Abstract
Pharmacokinetic and efficacy studies with levofloxacin were performed in the common marmoset (Callithrix jacchus) model of inhalational tularemia. Plasma levofloxacin pharmacokinetics were determined in six animals in separate single-dose and multidose studies. Plasma drug concentrations were analyzed using liquid chromatography-tandem mass spectrometry-electrospray ionization. On day 7 of a twice-daily dosing regimen of 40 mg/kg, the levofloxacin half-life, maximum concentration, and area under the curve in marmoset plasma were 2.3 h, 20.9 μg/ml, and 81.4 μg/liter/h, respectively. An efficacy study was undertaken using eight treated and two untreated control animals. Marmosets were challenged with a mean of 1.5 × 102 CFU of Francisella tularensis by the airborne route. Treated animals were administered 16.5 mg/kg levofloxacin by mouth twice daily, based on the pharmacokinetic parameters, beginning 24 h after challenge. Control animals had a raised core body temperature by 57 h postchallenge and died from infection by day 5. All of the other animals survived, remained afebrile, and lacked overt clinical signs. No bacteria were recovered from the organs of these animals at postmortem after culling at day 24 postchallenge. In conclusion, postexposure prophylaxis with orally administered levofloxacin was efficacious against acute inhalational tularemia in the common marmoset. The marmoset appears to be an appropriate animal model for the evaluation of postexposure therapies.
Francisella tularensis is a Gram-negative intracellular pathogen that is the causative agent of tularemia. The disease is prevalent in many countries in the northern hemisphere, including the United States (8), where the reported number of cases averages 100 to 200 annually (11). The pathogen is infectious by a number of routes, has a low infectious dose, and is readily transmitted by the airborne route and so is included on the CDC list of select agents that have potential to pose a major threat to public health and safety (http://emergency.cdc.gov/agent/agentlist-category.asp).
Currently, the recommended preferred treatment for adults with tularemia is systemic gentamicin or streptomycin or, alternatively, doxycycline or ciprofloxacin for 14 to 21 days (5; http://www.hpa.org.uk/deliberate_accidental_releases/biological). In the case of a mass bioweapon attack, prophylaxis with orally administered ciprofloxacin or doxycycline twice daily is suggested (5; http://www.hpa.org.uk/deliberate_accidental_releases/biological). Fluoroquinolones such as ciprofloxacin and levofloxacin are attractive alternative antibiotics for tularemia, as they have good in vitro activity, have bactericidal effects, and can be administered orally (13). Levofloxacin has a broad spectrum of activity, a good safety record, and an oral bioavailability of over 99% (10), while single daily dosing gives it a further advantage over the more commonly used antibiotic ciprofloxacin. Murine studies indicate that intraperitoneal administration of levofloxacin is 100% effective against intranasal tularemia challenge, even when administration is delayed up to 72 h postchallenge (17), although demonstration of efficacy following inhaled challenge in other models is lacking. Intravenous levofloxacin has been successful in treating human tularemia without relapse (1, 20). Levofloxacin has already proven utility in the biodefense arena and has been approved for the treatment of inhalational anthrax in both adults and children (6, 19), thus reducing the number of antibiotics required in the arsenal.
In order to determine the efficacy of pre- or postexposure therapies, there is a need to develop and characterize animal models of inhalational tularemia. To date, most research on tularemia has focused on developing murine models of infection (4). This has increased the understanding of disease progression and has identified important immunological responses to infection. However, in order to undertake the pivotal studies required for the licensure of any therapy, nonhuman primate models of infection are required. Previous work has demonstrated that the common marmoset (Callithrix jacchus) is susceptible to experimental inhalational tularemia and develops a lethal infection representative of human disease (21).
The purpose of this work was to investigate the use of orally administered levofloxacin as a postexposure therapy for tularemia using the common marmoset. Initially, this involved establishing the pharmacokinetic (PK) parameters in the marmoset and, using this data, testing the efficacy of levofloxacin against inhalational tularemia.
