 2003). Conversely, gamma oscillations are enhanced when these connections are cut (Gray and Skinner 1988; Martin et al. 2006). These phenomena point to beta oscillations as a larger-scale network phenomenon and gamma oscillations as an indicator of local population precision.
Beta oscillations have also been observed in other systems and have been associated with coupling cortical areas during sensory or cognitive processing. In motor systems, beta oscillations seem to be favored for long distance communication over higher frequency events (Hermer-Vazquez et al. 2007; Kopell et al. 2000; Tkach et al. 2007; van Wijk et al. 2009; Zhang et al. 2008). These oscillations have also been implicated in working memory processes in humans (Deiber et al. 2007). The frequency of beta is what defines the oscillation in the literature, and we do not yet know if beta oscillations represent a unified category within neural processing.
In our previous report on a portion of these data (OB and anterior pyriform cortex/aPC), we showed that OB gamma oscillations (65–100 Hz) were selectively enhanced when rats learned to discriminate similar (fine) versus dissimilar (coarse) odorant pairs (Beshel et al. 2007). However, odor discrimination learning in some go/no-go (GNG) tasks has been shown to enhance beta oscillations instead of gamma (Martin et al. 2004b, 2007). Here we report on underlying beta oscillatory activity and coherence patterns elicited during odor sampling in the gamma-dominated two-alternative choice (2AC) task. We include the OB and both the aPC and posterior PC (pPC) in our analyses to determine the frequency band involved in systemwide coupling. We show that beta oscillations represent highly cooperative activity among the OB, aPC, and pPC and that OB beta precedes similar activity in the aPC and pPC.
METHODS
Four adult male Sprague-Dawley rats were purchased from Harlan HSD (Indianapolis, IN) and housed in 14 h light/10 h dark (lights on at 8 am CST). All testing was done between 12 and 6 pm. Surgical and behavioral procedures were done with approval and oversight by the University of Chicago IACUC, according to AAALAC guidelines. A portion of the data from this study was published previously (power analysis of the gamma band in the OB and aPC) (Beshel et al. 2007).
Electrodes were implanted under pentobarbital anesthesia in the OB (8.5 mm anterior to bregma, 1.5 mm lateral to midline, ∼3–4 mm deep) and aPC (0.5 mm anterior to bregma, 3.0 mm lateral, ∼8–9 mm deep, 15° pitch from vertical) and pPC (−2.3 mm to bregma, 3.0 mm lateral, ∼7–10 mm deep, 15° pitch) as described previously (100 μm diameter stainless steel, formvar coated bipolar wires with a tip separation of ∼1.5 mm) (Beshel et al. 2007). After recovery, the rats were dieted to 85% of their ad libitum weight before training and were maintained at that weight during the several weeks of training and testing.
Behavior
Behavioral equipment was controlled by a Pentium IV computer running Graphic State 4.0 (Coulborn Instruments, Allentown, PA). The test chamber was a standard operant chamber (Med Associates, St. Albans, VT). Training of rats to the task was done prior to electrode implantation on a set of training odors that were not used for later testing. After recovery (1–2 wk), behavior was first reinforced on the training odors, and then the testing phase began. During testing, odorants were paired by odor class (alcohol or ketone) and discrimination type (coarse: odorants separated by 5 carbons in chain length within the odor class; fine: odorants separated by only one carbon in chain length; odorants listed in Table 1). Within each discrimination pair, the first odorant (odor A in Table 1) was associated with the right response lever, and the second odorant (odor B) was associated with the left response lever. Each behavioral trial was initiated by the rat with an enforced minimum 7 s intertrial interval. To initiate a trial, the rat pressed a lever in the rear of the cage, which enabled an odor port in the front of the cage. A nosepoke in this port initiated a 1.5 s odor stimulus (odor A or B pseudorandomly delivered). One second after the end of the odor pulse, two levers extended from the front of the behavioral chamber on either side of the odor port. At this time pressing the lever (right or left) associated with the odor stimulus (odor A or B, respectively) delivered a 40 mg sucrose pellet (BioServe, Frenchtown, NJ) in the reward cup beneath the odor port with 85% probability. Pressing the wrong lever resulted in an immediate termination of the trial, lights out and an extra 4 s delay until the next trial could be initiated. Criterion performance was set at two consecutive sessions ≥70% correct. We previously reported summary performance statistics from these rats (Beshel et al. 2007), and we summarize them here. Coarse discrimination took slightly fewer sessions to reach criterion performance than fine discrimination (sessions ± SE, coarse alcohols: 1.5 ± 0.65, coarse ketones: 1 ± 0.41, fine alcohols: 1.75 ± 0.85, fine ketones: 3.75 ± 1.38).
Table 1.
Odorant pairs
| Type | Class | Odor A | Odor B |
|---|---|---|---|
| Coarse | Alcohol | Propanol | Octanol |
| Ketone | Butanone | Nonanone | |
| Fine | Alcohol | Heptanol | Hexanol |
| Ketone | Octanone | Heptanone |
Odors A and B were right and left levers, respectively.
LFP signals were recorded during sessions of ∼200 trials lasting ∼2 h (Neuralynx Cheetah 32, with NB Labs unity gain headstage; 2,016 Hz sampling, 1–475 Hz analog filters, gain in most recordings was 4,000: AD gain of 2 and amplifier gain of 2,000).
Data analysis
Power spectral and coherence analyses were done using the MATLAB Chronux toolbox (http://www.chronux.org/; Mitra and Bokil 2008) using multitaper methods (time bandwidth 3, 5 tapers). Power spectra were computed on 1.2 s data segments (2,419 time points padded with 0s to 4,096 points) during the odor period, beginning 300 ms after the nosepoke which started odor delivery (Fig. 1). Power in the beta band was estimated from the mean of the power spectrum in the 15–35 Hz band for each trial. Because power varies dependent on electrode placement and quality of recording in a session, we normalized the power within a trial by the power in the 1.2 s prestimulus window from −1.5 to −0.3 s relative to zero at the time of the nosepoke. For statistical evaluation, we further transformed these values to dB using 10*Log10(beta power during odor sniffing/prestimulus beta power). We refer to this measure as the beta power ratio. Values close to zero signify no change in beta band power during odor sniffing, while values less than or greater than zero signify decreased or increased beta band power, respectively, relative to the prestimulus period.
