Abstract
The emergence of multidrug-resistant (MDR) microorganisms makes it increasingly difficult to treat infections. These infections include those associated with Pseudomonas aeruginosa, which are hard to eradicate, especially in patients with a compromised immune system. Naturally occurring membrane-active cationic antimicrobial peptides (CAMPs) serve as attractive candidates for the development of new therapeutic agents. Amphibian skin is one of the richest sources for such peptides, but only a few studies on their in vivo activities and modes of action have been reported. We investigated (i) the activity and mechanism underlying the killing of short CAMPs from frog skin (e.g., temporins and esculentin fragments) on an MDR clinical isolate of P. aeruginosa and (ii) their in vivo antibacterial activities and modes of action, using the minihost model of Caenorhabditis elegans. Our data revealed that in vivo, both temporin-1Tb and esculentin(1-18) were highly active in promoting the survival of Pseudomonas-infected nematodes, although temporin-1Tb did not show significant activity in vitro under the experimental conditions used. Importantly, esculentin(1-18) permeated the membrane of Pseudomonas cells within the infected nematode. To the best of our knowledge, this is the first report showing the ability of a CAMP to permeate the microbial membrane within a living organism. Besides shedding light on a plausible mode of action of frog skin CAMPs in vivo, our data suggest that temporins and esculentins would be attractive molecules as templates for the development of new therapeutics against life-threatening infections.
Pseudomonas aeruginosa is a ubiquitous and opportunistic Gram-negative bacterium capable of causing the most prevalent life-threatening infections, including otitis media (7), ocular keratitis (14), burn wound infections (28), and lung infections in cystic fibrosis patients (13, 34). Unlike other microorganisms, P. aeruginosa is a pathogen endowed with high intrinsic drug resistance, due in part to many elaborate virulence factors and the formation of a biofilm matrix, which makes it difficult for antibiotics and immune cells to attack. Importantly, clinical isolates of this bacterial species that are resistant to virtually all antibiotics have already emerged. Therefore, effective therapy to treat and defeat them remains a management dilemma for hospitalized individuals, especially those with a compromised immune system (10, 12). This has prompted researchers to seek new drugs. Membrane-active cationic antimicrobial peptides (CAMPs) are considered a new class of antibiotics with a new mode of action and promising therapeutic effects (17, 29, 40). Chemically, they are composed of 12 to 50 amino acids, and they are produced by almost all forms of life. They represent effector molecules of the innate defense mechanisms, with direct antimicrobial activity and/or a strong ability to modulate host adaptive immunity (17).
We recently demonstrated that amphibian CAMPs belonging to the temporin family, as well as the short fragment covering the first 18 N-terminal residues of the longer esculentin 1b [esculentin(1-18), or Esc(1-18)] from Pelophylax lessonae/ridibundus (previously classified as Rana esculenta [6]), possess potent antibacterial activity in vitro against multidrug-resistant (MDR) nosocomial pathogens (26). Nevertheless, except for the effects of a longer fragment of esculentin, Esc(1-21), on the regression of the clinical stage of mastitis in dairy cows (16), at present very little is known about the toxicities and antimicrobial activities of these peptides in vivo, both of which are fundamental requirements for the potential development of CAMPs as new therapeutic agents.
To gain this knowledge, we analyzed (i) the activities of different CAMPs from frog skin [temporin-1Tb, -1Tf, and -1Tl, Esc(1-18), and Esc(1-21)] on a clinical isolate of P. aeruginosa that is resistant to a broad spectrum of antibiotics and the mechanism underlying the bacterial killing of the most active peptides by means of in vitro assays and (ii) their toxicities and antibacterial activities in vivo, using the well-characterized invertebrate Caenorhabditis elegans and also considering the plausible mode of action of Esc(1-18). C. elegans is a differentiated worm with a specialized nervous system, intestine, and reproductive organs; it possesses relevant biological processes, i.e., absorption, transport, and distribution of nutrients through various tissues and cell types. Different species of bacteria (including P. aeruginosa) can pass through the mouth of the worm, ultimately reaching and invading its gut, where they proliferate and kill the animal in an infection-like process. Interestingly, many virulence factors used by these bacteria to kill C. elegans play important roles in inducing pathogenesis in mammals, as well (31, 37, 38). In light of these properties, and taking into account the simple structure, transparency, and short life cycle of the nematode (less than 3 days) and the ability to grow it easily in the laboratory, C. elegans serves as a very suitable and convenient animal model for identifying antimicrobial compounds lacking toxic effects in vivo (30).