MATERIALS AND METHODS
Animals.
Healthy, sexually mature common marmosets (C. jacchus) were obtained from the Defence Science and Technology Laboratory Porton Down breeding colony and housed in female and vasectomized male pairs. Animals were aged 25 to 64 months and weighed between 310 and 560 g at the time of study. The animals were allowed free access to food and water, as well as environmental enrichment. Prior to use in challenge studies, all animals were surgically implanted intraperitoneally (i.p.), under general anesthesia (ketamine/metetomidine/isofluorane), with a Remo 200 device to record core body temperature (Tc) remotely (Remo Technologies Ltd., Salisbury, United Kingdom). Data were transmitted from the Remo 200 devices at 30-s intervals to locally placed antennas and relayed to receivers. Data were analyzed using the eDacq software to provide real-time and recordable Tc. All animals had Actiwatch-Mini devices (Cambridge Neurotechnology Ltd., United Kingdom) attached to their collars to monitor activity. All animal studies were carried out in accordance with the United Kingdom Animals (Scientific Procedures) Act of 1986 and the Codes of Practice for the Housing and Care of Animals Used in Scientific Procedures 1989. Following challenge with F. tularensis, all animals were handled under United Kingdom Advisory Committee on Dangerous Pathogens (ACDP) animal containment level 3 conditions (equivalent to CDC/NIH biosafety level 3), within a half-suit isolator compliant with British standard BS5726.
Determination of the bioavailability of levofloxacin in the common marmoset.
Two studies were performed, a single-dose study and a multidose study. All blood samples were collected in tubes containing lithium heparin anticoagulant and thoroughly mixed prior to centrifugation for 5 min at 13,000 rpm. Plasma was collected in a labeled, sterile Eppendorf tube and stored at −70°C until analysis.
Single-dose study.
A cohort of six animals was assigned to either group A or group B (three animals per group). Both groups of animals received a single oral dose of levofloxacin of 40 mg/kg in a banana milkshake. The dose was calculated to be equivalent to a human dose on the basis of individual body weight and surface area (16). This assumes an average human body surface area of 1.73 m2 and a standard human levofloxacin dose of 500 mg and uses the following formula: body surface area = 11.7 × weight2/3. Animals in group A were bled at 0.5, 2, and 6 h postdosing, and animals in group B were bled at 1, 4, and 8 h postdosing. This approach was taken due to the limitations on the bleed intervals for individual animals.
Multidose study.
Animals were divided into groups as described above and dosed with 40 mg/kg levofloxacin per dose twice daily in a banana milkshake for 7 consecutive days. Animals in group A were bled on day 1 and day 7 at 0 h (i.e., predosing) and 1, 4, and 8 h postdosing. Animals in group B were bled on day 1 and day 7 at 0.5, 2, 6, and 12 h postdosing.
Quantification of levofloxacin concentrations in marmoset plasma.
The levels of levofloxacin in plasma were determined by a modification of the method described by Wong et al. (22). Working standards of levofloxacin were prepared in water and used to spike control marmoset plasma (50 μl) to produce a calibration curve over a range of 0.1 to 50 μg/ml. Ciprofloxacin was used as an internal standard and was spiked into each of the levofloxacin plasma standards at 10 μg/ml prior to extraction. Phosphate buffer (50 μl) was added to either the standard or marmoset test plasma, which was then extracted with 1 ml dichloromethane. Extracts (500 μl) were evaporated to dryness and reconstituted in 100 μl of deionized water, and 10-μl aliquots were analyzed by liquid chromatography-tandem mass spectrometry-electrospray ionization. Relevant PK parameters were calculated using WinNonlin software (v5.2; Pharsight Corporation).
Bacterial strain and culture.