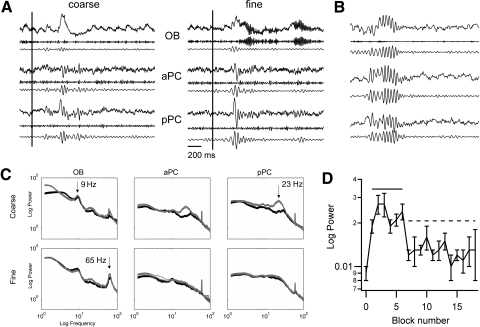
Fig. 1.
Beta oscillations are present in all 3 structures. A: sample data (2 s for each panel) from olfactory bulb (OB), anterior pyriform cortex (aPC), and posterior PC (pPC) during odor sampling for coarse/nonanone and fine/octanone odor sampling, vertical line indicates nosepoke which triggers the 1.5 s odor pulse. Top: raw local field potential (LFP); middle: gamma-filtered; bottom: beta-filtered. B: beta oscillations from a go/nogo (GNG) task for comparison, nosepoke is at the beginning of the 2 s sample (unpublished data from D. Frederick and L. M. Kay). Data for both A and B are normalized to the SD of the nonbeta periods surrounding the beta burst region. This allows visual comparison of the relative amplitudes of the beta event across rats and behavioral conditions. C: power spectra for all 3 areas during ketone discrimination (butanone/nonanone and octanone/heptanone). In each plot the black curve refers to odor A (butanone and octanone) and the gray curve to odor B (nonanone and heptanone). 95% confidence limits from the jackknife method are barely discernable about the spectral plots. Spectra are from data normalized by the SD of a preexperiment quiet period, so units are dimensionless. D: nonoverlapping blocks of 10 successive trials (both odors combined) show a fast rise in beta power at the beginning of each session. Data are from 1 session, fine ketone discrimination, but the rise is repeated across subjects and odor sets. The subsequent decrease in beta power in this example is not repeated in all rats and odor sets. The solid line signifies blocks that are elevated relative to the first block. The dashed line indicates blocks that are decreased relative to the blocks 3 and 4.
Coherence spectra were computed from the same time windows as the power and also used multitaper methods. Because coherence on single trials can produce values very close to 1, we transformed coherence using the Fisher z-transform as we have previously (Kay and Freeman 1998), with z-coherence = arctanh(coherence). This produces values from zero to infinity, instead of zero to one. We do not divide the z-coherence (Zcoh) values by the prestimulus windows because coherence is already normalized by power, and values can be directly averaged across subjects. Time-frequency coherence plots were computed using 0.5 s windows stepped by 10 ms beginning 0.75 s before the nosepoke.
To analyze whether the level of beta power or coherence was related to increases in gamma power, we estimated correlation coefficients between beta power or coherence and OB gamma power over all the trials within a session.
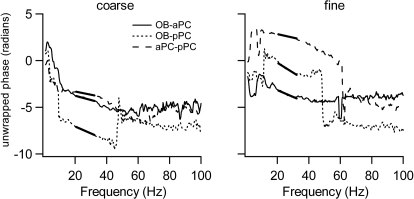
Phase analysis was used to calculate estimated delays of the coherent signal between pairs of brain regions. We used the slope of the unwrapped phase spectrum, as we have previously (Kay and Freeman 1998). The phase spectrum is obtained from the cross spectrum by φ = arctan(Im/Re), where Im and Re are the imaginary and real parts of the cross spectrum. Unwrapping the phase means that the phase is not reset to zero as it crosses 2π. Instead, sudden changes in phase across the spectrum are unwrapped by adding or subtracting 2π. The unwrapped spectrum allows us to estimate delays between regions within various frequency bands. If a highly coherent wide frequency band event occurs at a constant delay from one region to another, that constant delay will translate to a linear phase spectrum over the coherent band. For example, a delay of 5 ms from the OB to the PC will translate to a 0.2π phase difference at 20 Hz, 0.3π at 30 Hz, and 0.4π at 40 Hz (slope of 0.2π/20 Hz). Thus if the phase spectrum has a linear region over a wide band highly coherent region, like the beta oscillation band, we may assume this is created by a constant delay from one region to the other. The advantage of this method is that it is insensitive to changes in polarity of a signal, but it requires that the coherent event be spread over many adjacent frequencies on average over the session. (When the polarity of one of the signals is flipped, the slope of the unwrapped spectrum stays the same but it is translated to the plane on the opposite side of the abscissa.)
We used the 20–32 Hz region of the beta band to be sure that the coherence in that range was robust enough to give a reliable phase estimate. The slope of the linear part of the unwrapped spectrum was then converted to an estimated delay in seconds by delay = slope/2π with phase measured in radians and frequency in cycles/s. A negative slope indicates that the first structure leads the second.
Because the distributions of coherence and power values did not correspond to a normal distribution, we used nonparametric methods to compute ANOVAs, post hoc comparisons, and one sample tests against baseline values [Kruskal-Wallis, Kolmogorov-Smirnov (K-S test), the Wilcoxon signed-rank test, and the 1 sample sign test, as described in the text, with alpha = 0.05]. Because we had approximately equal numbers of trials for each rat within sessions (∼175–200 trials per session, 50% for each of the 2 odors), and beta power did not show specific longitudinal changes within sessions, statistics were performed using trials as independent measures within conditions. We compared the first and final sessions for each discrimination pair because the rats took different numbers of sessions to reach criterion performance.
RESULTS
LFPs from the OB, aPC, and pPC were collected during 2AC odor discrimination behavior from four rats. We had previously described increases in OB gamma oscillation power associated with learned discrimination of high overlap odorant pairs (Beshel et al. 2007). While we had noted a general increase in beta oscillation power, we had not characterized beta oscillations except to determine that OB beta power was not related to odor discrimination learning as has been reported for some GNG tasks (Martin et al. 2004b, 2007). To characterize oscillatory activity in the beta frequency band in this study, we analyzed these signals for beta band power, coherence, and phase. We assessed influences on these measures by behavioral condition: discrimination type (coarse or fine), odorant class (alcohol or ketone), and individual odorant (A or B within each discrimination pair in Table 1). Analysis of spectral power provided information on the relative strength of beta oscillations within each region and condition, coherence gave information on whether covariance of the signals was modulated by behavioral factors, and phase analysis showed in which of the three recorded brain regions activity seemed to originate and which path it took through the circuit. Our aim was to determine to what extent the robust beta oscillatory network seen in other types of olfactory discrimination tasks was present in this 2AC task, even though beta oscillations did not dominate the OB LFP signal (Beshel et al. 2007).