Our results indicate that, with the exception of temporin-1Tl, all of the peptides increased the survival rate of Pseudomonas-infected worms compared with non-peptide-treated animals and that Esc(1-18) and temporin-1Tb were the most active molecules. In addition, Esc(1-18) exhibited the same mode of action on P. aeruginosa under both in vitro and in vivo conditions. Although previous studies have shown the therapeutic potential of frog skin peptides (e.g., magainin and dermaseptins [5, 32]) against animals infected by this pathogen, to the best of our knowledge, the present report is the first describing both the ability of amphibian CAMPs to reduce the mortality of Pseudomonas-infected animals and their in vivo membrane-active properties.
MATERIALS AND METHODS
Materials.
Synthetic temporin-1Tb, -1Tf, and -1Tl, Esc(1-18), and Esc(1-21) were purchased from Genepep (Prades le Lez, France). The purity of the peptides and their sequences and concentrations were determined as previously described (24). Culture media were all purchased from Sigma (St. Louis, MO). Sytox Green was from Molecular Probes, Invitrogen (Carlsbad, CA). All other chemicals were reagent grade.
Bacterial and nematode strains.
Escherichia coli strain OP50 (the C. elegans food source), a clinical isolate of P. aeruginosa (kindly provided by Anna Rita Blanco, SIFI Pharmaceutical Company, Catania, Italy), and the reference strain of P. aeruginosa, ATCC 15692, were used.
The clinical isolate of P. aeruginosa was considered an MDR bacterium because it was resistant to more than three drugs from distinct classes of antibiotics. Its resistance profile was determined via antibiotic susceptibility tests using Vitek 2 automatic instruments (bioMérieux, Lyon, France) (see Table 2, note a).
TABLE 2.
In vitro antibacterial activities of amphibian CAMPs on the MDR strain of P. aeruginosaa
| Peptide | Concn (μM) for antibacterial activity |
||
|---|---|---|---|
| MIC | Bactericidal activityb |
||
| MHB | PBS | ||
| Temporin-1Tb | >64 | >64 | >64 |
| Temporin-1Tf | >64 | >64 | >64 |
| Temporin-1Tl | 64 | >64 | 16 |
| Esc(1-18) | 8 | 64 | 16 |
| Esc(1-21) | 4 | 16 | 4 |
Resistance phenotype: ampicillin, cefepime, cefotaxime, ceftazidime, cefuroxime, ciprofloxacin, gentamicin, meropenem, piperacillin, tobramycin, trimethoprim-sulfamethoxazole.
The bactericidal activity is expressed as the concentration of peptide that is sufficient to reduce the number of viable bacteria by ≥3 log10 CFU/ml after 90 min of incubation.
For fluorescence microscopy experiments, P. aeruginosa ATCC 15692 was transformed by electroporation with the plasmid pSMC21 (a kind gift from R. Kolter, Boston, MA) containing a gene for the expression of the green fluorescent protein (GFP) and a gene for the enzyme β-lactamase, which provides resistance to ampicillin. Indeed, β-lactamase, which is produced and secreted by bacteria that contain the plasmid, inactivates the ampicillin present in nutrient agar plates, allowing bacterial growth. Only transformed bacteria that contain the plasmid and express β-lactamase can survive on plates supplemented with ampicillin. P. aeruginosa ATCC 15692 was found to be resistant to ceftazidime, cefuroxime, and meropenem.
The wild-type C. elegans strain N2 was used in all experiments.
Antibacterial activities of the peptides.