Glycerol stocks of F. tularensis strain SCHU S4 were obtained from DynPort Vaccine Company LLC (Frederick, MD). Bacteria were recovered from the vial on blood cysteine glucose agar (BCGA) plates and incubated at 37°C for 24 h prior to recovery in phosphate-buffered saline (PBS), pH 7.3. The optical density at 590 nm (OD590) of the suspension was adjusted to 0.1, equivalent to approximately 1 × 108 CFU ml−1, 1 ml of which was used to inoculate 100 ml of modified cysteine partial hydrolysate broth. The broth was shaken at 180 rpm for 48 h at 37°C. Prior to challenge, the OD590 of the culture was adjusted to 0.1 and it was serially diluted in PBS to the appropriate concentration for challenge. Viable counts were performed by subculture on BCGA retrospectively. Plates were incubated at 37°C for 72 h prior to enumeration. All bacteriological procedures were carried out at ACDP CL3 in class 3 microbiological safety cabinets compliant with British standard BS5726.
Challenge.
Marmosets were anesthetized with 25 mg kg−1 ketamine administered intramuscularly prior to exposure and challenged in pairs by the airborne route (18, 21). Briefly, a Collison nebulizer containing 20 ml F. tularensis and 3 drops of Antifoam 289 (Sigma) was used to generate aerosol particles of approximately 1 to 3 μm. The aerosol was conditioned in a modified Henderson apparatus (7). Marmosets were placed in a head-only exposure chamber (plethysmograph tube) and exposed for 10 min to a dynamic aerosol maintained at 50 to 55% relative humidity and 18 to 20°C. The total accumulated tidal volume for each animal during challenge was determined by whole-body real-time plethysmography with a Fleisch pneumotacograph (EMMS, Bordon, Hampshire, United Kingdom). The concentration of the aerosol cloud was quantified after sampling from a sample port into an all-glass impinger (AGI-30) by serial dilution and plating onto BCGA.
Antibiotic dosing schedule.
All of the animals receiving antibiotic were given 16.5 mg/kg levofloxacin twice daily in a banana milkshake for 10 consecutive days. An additional two control animals in each study received the milkshake without antibiotic for the same duration. Animals were monitored for a period of 14 days after antibiotic dosing was stopped. Animals were culled on reaching the humane endpoint or at the scheduled end of the study.
Postmortem.
Postmortem examinations were performed on all of the animals upon culling or death from disease; organs were removed aseptically and assessed for bacteriology. Blood was removed from anesthetized marmosets by cardiac puncture for assessment of bacteremia, clinical chemistry, hematology, and immunology.
Bacteriology.
Bacterial loads in the blood, liver, spleen, kidneys, and lungs were assessed for all of the animals. Weighed organ sections were homogenized in 5 ml PBS using cell sieves and a plunger. The organ homogenates and blood were serially diluted in PBS, and 250-μl aliquots in duplicate appropriate dilutions were subcultured onto BCGA plates, which were incubated at 37°C for 72 h prior to enumeration.
Hematology, clinical chemistry, and electrolytes.
Blood was collected at defined time points in tubes containing EDTA, and key parameters were measured by the use of a laser flow cytometry-based hematological analyzer (LaserCyte; IDEXX Laboratories Ltd., Bucks, United Kingdom), including the total white blood cell count, platelet count, packed cell volume (hematocrit), and hemoglobin. Plasma (lithium-heparin) concentrations of albumin, alkaline phosphatase, total bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatine kinase, glucose, and creatinine were determined using a “dry-slide” technology biochemistry analyzer (VetTest; IDEXX Laboratories Ltd.). An ion-selective electrode-based electrolyte analyzer (VetLyte; IDEXX Laboratories Ltd.) was used to measure K+ and Na+ concentrations. Data collected postmortem were compared to the mean data from the same group of animals analyzed prechallenge (baseline data).
Statistical analysis.
Two-way analysis of variance was used to compare blood parameters and the activity of animals using GraphPad PRISM V5.0.
RESULTS
Bioavailability studies. (i) Single-dose study.