We report in the following two subsections statistical analysis of the trials with correct performance for the first and final sessions for each odor discrimination pair. Differences between correct and incorrect trials are significant and are included after these results.
Beta oscillation power
Beta oscillations are typically defined in the 15–35 Hz band with average peak frequencies of 18–25 Hz in the olfactory system (Lowry and Kay 2007; Martin et al. 2004b). We tested whether beta band power in any of the three regions studied was modified by any of our task specifics (discrimination type- fine or coarse; odor class, alcohol or ketone; odor, A or B within each pair; see Table 1 for odors).
Raw data from all three brain regions are shown in Fig. 1A. Gamma oscillations, which we previously reported to increase during fine odor discrimination, are evident in the OB signal. Relatively irregular beta oscillations can be observed in the aPC and pPC. However, these beta oscillations are of lower amplitude than those typically observed in our lab during GNG tasks (Fig. 1B; unpublished data from D. Frederick and L. M. Kay). Power spectra for all rats and all odor sets show a significant increase in beta power in the olfactory system during odor sniffing (final criterion sessions for 1 rat are illustrated in Fig. 1C). Beta power did not rise with performance on this 2AC odor discrimination task, as it did in other reports using GNG tasks (Martin et al. 2004b, 2007) but instead rose at the beginning of each session within the first few trials, sometimes falling to lower levels later in the session (Fig. 1D).
Beta band power varied across session (1st vs. final), brain region, discrimination type (fine vs. coarse), odor class (alcohol vs. ketone), and odor within a discrimination pair.
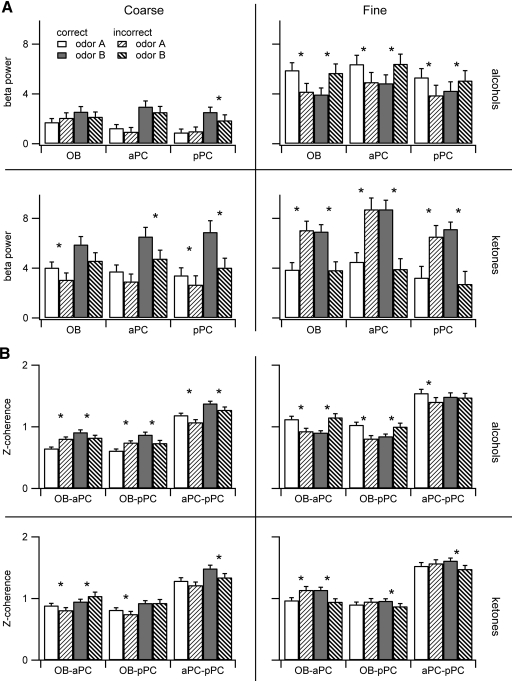
In the first session, beta power ratio values varied across brain region, discrimination type, odor class, and individual odor (Kruskal-Wallis test for each region across the 8 conditions, P < 0.0001; Fig. 2A). Every odor and brain region showed significantly higher power during odor sniffing than during the prestimulus 1.5 s (1 sample sign test of Log10 power ratio value against 0; P < 0.0001 for all bars in Fig. 2A). Beta power was higher for fine than coarse discrimination in all three olfactory areas in the first session for both alcohols and ketones (fine vs. coarse, K-S test, OB ketones P = 0.02; all other comparisons P < 0.0001).
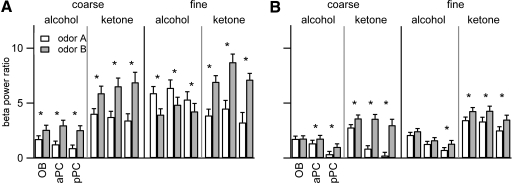
Fig. 2.
Elevation of beta power during odor sniffing is quantified by the beta power ratio [10*Log10(beta power during odor sniffing/beta power prior to odor sniffing) in decibels]. A: power ratio for each odorant averaged across rats and trials from the 1st sessions for each odor pair. Error bars show 95% confidence intervals; 0 indicates no change from the prestimulus period, and positive values indicate increases (no values were negative). B: power ratios from the final sessions. All values are significantly different from 0; *, significant differences in the power ratio between the 2 odorants in a discrimination pair (Kolmogorov-Smirnov test, see text for details). Only trials in which the rats responded correctly are included in this figure (see Fig. 5 for a comparison of correct and incorrect trials). See Table 1 for the identities of odors A and B for each discrimination pair.
Beta power has been shown to vary dependent on odorant identity and volatility absent any associative learning (Lowry and Kay 2007). We therefore expected that the magnitude of beta power would be affected by the specific odorants in a discrimination pair, with beta oscillation power largest for odorants in the 1–120 mmHg theoretical vapor pressure (VP) range as we had found in this earlier study. We saw here that beta power was different between the two odors of a discrimination pair for every odor pair in each of the three brain regions (K-S test, P < 0.006 for all comparisons). However, the relative magnitudes did not map onto the relationships seen during passive presentations of odorants. First, all odorants enhanced beta oscillations over the prestimulus background, which was not the case in the previous study (Lowry and Kay 2007). Second, increased beta oscillations for fine discrimination odors contradict the passive odor stimulation results. Fine alcohols hexanol and heptanol have theoretical VP values <1 mmHg, whereas propanol (26.3 mmHg) is squarely within the 1–120 mmHg range. Finally, the odorants in the current study were diluted and delivered in an airstream, decreasing airborne concentration significantly, while those in the passive exposure study were delivered in pure liquid form on a saturated cotton swab placed under the rat's nose.
In the final session, beta power ratio values were lower than in the first session (K-S test for each brain region individually, final vs. 1st session, P < 0.0001; Fig. 2B). When broken down by odor, the beta power decrease for propanol was not significant for the OB and aPC (P > 0.3). For all other odors, beta power decreased significantly from the first session to the last within each of the three brain regions (P < 0.0001).
As in the first session, in the final session there were significant differences in beta power ratio values within each brain region across odors (Kruskal-Wallis test for each region across the 8 conditions, P < 0.0001; Fig. 2B). Beta power ratio values for each brain region and odorant were significantly above zero, meaning that beta power in the odor sniffing period was greater than in the prestimulus period (1 sample sign test, P < 0.0001 for all bars in Fig. 2B). Fine discrimination showed larger power than coarse, but there were some differences across brain regions and odor classes. In the OB and pPC fine discrimination odors produced higher beta power than coarse for both alcohols (OB: P = 0.001; pPC: P = 0.05) and ketones (P < 0.0001). In the aPC, this was not significant for alcohols (P = 0.08) but was significant for ketones (P < 0.0001).