Susceptibility testing of peptides was performed by adapting the broth microdilution method outlined by the Clinical and Laboratory Standards Institute using sterile 96-well plates (Falcon, NJ). Stock solutions of peptides were prepared in serial 2-fold dilutions in 20% ethanol; 4 μl was then added to 46 μl of Mueller-Hinton broth (MHB) (Oxoid, Cambridge, United Kingdom) previously put into the wells of the microtiter plate. Then, aliquots (50 μl) of bacteria in mid-log phase, at a concentration of 2 × 106 CFU/ml, were added to each well.
The ranges of peptide dilutions used were from 1 to 64 μM. Inhibition of growth was determined by measuring the absorbance at 595 nm with a microplate reader (Infinite M200; Tecan, Salzburg, Austria) after 18 to 20 h of incubation at 37°C. Antibacterial activities were expressed as the MIC, the minimal concentration of peptide causing 100% inhibition of bacterial growth.
Bactericidal activity.
The bactericidal activities of amphibian peptides against the MDR clinical isolate of P. aeruginosa were evaluated as previously described (26). Briefly, exponentially growing bacteria in MHB were harvested by centrifugation and then resuspended in fresh MHB or phosphate-buffered saline (PBS) to obtain a density of 1 × 107 CFU/ml. Ten microliters of the bacterial suspension was incubated at 37°C for 90 min in the presence of different concentrations of peptide (serial 2-fold dilutions ranging from 2 to 64 μM) dissolved in MHB or PBS to a final volume of 100 μl. Following incubation, appropriate aliquots were plated on Luria-Bertani (LB) agar plates to accurately determine the 99.9% killing. The number of surviving bacteria was determined after overnight incubation at 37°C. Bactericidal activity was expressed as the peptide concentration necessary to induce a reduction in the number of viable bacteria of ≥3 log10 CFU/ml (22). Controls were run without peptide and in the presence of peptide solvent (20% ethanol) at a final concentration of 0.6%.
Time-kill.
About 1 × 105 CFU in 100 μl PBS were incubated at 37°C with Esc(1-18) or Esc(1-21) at their MICs (8 and 4 μM, respectively). Aliquots of 10 μl were withdrawn at different intervals, diluted in MHB, and spread onto LB agar plates. After overnight incubation at 37°C, the CFU were counted. Controls were run without peptide and in the presence of peptide solvent (20% ethanol) at a final concentration of 0.6%.
Permeation of the bacterial IM.
To assess the ability of esculentin fragments to alter the permeability of the inner membrane (IM) of P. aeruginosa, 1 × 105 cells in 100 μl of PBS were mixed with 1 μM Sytox Green for 5 min in the dark. After peptide was added, the increase in fluorescence, owing to the binding of the dye to intracellular DNA, was measured at 37°C in the microplate reader, using 485-nm and 535-nm filters for excitation and emission wavelengths, respectively. The peptide concentrations ranged from 1 to 16 μM. Controls were cells without peptide, whereas the maximal membrane perturbation was obtained after lysing bacteria with 1 mM EDTA-0.5% Triton X-100.
Hemolytic activity.
The hemolytic activities of the peptides were determined using fresh human erythrocytes from healthy donors. The blood was centrifuged, and the erythrocytes were washed three times with 0.9% NaCl. Peptides dissolved in 20% ethanol at concentrations ranging from 2 to 64 μM were added to the erythrocyte suspension to reach a final volume of 100 μl (final erythrocyte suspension, 5% [vol/vol]). Samples were incubated with agitation at 37°C for 40 min. The release of hemoglobin was monitored by measuring the absorbance (Abs) of the supernatant at 540 nm. Control for zero hemolysis (blank) consisted of erythrocytes suspended in the presence of peptide solvent (20% ethanol at a final concentration of 0.6%). Hypotonically lysed erythrocytes were used as a standard for 100% hemolysis. The percent hemolysis was calculated using the following equation: percent hemolysis = [(Abssample − Absblank)/(Abstotal lysis − Absblank)] × 100.
Toxic activities of CAMPs on C. elegans.