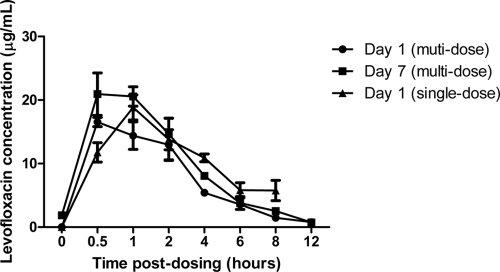
Plasma levofloxacin concentrations with time following a single dose are shown in Fig. 1. The half-life (t1/2) of levofloxacin in marmoset plasma was 3.9 h, the maximum concentration (Cmax) was 18.8 μg/ml, and the area under the curve (AUC) was 112.4 μg/ml/h.
FIG. 1.
Comparison of marmoset plasma levofloxacin concentrations following single-dose and multiple-dose regimens.
(ii) Multidose study.
Plasma levofloxacin concentrations with time following multiple doses are shown in Fig. 1. The levofloxacin t1/2, Cmax, and AUC in marmoset plasma on day 1 were 2.4 h, 16.5 μg/ml, and 63.6 μg/ml/h, respectively, and on day 7 they were 2.3 h, 20.9 μg/ml, and 81.4 μg/ml/h, respectively.
Efficacy studies. (i) Control animals.
The two untreated control animals were exposed to either 1.4 × 102 or 3.9 × 102 CFU of F. tularensis strain SCHU S4 and succumbed to infection on day 4 (male) and day 5 (female). High bacterial numbers were isolated from all of the organs of these animals, reaching approximately 108 CFU g−1 in the spleen, 107 in the lungs, and 106 in the liver. The highest numbers of bacteria were recovered from the spleens of both animals. Bacteremia was also evident in both animals (reaching approximately 106 CFU ml−1). Gross pathology was only evident in the lungs of these animals with areas of hemorrhaging and/or mottled tissue.
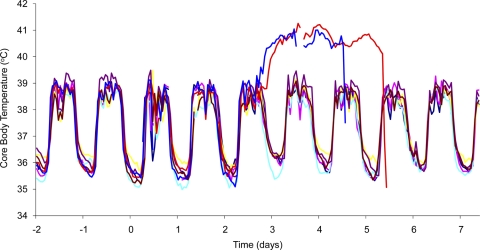
The temperatures of both animals remained within the normal diurnal rhythm until 53 h postchallenge (male) and 57 h (female), when the temperatures increased by >1°C over the baseline (Fig. 2). The temperatures gradually increased and remained at >40°C, decreasing rapidly immediately prior to death.
FIG. 2.
Marmoset temperature profiles after infection with approximately 102 CFU of F. tularensis by the airborne route. Animals 23L (light blue), 103K (pink), 51L (purple), 138K (brown), 22L (green), and 80K (yellow) all received 16.5 mg/kg levofloxacin twice daily for 10 days. Animals 100K (dark blue) and 140K (red) received no antibiotic.
The activity of the control animals decreased significantly, compared to that of treated animals, at day 3 postchallenge, from approximately 95% on day 2 to approximately 13% on day 3 (P < 0.001).
Notable postmortem blood findings included a significantly reduced platelet count (P < 0.05), a significantly increased alkaline phosphatase concentration (P < 0.01), and increased ALT and K+ and reduced glucose concentrations (not statistically significant).
(ii) Antibiotic-treated animals.
Eight test animals were exposed to a mean of 3.8 ×102 ± 77 CFU of F. tularensis strain SCHU S4 and received antibiotics twice daily for 10 days postchallenge, beginning approximately 24 h after challenge. All of the test animals received all of the antibiotic doses at the scheduled times and survived until the end of the study (day 24 postchallenge). The temperature profile of six of the eight test animals is depicted in Fig. 2. All of the test animals maintained their normal diurnal rhythm throughout the study, and no overt clinical signs or behavioral changes were observed in these animals. On postmortem, no gross pathology was observed in any organ of the animals and no bacteria were recovered from any organ. Notable postmortem blood findings included a reduced platelet count from a mean of 460 × 103/μl in two animals and raised creatine kinase levels in all of the animals tested. Glucose, alkaline phosphatase, plasma creatinine, and ALT concentrations were within the normal range in all of the animals (23).