Differences in beta power between the two odors in a discrimination pair were similar to the first session patterns (Fig. 2B, asterisks), but due to the general decrease in beta power, many comparisons were no longer significant. In the OB, beta power differed between the two odors only for ketone pairs (K-S test, P < 0.002). In the aPC, all odor pairs except fine alcohols showed significant differences between the two odors (K-S tests: coarse alcohols, P = 0.04; coarse ketones, P < 0.0001; fine alcohols, P = 0.11; fine ketones, P = 0.02). In the pPC, all odor pairs showed significant differences between the two odors in the final session (P < 0.006 for all comparisons).
In summary, beta band power decreased from the first to the final session for each discrimination pair with some differences across odors and brain regions. Within each brain region and discrimination type, alcohols showed lower power than ketones, consistent with their lower volatility (see discussion). In both the first and last sessions, beta power was higher for fine than coarse discrimination, but the effect was decreased in the final session where odor class (ketones > alcohols) was a better determinant of increased beta power (Fig. 2B). Differences in beta power between the two odors in a discrimination pair were significant for all odor pairs in the first session. In the final session, most significant differences were confined to the fine and coarse ketone pairs.
Coherence
Coherence measures the cooperative activity (covariance) within pairs of structures. To analyze and compare magnitudes across pairs of brain regions and odor discrimination conditions, we transformed coherence using Fisher's z-transform (Zcoh; see methods). We assessed changes in the magnitude of Zcoh for all pairs (OB-aPC, OB-pPC, and aPC-pPC) and whether these changes might be modified by discrimination type or odor identity.
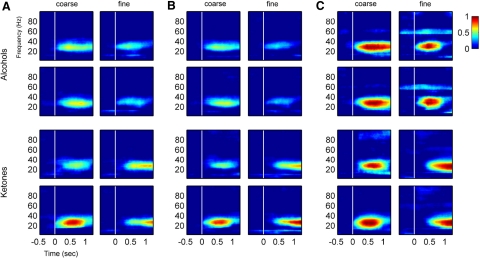
Time-frequency plots show that the beta band is the only consistently coherent band in this set of structures during odor sniffing (Fig. 3). There is obvious temporal structure to the coherence patterns during the odor sniffing period specific to each odorant across sessions within subjects, but the patterns are not consistent across subjects.
Fig. 3.
Time-frequency coherence plots from the final sessions for each odor pair (data shown are from 1 rat, averages from ∼100 trials for each odorant). Zero on the time axis is the time of the nosepoke trigger of the odor (vertical white line); the odor takes 200–300 ms to reach the odor port. For each plot, 0 on the color scale indicates the upper 95% confidence value from the matched prestimulus z-coherence (Zcoh) for each frequency from the time period 1.5–0.5 s before the nosepoke. A: OB-aPC Zcoh; alcohols and ketones, fine and coarse discrimination are marked. B: OB-pPC Zcoh. C: aPC-pPC Zcoh. Note the difference in temporal patterns in Zcoh for different odorant pairs.
Beta band Zcoh was elevated in both the first and final sessions across all pairs of structures (OB-aPC, OB-pPC, aPC-pPC) as compared with baseline levels before the odor sniffing period for each odor type and class (Wilcoxon signed-rank test for each odor and pair of structures, P < 0.0001; Fig. 4). All Zcoh values (prestimulus, odor sniffing, first session, and final session for each odor individually) were significantly above values from mismatched trials' Zcoh estimates (1 sample sign test for each against the upper 95% confidence value from shuffled data = 0.483; P < 0.0001 for all comparisons).
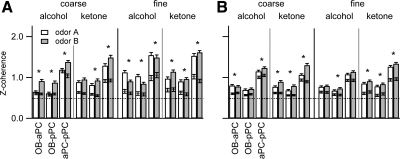
Fig. 4.
Beta band (15–35 Hz) coherence averaged across all rats for each pair of olfactory areas and each odor discrimination pair, correct trials only. A: 1st session averages across all rats and trials; B: last session averages. All mean values during odor sniffing are significantly above the mean for the prestimulus period (black horizontal line with error bars on each bar). The largest coherence values both before and during odor sniffing occur between the 2 pyriform areas (aPC-pPC). All prestimulus and odor sniffing coherence averages are significantly above the value from resampled data (dashed horizontal, upper 95% confidence level from mismatched trials' Zcoh). Asterisks indicate significant differences in Zcoh between the 2 odors in a pair (K-S test; see text for details on specific comparisons). See Table 1 for the identities of odors A and B for each discrimination pair.
In the first session, for each odor pair, Zcoh varied significantly across pairs of brain regions, discrimination type, odor class, and individual odor (Kruskal-Wallis test for each pair of regions across the 8 conditions, P < 0.0001; Fig. 4A). Zcoh varied between fine and coarse discriminations in all three pairs of brain regions for both alcohols and ketones with fine odor discrimination showing higher Zcoh than coarse (K-S test, P < 0.0001). Zcoh varied across individual odors within a discrimination pair for all brain region pairs (K-S test, odor A vs. odor B, P < 0.02, most P < 0.0001) except OB-aPC coarse ketones (P = 0.10).
In the final session, Zcoh values dropped significantly relative to the first session for all pairs of brain regions and odors except propanol (K-S test, 1st vs. final session, P < 0.0005). aPC-pPC Zcoh was not lower in the final session for propanol (propanol, P = 0.13).
Analysis of the final session alone showed that Zcoh varied significantly across pairs of brain regions, discrimination type, odor class, and odor (Kruskal-Wallis test for each pair of regions across the 8 conditions, P < 0.0001; Fig. 4B). Zcoh varied between fine and coarse discrimination in all three pairs of brain regions (K-S test, P < 0.009). This was true for both ketones and alcohols in the aPC-pPC Zcoh values (K-S test, P < 0.0001), but only for ketones in the OB-aPC and OB-pPC values (P > 0.4 for alcohols, P < 0.0001 for ketones). There were significant differences in Zcoh between the odors within discrimination pairs. For OB-aPC Zcoh, there was a difference between the two odors for all odor pairs except fine alcohols (K-S test; P < 0.04, fine alcohols, P = 0.23). For OB-pPC, Zcoh was different between the two odors for all discrimination pairs (K-S test; P < 0.004). For aPC-pPC, Zcoh was different between the two odors for all discrimination pairs except fine alcohols (K-S test, P < 0.03, P = 0.13 for fine alcohols).
In summary, Zcoh showed differences between fine and coarse discrimination, across pairs of olfactory regions and between first and final sessions for a given odor discrimination pair. In the first session, Zcoh was higher for fine than coarse discrimination, and there were smaller relative differences between odor pairs than for beta power. This is due to the insensitivity of the coherence measure to power differences. Overall, the two pyriform areas (aPC-pPC) showed higher coherence than either area with the OB. Zcoh decreased in the final sessions for most odors, but patterns were very similar to the first session (Fig. 4).
Comparison of correct and incorrect trials
The differences between correct and incorrect trials in the first session for each odor discrimination pair showed unique patterns for fine versus coarse discrimination beta power. Comparison of beta power ratios for coarse discrimination pairs showed that incorrect trials had on average lower beta power than correct trials, but this was driven primarily by significant differences for the coarse ketones (K-S test, coarse odor pairs, P < 0.02 for OB, aPC, and pPC; coarse alcohols, P > 0.1; coarse ketones, P < 0.0009; Fig. 5A, left).
Fig. 5.
Comparisons between correct and incorrect trials. Means from the first session for each odor discrimination pair are shown. A: beta power ratio for labeled odor discrimination pairs. Solid bars signify correct trials for each olfactory region, and hatched bars signify incorrect trials. Note that for significant differences in coarse discrimination incorrect trial means have lower power than correct trial means. For significant differences in fine discrimination incorrect trials produce power ratio means that are the same as the means from correct trials for the other odor in the discrimination pair. B: Zcoh correct vs. incorrect trial comparisons of means from the same sessions as in A. Differences appear smaller than for power but the same general patterns are observed. See Table 1 for the identities of odors A and B for each discrimination pair.
Fine odor discrimination pairs in the first session for each odor pair showed a different pattern from coarse discrimination. Incorrect response trials showed beta oscillation power that was significantly different from the correct response trials for that odor but was similar to the correct responses for the other odor in the pair (Fig. 5A, right). Ketones and alcohols showed the same effect, but correct odor B and incorrect odor A had higher power for alcohols, and the opposite for ketones. In the second and final sessions, the differences between correct and incorrect trials were far smaller and the variance for incorrect trials was larger, due to the smaller number of such trials (see Supplementary Fig. S11 for a comparison of 1st, 2nd, and final sessions for each odor discrimination pair).
There was also significant modulation of Zcoh in the first session depending on whether the rats' responses were correct or incorrect, and these patterns generally reflected those seen in the power profile in the first session (Fig. 5B). For OB-aPC, every odor showed a significant difference in Zcoh when comparing correct versus incorrect trials (P < 0.007). For OB-pPC Zcoh, every odor except coarse ketone B and fine ketone A showed significant differences (P < 0.013). For aPC-pPC, five of eight odors showed differences in Zcoh between correct and incorrect trials (P < 0.003). As with beta power, coherence measures were more homogeneous in the final session, but there were some significant differences between correct and incorrect trials. In the final sessions OB-aPC Zcoh showed significant differences for coarse alcohol A (P = 0.001) and coarse ketone B (P < 0.0001). OB-pPC Zcoh showed significant differences for coarse ketone B (P < 0.0001). aPC-pPC Zcoh showed significant differences for fine ketone B (P = 0.0006).
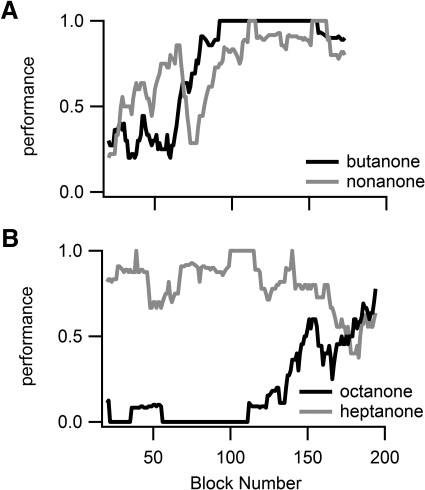
The differences in power across fine and coarse discrimination reflect a qualitative difference in behavioral strategy between the two in the first session for each odor pair. In coarse discrimination, rats were likely to alternate favoring one lever over another or to improve performance with each odor simultaneously or successively (Fig. 6A; only 2 sessions of the 8 first coarse discrimination sessions across 4 rats and 2 odor sets showed strong favoring of a single response lever). In fine discrimination, rats were more likely to favor a single lever (Fig. 6B; 6 of 8 1st fine discrimination sessions showed rats strongly favoring 1 lever over the other). The rats were evenly divided whether they favored one lever or the other in the first session.
Fig. 6.
Examples of behavioral strategies in the 1st session for 2 odor pairs in one rat. Figures track performance statistics for each odor in the pair in 20 trial blocks, stepped by 1 trial. A: during coarse ketone discrimination the rat increased performance levels for both odors during the session (1st of 3 butanone-nonanone sessions). B: during fine ketone discrimination, the rat pressed the heptanone lever for most trials.
In summary, the largest differences in correct versus incorrect trials occurred in the first session. In this session, coarse discrimination showed larger power for correct than incorrect trials. Correct fine discrimination trials for one odor showed the same beta power as incorrect trials for the other (Fig. 5A). Coherence profiles (Fig. 5B) resembled those seen for power with somewhat reduced differences. Differences between correct and incorrect trials were reduced beginning with the second session for each odor (Fig. S1).
Phase
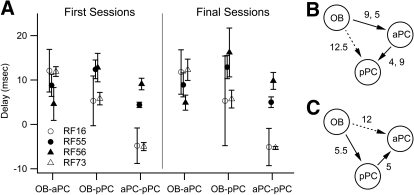
Phase differences were estimated from the real and imaginary parts of the cross spectrum, and the slope of the phase spectrum was used to estimate the time delay from one signal to the other. This estimate is particularly powerful when coherence is high and covers many frequencies, because a time delay translates to linear phase difference increases or decreases across neighboring frequencies (Fig. 7). These analyses yielded a picture in which the OB drove beta band activity in the other structures. Averaging all rats' delays, the delay from the OB to the aPC was 9.4 ± 4.2 ms; the delay from OB to the pPC was 9.5 ± 6.0 ms; the delay from the aPC to the pPC was 1.0 ± 6.7 ms. When examined more closely, two patterns emerged (Table 2 and Fig. 8). In all rats, the OB led both pyriform areas. In two rats (RF55 and RF56) the OB led the aPC and the aPC led the pPC, and the delay from the OB to the pPC was approximately the sum of the OB to aPC and aPC to pPC delays (average differences between these 2 measures were 0.8–1.5 ms). In the other two rats (RF16 and RF73), the OB led the pPC, which led the aPC, and the delay from the OB to the aPC was approximately the sum of the other two delays (average differences were 1.2–2.0 ms).