DISCUSSION
The development of appropriate animal models to assess therapies against potential biodefense agents is an essential prerequisite to achieve licensure of products via the U.S. Food and Drug Administration's animal rule. The aim of this study was to investigate the efficacy of levofloxacin as postexposure therapy against F. tularensis in the marmoset model. This involved both investigation of the plasma PK of levofloxacin and efficacy studies.
The PK of levofloxacin was investigated in two studies (single dose and multidose), and the data were compared to the PK parameters of rhesus monkeys and humans (2, 3, 12, 15). These are shown in Table 1.
TABLE 1.
Comparison of PK parameters of levofloxacin in common marmosets, rhesus monkeys, and humans
| Species (dose) | t1/2 (h) | tmax (h) | Cmax (μg/ml) | AUCINF obs (μg/liter/h) | Reference |
|---|---|---|---|---|---|
| Marmoseta (40 mg/kg) | 2.3 | 0.5 | 20.9 | 81.4 | This study |
| Human (500 mg) | 7.6 | 1.1 | 5.7 | 47.5 | 3 |
| Human (500 mg) | 6.4 | 1.5 | 8.5 | 56.3 | 2 |
| Rhesus monkey (15 + 4 mg/kg)c | 3.4b | 2.2b | 5.3b | 33.4b | 15 |
| Rhesus monkey (15 mg/kg) | 2 | 1.5b | 5.1b | 21.3b | 12 |
Day 7 data from the multidose study.
Mean of male and female data.
A 15-mg/kg dose was followed by a 4-mg/kg dose administered 12 h later.
The human dose of levofloxacin is 500 mg/day, achieving a serum Cmax of 6.2 μg/ml and an AUC of 48.3 μg/ml/h. Therefore, in order to match the marmoset dosage to the human dosage in the efficacy study, it was determined that the marmoset dose should be reduced from 40 to 16.5 mg/kg, based on the Cmax. Assuming a linear relationship, this gives a marmoset plasma Cmax of 6.2 μg/ml. This also lowers the predicted AUC (24 μg/ml/h) to below the human target AUC, but dosing at shorter intervals would have been necessary to more accurately match this parameter.
Interestingly, the t1/2 in rhesus monkeys (2 h) and marmosets (2.4 h) is lower than the human value of approximately 7 h, indicating more rapid clearance (Table 1). However, the Cmax and AUC for marmosets are higher than the human values, which in turn are higher than for rhesus monkeys. This may, at least in part, be related to the higher dose used in the marmoset studies (2, 3, 12, 15).
In the current study, the time to death, activity, temperature profile, and bacterial load at postmortem in the nontreated animals were consistent with previously reported data (21). Clinical parameters were investigated in the current study and showed a statistically significant decrease in the platelet counts and an increase in the alkaline phosphatase levels, as well as notable changes in the levels of potassium, ALT, and glucose from the baseline. This is consistent with human tularemia (9, 14). Following a 10-day dosing regimen, there was 100% survival in the levofloxacin-treated group, with no evidence of overt clinical signs. The temperature and activity of these animals remained comparable to the baseline data, and at postmortem, 14 days after the cessation of antibiotic dosing, no bacteria were recovered from any of the organs of the animals assessed. However, slight changes in the clinical parameters were noted, including some mild liver dysfunction and a drop in platelet levels.