Fig. 7.
Unwrapped phase spectra from 1 rat for coarse (left) and fine (right) alcohols (octanol and hexanol, respectively). The dark line fit to the 20–32 Hz portion of the spectrum is used for delay estimates for the beta frequency band. Negative slope indicates that the 1st structure leads the second.
Table 2.
Delays in ms for each pair of structures for each rat averaged across all first and final sessions for that rat
| First Sessions |
Final Sessions |
|||||
|---|---|---|---|---|---|---|
| Rat | OB to aPC | OB to pPC | aPC to pPC | OB to aPC | OB to pPC | aPC to pPC |
| RF16 | 12.1 ± 4.8 | 5.3 ± 5.6 | −4.8 ± 4.0 | 11.8 ± 5.0 | 5.3 ± 10.1 | −5.1 ± 4.2 |
| RF55 | 8.8 ± 2.5 | 12.4 ± 2.1 | 4.4 ± 0.6 | 8.9 ± 2.7 | 12.9 ± 2.6 | 5.0 ± 1.2 |
| RF56 | 4.6 ± 3.7 | 12.8 ± 3.2 | 9.1 ± 1.3 | 4.9 ± 1.7 | 16.2 ± 5.5 | 9.8 ± 1.9 |
| RF73 | 11.9 ± 1.1 | 5.8 ± 1.4 | −5.0 ± 0.9 | 12.3 ± 2.4 | 5.7 ± 2.0 | −5.3 ± 0.3 |
Negative values indicate that the second structure in the pair leads the first. Values are means ± SD. OB, olfactory bulb; aPC and pPC, anterior and posterior pyriform cortex, respectively.
Fig. 8.
Summary of beta band delays between brain regions from the 4 rats. A: within rats, delays across odors and odor sets were very similar for each rat (see Table 3 for details). The values shown here are the averages and SDs of the delays computed across the 4 odor discrimination sessions (1st or final session for each odor pair) for each pair of brain regions. Note the low variance in delays between regions for 3 of the 4 rats. Positive values indicate that the 1st region in the pair leads the 2nd, and negative that the 2nd leads the 1st. B: delay circuit for rats RF55 and RF56. The 2 rats showed different delay times between OB-aPC and aPC-pPC (the 2 values from the 2 rats are indicated) but similar delay times for OB-pPC. The dashed arrow indicates the delay that is close to the sum of the other 2 delays. C: delay circuit for rats RF16 and RF73. The 2 rats showed similar delays for all 3 pairs of regions, and the sum of the delays from OB to pPC and pPC to aPC is approximately equal to the delay from OB to aPC (dashed arrow).
Relationship between gamma and beta oscillations
We previously reported a significant increase in gamma oscillation power during criterion performance of fine odor discrimination. We now address the relationship between beta and gamma oscillations within and across olfactory areas. If the beta oscillation network is a default network that must be overcome to perform either a fine odor discrimination or to perform the 2AC task, then we expect that as gamma oscillations increase, beta oscillation power or coherence should decrease. If beta oscillations support the increase in OB gamma power, then we expect that beta and gamma should increase together. We therefore computed correlation coefficients comparing gamma power in the OB to beta power in each of the three areas and to coherence magnitude of each of the three pairs of areas within this set of olfactory structures.
Many correlations were significant, and some of these supported the hypothesis that beta oscillation power or coherence decreased as gamma power increased. However, there were just as many that supported the opposite hypothesis. Within rats, across sessions and odor sets, both positive and negative correlations were seen. In summary, there was no consistent relationship between OB gamma power and OB, aPC, or pPC beta power, and there was no consistent relationship between OB gamma power and OB-aPC, OB-pPC, or aPC-pPC beta oscillation coherence.
DISCUSSION
There are conflicting data regarding the frequencies most strongly involved in odor processing in rats performing odor discriminations. Gamma oscillations have long been considered the functional substrate for sensory processing within the mammalian olfactory bulb (Beshel et al. 2007; David et al. 2009; Eeckman and Freeman 1990; Gray and Skinner 1988; Nusser et al. 2001; Rojas-Líbano and Kay 2008; Stopfer et al. 1997). However, some recent studies have challenged this view, arguing that beta oscillations may instead be the relevant frequency for information processing (Fuentes et al. 2008; Martin et al. 2004b, 2007). Beta and gamma oscillations are not simply different frequencies but also show some opposing effects in the olfactory network. Gamma oscillations are increased when centrifugal input to the OB is removed (Gray and Skinner 1988; Martin et al. 2004a, 2006), but beta oscillations are decreased (Martin et al. 2004a, 2006). We speculated in another report (Kay et al. 2009) that the gamma/beta oscillation difference may be driven by the operant task structure, with gamma oscillations dominating in a 2AC task and beta oscillations dominating in a GNG task in our laboratory (Beshel et al. 2007; Martin et al. 2007).
We do not directly address the possible role of tasks differences here, but we address whether beta and gamma oscillations really occur in this dichotomous fashion. The main result of the present study is that beta oscillations are robust within the olfactory network even in this 2AC task, when gamma, not beta, oscillations dominate the OB LFP signal. We showed that oscillations in the 15–35 Hz range were significantly above baseline levels in all three areas studied (OB, aPC, and pPC), and this effect was strongest in the first session and during fine odor discrimination (Figs. 1 and 2). These oscillations showed significant pairwise coherence among all three pairs of structures during prestimulus as well as odor sniffing periods with coherence during odor sniffing stronger than during prestimulus periods and coherence between the two pyriform areas stronger than between the OB and either pyriform area (Figs. 3 and 4). Increases in beta activity did not correlate with the onset of criterion performance as has been shown in studies employing GNG tasks, but the beta frequency band was the only band that was consistently more coherent than prestimulus levels among all pairs of structures in this 2AC task (Fig. 3). Thus, even when gamma oscillation power is strong as in fine odor discrimination (Fig. 1A) beta oscillations may serve to link different olfactory areas.
Analysis of correct versus incorrect trials showed strong differences in the first session for each odor discrimination pair that decreased by the final sessions. One striking result is a qualitative difference between coarse and fine discrimination (Fig. 5). In the first sessions, coarse discrimination errors resulted in overall lower power beta oscillations than for correct trials, although this was more evident for ketones than alcohols. In fine discrimination, the results showed instead that beta power during error trials for each odor was the same as beta power during correct trials for the other odor in the discrimination pair. There was a parallel qualitative difference in behavioral strategy in the first session. In coarse discrimination, the rats appeared to respond to each of the odors individually as if they were learning two separate odors simultaneously (Fig. 6A). In the first sessions for fine discrimination, the rats overwhelmingly responded to a single odorant by pressing a single lever on each trial for long time periods (Fig. 6B). This behavior resulted in near perfect performance for one odor and near zero performance for the other.
These results suggest that beta oscillations may be associated with something besides simple sensory processing. The results are consistent with expectation of reward or outcome. If in fine discrimination the rats cannot discriminate the two odors in the first session, then pressing one lever would be accompanied by the expectation of reward (e.g., the right lever, which is correct for odor A and incorrect for odor B), while pressing the other lever would result in the expectation of no reward in response to what is perceived as the same odor (e.g., the left lever, which is correct for odor B and incorrect for odor A). Mapping this onto the coarse discrimination sessions, if the rats know both odors and at some level which lever is correct for each, then this would be accompanied by higher amplitude beta/expectation of reward for correct responses, but wrong responses (for whatever reason) would result in the expectation of no reward. The final story from future studies is unlikely to be as simple as this because the rats were evenly divided on which lever they favored, and the beta power pattern was opposite for the two fine odor sets. Other interpretations are possible, such as that in fine discrimination the rats simply mistake one odor for the other on the incorrect trials, but this does not map well onto the results for coarse discrimination.
Physico-chemical interactions with beta power
Power differences between and across odorant pairs appear to be affected by the odorants themselves. We showed previously that odorants with volatilities in the 1–120 mmHg range selectively enhance beta oscillations over prestimulus levels on repeated presentation without any behavioral conditioning (Lowry and Kay 2007), and some of our odorants fall within this range (propanol, octanone, heptanone, and butanone). Because increased beta power associated with odor sampling was observed for all of the odorants used here, and not only those that are highly volatile, there is likely a complex interaction between physicochemical odorant properties and central processes associated with odor learning and discrimination. This is the object of future studies.
Network properties associated with phase
Beta oscillations in the GNG task have been shown to be a network phenomenon; disrupting feedback to the OB in the GNG task decreases beta oscillations (Martin et al. 2006). However, even in a reciprocally connected set of oscillators, one node may entrain another, showing up as a phase lead for that node. One node can also drive another but still require input from elsewhere to maintain the correct dynamic range for oscillatory activity (Freeman 1968). We therefore examined whether there is a consistent phase gradient for beta oscillations to determine whether a single region might lead the system in this frequency band. Our phase analysis suggests that the OB may either drive or entrain beta activity in the aPC and pPC; in two animals the aPC led the pPC and in two the pPC led the aPC (Figs. 7 and 8; Table 2). This difference in phase pattern across rats is almost certainly due to differences in electrode placements relative to the lateral olfactory tract because signals can slow considerably as they travel along association fibers within the aPC and pPC. We have recently shown that beta oscillations in the hippocampus that arise in the process of odor discrimination learning in a GNG task are led by OB beta activity (Gourévitch et al. 2010). Thus the results from these two studies suggest that the source, or at least the dominant force, for entraining beta oscillations in this system is the OB.
What are beta oscillations?
We still know very little about what beta oscillations represent and what the mechanisms are that support them (Kay et al. 2009). We do not know the cellular substrates for beta oscillations in the olfactory system. We also do not know from which synaptic layers these oscillations emerge in any of the olfactory cortical areas, although one study in urethan-anesthetized rats suggests that in the OB they may have the same source as gamma oscillations (Neville and Haberly 2003). Plasticity may occur at several points in this circuit including at the point of feedback to the OB granule cell layer (Gao and Strowbridge 2009; Patneau and Stripling 1992; Stripling and Patneau 1999), within the PC association fiber network connected with olfactory rule learning (Lebel et al. 2001), at OB to PC synapses associated with odor learning (Best and Wilson 2004), and among connections with distant and higher order areas, such as the orbitofrontal cortex (Cohen et al. 2008). Each of these points of plasticity affects network connections, which may support beta oscillations.
We may have more insight into cognitive than cellular features of beta oscillations. The differences between correct and incorrect odor discrimination responses that we report here suggest that these oscillations may represent processes associated with recognition, response selection or reward expectation. These possibilities point to beta oscillations' possible role in binding across cortical areas.
Conclusion
There is a long held intellectual tradition that associates gamma oscillations with local sensory processing in the OB (reviewed in Rojas-Líbano and Kay 2008). Beta oscillations have recently been proposed to be a substrate for pattern discrimination in olfactory areas (Martin et al. 2004b). The results presented here suggest that beta oscillations in olfactory cortical areas may be well suited to information transfer and systemwide coupling, even in a behavioral context in which local gamma oscillations may be enhanced.
From the current study and others, a picture is emerging in which beta oscillations involve multiple cortical areas, supporting precise coupling over relatively long distances. How sensitive cortical areas are to this type of spatial and temporal structure in information flow and whether local neurons use this frequency for binding cell assemblies are questions that should be addressed by future studies.