These results indicate that orally administered levofloxacin is highly effective in preventing the onset of acute inhalational tularemia. They also suggest that bacterial clearance has been achieved and that relapse of infection following treatment is unlikely. Murine studies indicate that intraperitoneal administration of levofloxacin is 100% effective against intranasal challenge, even when administration is delayed up to 72 h postchallenge (17). Moreover, it has previously been reported that intravenous administration of 500 mg of levofloxacin has been successful in treating human tularemia without relapse (1, 20). As the bioavailability of levofloxacin is similar when it is administered orally or intravenously to humans (3), the marmoset PK and efficacy results can be extrapolated to humans.
In conclusion, orally administered levofloxacin was efficacious as postexposure prophylaxis against acute inhalational tularemia in the common marmoset. In addition, the marmoset appears to be an appropriate animal model for use in the evaluation of postexposure therapies.
Acknowledgments
This project has been funded with federal funds from the National Institute of Allergy and Infectious Diseases under contract N01-AI-30062 through our prime contractor, the Health Protection Agency (HPA), Porton Down, United Kingdom.
We thank Allen Roberts and Kristin DeBord for their support. We thank all Defence Science and Technology Laboratory employees who helped support this work, Victoria Savage for telemetry support, Sarah Stubbs for mass spectrometry analysis, and Ed Gosden for PK analysis. We also thank Donna Hopkins, Graham Hatch, and Robert Fretwell at the HPA for performing the clinical analyses on blood samples.
Footnotes
Published ahead of print on 12 July 2010.
REFERENCES
- 1.Aranda, E. A. 2001. Treatment of tularemia with levofloxacin. Clin. Microbiol. Infect. 7:167-168. [DOI] [PubMed] [Google Scholar]
- 2.Carrasco-Portugal, M. D. C., and F. J. Flores-Murrieta. 2009. Comparative bioavailability of two oral formulations of levofloxacin in healthy Mexican volunteers. Int. J. Clin. Pharmacol. Ther. 47:283-286. [DOI] [PubMed] [Google Scholar]
- 3.Chien, S. C., M. C. Rogge, L. G. Gisclon, C. Curtin, F. Wong, J. Natarajan, R. R. Williams, C. L. Fowler, W. K. Cheung, and A. T. Chow. 1997. Pharmacokinetic profile of levofloxacin following once-daily 500-milligram oral or intravenous doses. Antimicrob. Agents Chemother. 41:2256-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conlan, J. W., W. Chen, H. Shen, A. Webb, and R. KuoLee. 2003. Experimental tularemia in mice challenged by aerosol or intradermally with virulent strains of Francisella tularensis: bacteriologic and histopathologic studies. Microb. Pathog. 34:239-248. [DOI] [PubMed] [Google Scholar]
- 5.Dennis, D. T., T. V. Inglesby, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, A. M. Friedlander, J. Hauer, M. Layton, et al. 2001. Tularemia as a biological weapon—medical and public health management. JAMA 285:2763-2773. [DOI] [PubMed] [Google Scholar]
- 6.Deziel, M. R., H. Heine, A. Louie, M. Kao, W. R. Byrne, J. Basset, L. Miller, K. Bush, M. Kelly, and G. L. Drusano. 2005. Effective antimicrobial regimens for use in humans for therapy of Bacillus anthracis infections and postexposure prophylaxis. Antimicrob. Agents Chemother. 49:5099-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Druett, H. A. 1969. A mobile form of the Henderson apparatus. J. Hyg. (Lond) 67:437-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis, J., P. C. F. Oyston, M. Green, and R. W. Titball. 2002. Tularemia. Clin. Microbiol. Rev. 15:631-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans, M. E., D. W. Gregory, W. Schaffner, and Z. A. McGee. 1985. Tularemia: a 30-year experience with 88 cases. Medicine 64:251-269. [PubMed] [Google Scholar]