GRANTS
Funding was provided by National Institute of Deafness and Other Communication Disorder Grants R01-DC-007995 (CRCNS program) to L. M. Kay and F31DC-008467 to J. Beshel.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank D. Frederick for help with MATLAB programming and data for Fig. 1B and N. Kopell, D. Frederick, and D. Rojas-Líbano for comments on the manuscript.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Beshel J, Kopell N, Kay LM. Olfactory bulb gamma oscillations are enhanced with task demands. J Neurosci 27: 8358–8365, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best AR, Wilson DA. Coordinate synaptic mechanisms contributing to olfactory cortical adaptation. J Neurosci 24: 652–660, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Y, Reuveni I, Barkai E, Maroun M. Olfactory learning-induced long-lasting enhancement of descending and ascending synaptic transmission to the piriform cortex. J Neurosci 28: 6664–6669, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David FO, Hugues E, Cenier T, Fourcaud-Trocme N, Buonviso N. Specific entrainment of mitral cells during gamma oscillation in the rat olfactory bulb. PLoS Comput Biol 5: e1000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiber M-P, Missonnier P, Bertrand O, Gold G, Fazio-Costa L, Ibanez V, Giannakopoulos P. Distinction between perceptual and attentional processing in working memory tasks: a study of phase-locked and induced oscillatory brain dynamics. J Cognit Neurosci 19: 158–172, 2007 [DOI] [PubMed] [Google Scholar]
- Eeckman FH, Freeman WJ. Correlations between unit firing and EEG in the rat olfactory system. Brain Res 528: 238–244, 1990 [DOI] [PubMed] [Google Scholar]
- Freeman WJ. Effects of surgical isolation and tetanization on prepyriform cortex in cats. J Neurophysiol 31: 349–357, 1968 [DOI] [PubMed] [Google Scholar]
- Freeman WJ. Mass Action in the Nervous System. New York: Academic, 1975 [Google Scholar]
- Fuentes RA, Aguilar MI, Aylwin ML, Maldonado PE. Neuronal activity of mitral-tufted cells in awake rats during passive and active odorant stimulation. J Neurophysiol 100: 422–430, 2008 [DOI] [PubMed] [Google Scholar]
- Gao Y, Strowbridge BW. Long-term plasticity of excitatory inputs to granule cells in the rat olfactory bulb. Nat Neurosci 12: 731–733, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goense JBM, Logothetis NK. Neurophysiology of the BOLD fMRI signal in awake monkeys. Curr Biol 18: 631–640, 2008 [DOI] [PubMed] [Google Scholar]
- Gourévitch B, Kay LM, Martin C. Directional coupling from the olfactory bulb to the hippocampus during a go/no-go odor discrimination task. J Neurophysiol 103: 2633–2641, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CM, Skinner JE. Centrifugal regulation of neuronal activity in the olfactory bulb of the waking rabbit as revealed by reversible cryogenic blockade. Exp Brain Res 69: 378–386, 1988 [DOI] [PubMed] [Google Scholar]
- Grossman SE, Fontanini A, Wieskopf JS, Katz DB. Learning-related plasticity of temporal coding in simultaneously recorded amygdala-cortical ensembles. J Neurosci 28: 2864–2873, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermer-Vazquez R, Hermer-Vazquez L, Srinivasan S, Chapin J. Beta- and gamma-frequency coupling between olfactory and motor brain regions prior to skilled, olfactory-driven reaching. Exp Brain Res 180: 217–235, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM, Beshel J, Brea J, Martin C, Rojas-Líbano D, Kopell N. Olfactory oscillations: the what, how and what for. Trends Neurosci 32: 207–214, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM, Freeman WJ. Bidirectional processing in the olfactory-limbic axis during olfactory behavior. Behav Neurosci 112: 541–553, 1998 [DOI] [PubMed] [Google Scholar]
- Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci USA 97: 1867–1872, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel D, Grossman Y, Barkai E. Olfactory learning modifies predisposition for long-term potentiation and long-term depression induction in the rat piriform (olfactory) cortex. Cereb Cortex 11: 485–489, 2001 [DOI] [PubMed] [Google Scholar]
- Lowry CA, Kay LM. Chemical factors determine olfactory system beta oscillations in waking rats. J Neurophysiol 98: 394–404, 2007 [DOI] [PubMed] [Google Scholar]
- Martin C, Beshel J, Kay LM. An olfacto-hippocampal network is dynamically involved in odor-discrimination learning. J Neurophysiol 98: 2196–2205, 2007 [DOI] [PubMed] [Google Scholar]
- Martin C, Gervais R, Chabaud P, Messaoudi B, Ravel N. Learning-induced modulation of oscillatory activities in the mammalian olfactory system: the role of the centrifugal fibres. J Physiol 98: 467–478, 2004a [DOI] [PubMed] [Google Scholar]
- Martin C, Gervais R, Hugues E, Messaoudi B, Ravel N. Learning modulation of odor-induced oscillatory responses in the rat olfactory bulb: a correlate of odor recognition? J Neurosci 24: 389–397, 2004b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Gervais R, Messaoudi B, Ravel N. Learning-induced oscillatory activities correlated to odor recognition: a network activity. Eur J Neurosci 23: 1801–1810, 2006 [DOI] [PubMed] [Google Scholar]
- Mitra P, Bokil H. Observed Brain Dynamics. New York: Oxford, 2008 [Google Scholar]
- Neville KR, Haberly LB. Beta and gamma oscillations in the olfactory system of the urethan-anesthetized rat. J Neurophysiol 90: 3921–3930, 2003 [DOI] [PubMed] [Google Scholar]
- Nusser Z, Kay LM, Laurent G, Homanics GE, Mody I. Disruption of GABA(A) receptors on GABAergic interneurons leads to increased oscillatory power in the olfactory bulb network. J Neurophysiol 86: 2823–2833, 2001 [DOI] [PubMed] [Google Scholar]
- Patneau DK, Stripling JS. Functional correlates of selective long-term potentiation in the olfactory cortex and olfactory-bulb. Brain Res 585: 219–228, 1992 [DOI] [PubMed] [Google Scholar]
- Plailly J, Howard JD, Gitelman DR, Gottfried JA. Attention to odor modulates thalamocortical connectivity in the human brain. J Neurosci 28: 5257–5267, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo C, Isaacson JS. Odor representations in olfactory cortex: “sparse” coding, global inhibition, and oscillations. Neuron 62: 850–861, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Líbano D, Kay LM. Olfactory system gamma oscillations: the physiological dissection of a cognitive neural system. Cognit Neurodynam 2: 179–194, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopfer M, Bhagavan S, Smith BH, Laurent G. Impaired odor discrimination on desynchronization of odour- encoding neural assemblies. Nature 390: 70–74, 1997 [DOI] [PubMed] [Google Scholar]
- Stripling JS, Patneau DK. Potentiation of late components in olfactory bulb and piriform cortex requires activation of cortical association fibers. Brain Res 841: 27–42, 1999 [DOI] [PubMed] [Google Scholar]
- Tkach D, Reimer J, Hatsopoulos NG. Congruent activity during action and action observation in motor cortex. J Neurosci 27: 13241–13250, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk BCM, Daffertshofer A, Roach N, Praamstra P. A role of beta oscillatory synchrony in biasing response competition? Cereb Cortex 19: 1294–1302, 2009 [DOI] [PubMed] [Google Scholar]
- Xing D, Yeh C-I, Shapley RM. Spatial spread of the local field potential and its laminar variation in visual cortex. J Neurosci 29: 11540–11549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen Y, Bressler SL, Ding M. Response preparation and inhibition: the role of the cortical sensorimotor beta rhythm. Neuroscience 156: 238–246, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.