- 10.Fish, D. N. 2003. Levofloxacin: update and perspectives on one of the original ‘respiratory quinolones’. Expert Rev. Anti Infect. Ther. 1:371-387. [DOI] [PubMed] [Google Scholar]
- 11.Hayes, E., S. Marshall, D. Dennis, and K. Feldman. 2002. Tularemia—United States, 1990-2000. MMWR Morb. Mortal. Wkly. Rep. 51:181-184. [PubMed] [Google Scholar]
- 12.Hemeryck, A., R. N. V. S. Mamidi, M. Bottacini, D. Macpherson, M. Kao, and M. F. Kelley. 2006. Pharmacokinetics, metabolism, excretion and plasma protein binding of 14C-levofloxacin after a single oral administration in the rhesus monkey. Xenobiotica 36:597-613. [DOI] [PubMed] [Google Scholar]
- 13.Hepburn, M. J., and A. J. Simpson. 2008. Tularemia: current diagnosis and treatment options. Expert Rev. Anti Infect. Ther. 6:231-240. [DOI] [PubMed] [Google Scholar]
- 14.Kaiser, A. B., D. Rieves, A. H. Price, M. R. Gelfand, R. E. Parrish, M. D. Decker, and M. E. Evans. 1985. Tularemia and rhabdomyolysis. JAMA 253:241-243. [PubMed] [Google Scholar]
- 15.Kao, L. M., K. Bush, R. Barnewall, J. Estep, F. W. Thalacker, P. H. Olson, G. L. Drusano, N. Minton, S. Chien, A. Hemeryck, and M. F. Kelley. 2006. Pharmacokinetic considerations and efficacy of levofloxacin in an inhalational anthrax (postexposure) rhesus monkey model. Antimicrob. Agents Chemother. 50:3535-3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly, D. J., J. D. Chulay, P. Mikesell, and A. M. Friedlander. 1992. Serum concentrations of penicillin, doxycycline, and ciprofloxacin during prolonged therapy in rhesus monkeys. J. Infect. Dis. 166:1184-1187. [DOI] [PubMed] [Google Scholar]
- 17.Klimpel, G. R., T. Eaves-Pyles, S. T. Moen, J. Taormina, J. W. Peterson, A. K. Chopra, D. W. Niesel, P. Carness, J. L. Haithcoat, M. Kirtley, and A. B. Nasr. 2008. Levofloxacin rescues mice from lethal intra-nasal infections with virulent Francisella tularensis and induces immunity and production of protective antibody. Vaccine 26:6874-6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lever, M. S., A. J. Stagg, M. Nelson, P. Pearce, D. J. Stevens, E. A. Scott, A. J. H. Simpson, and M. J. Fulop. 2008. Experimental respiratory anthrax infection in the common marmoset (Callithrix jacchus). Int. J. Exp. Pathol. 89:171-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, F., P. Nandy, S. Chien, G. J. Noel, and C. W. Tornoe. 2010. Pharmacometrics-based dose selection of levofloxacin as a treatment for postexposure inhalational anthrax in children. Antimicrob. Agents Chemother. 54:375-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Limaye, A. P., and C. J. Hooper. 1999. Treatment of tularemia with fluoroquinolones: two cases and review. Clin. Infect. Dis. 29:922-924. [DOI] [PubMed] [Google Scholar]
- 21.Nelson, M., M. S. Lever, V. L. Savage, F. J. Salguero, P. C. Pearce, D. J. Stevens, and A. J. H. Simpson. 2009. Establishment of lethal inhalational infection with Francisella tularensis (tularemia) in the common marmoset (Callithrix jacchus). Int. J. Exp. Pathol. 90:109-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong, F. A., S. J. Juzwin, and S. C. Flor. 1997. Rapid stereospecific high-performance liquid chromatographic determination of levofloxacin in human plasma and urine. J. Pharm. Biomed. Anal. 15:765-771. [DOI] [PubMed] [Google Scholar]
- 23.Yarbrough, L. W., J. L. Tollett, R. D. Montrey, and R. J. Beattie. 1984. Serum biochemical, hematological and body measurement data for common marmosets (Callithrix jacchus jacchus). Lab. Anim. Sci. 34:276-280. [PubMed] [Google Scholar]