C. elegans strain N2 was grown on nematode growth medium (NGM) agar plates (9-cm diameter) and fed with E. coli OP50 (39). Adult nematodes were transferred to 96-well plates (20 worms/well), each well containing 50 μl of S medium with E. coli OP50, according to the method of Stiernagel (39). CAMPs, at different concentrations, were added or not to the wells, as indicated. To score for worm survival after 48 h of peptide treatment, the plates were hand shaken, and the worms were considered to be dead if they did not move or exhibit muscle tone. Living nematodes maintain a sinusoidal shape, whereas dead nematodes appear as straight, rigid rods as the corpse becomes filled with bacteria (30).
C. elegans infections.
P. aeruginosa cultures were grown overnight in LB at 37°C. Bacterial lawns used for C. elegans infection assays were prepared by spreading 200 μl of the overnight culture of P. aeruginosa on modified NGM (50 mM NaCl and 0.35% peptone) agar plates. The plates were incubated at 37°C for 12 h before being seeded with young adult hermaphrodite nematodes, grown at 16°C, from a synchronized culture (39). The infections were performed at 25°C for several hours, as indicated.
Survival assays of infected C. elegans.
Nematode survival was evaluated by liquid or agar medium assay.
For the liquid assay, adult worms infected for 6 h with the MDR strain of P. aeruginosa (36) were washed three times with M9 buffer (39) and transferred into 96-well plates (15 worms/well). Each well contained 50 μl of S medium with E. coli OP50 (39). CAMPs were added or not to the wells, as indicated. The plates were incubated at 25°C for 40 h. Worm survival was estimated as described above. For the agar plate assay, which was used to examine the peptides' effects on both nematode survival and the bacterial counts within the worm gut (see below), 15 infected animals were washed as described above and transferred each day to new 3.5-cm-diameter NGM plates containing E. coli OP50. Two hundred microliters of a peptide solution, at the indicated concentration, was prepared every day and evenly distributed on the NGM plates before the nematodes were seeded. Worm death was scored by the absence of touch-provoked movement.
Estimation of bacterial CFU within the nematode gut.
Adult infected worms were washed as described above and transferred to E. coli OP50-supplemented-NGM plates, to which a solution of 0.5 nM Esc(1-18) or temporin-1Tb was directly added. The numbers of live P. aeruginosa bacteria in the worm intestine were determined before and after 1 day of peptide treatment, as described in reference 8. Briefly, for each replicate, 10 infected worms were put into a 1.5-ml Eppendorf tube and washed three times with M9 buffer (500 μl) containing 20 μg/ml gentamicin to remove surface E. coli bacteria. The nematodes were broken with 50 μl of M9 buffer-1% Triton X-100. Appropriate dilutions of whole-worm lysates were plated on selective medium (LB agar plates containing 100 μg/ml ampicillin to avoid growth of E. coli). The CFU were counted after overnight incubation at 37°C.
Fluorescence microscopy analysis of Pseudomonas-infected nematodes.
Nematodes infected for 16 h with the reference strain of P. aeruginosa, ATCC 15692, expressing GFP were transferred to NGM agar plates (3.5-cm diameter) containing E. coli OP50 and supplemented or not with the peptide, as reported above. After 90 min of peptide treatment, the worms were observed under a Leica MZ10F stereomicroscope equipped for epifluorescence (GFP2 filter) at ×60 magnification.
In vivo bacterial-membrane permeation of Esc(1-18).
Adult worms infected or not with the clinical isolate of P. aeruginosa for 16 h were washed three times with M9 buffer and then incubated in K medium (32 mM KCl plus 50 mM NaCl) agar plates (9-cm diameter) for approximately 24 h at 25°C. In the case of infected animals, this allowed intestinal Pseudomonas cells to proliferate, thereby increasing their number (about 1,000 CFU per worm), which is extremely important for optimal detection of bacterial-membrane permeabilization by the Sytox Green assay. Then, using a wire pick, nematodes were put into a 24-well plate (200 worms per well), each well containing 300 μl of K medium. All samples were mixed with 1 μM Sytox Green for 5 min in the dark, and the fluorescence intensity was monitored at 25°C in the microplate reader using 485-nm and 535-nm filters for excitation and emission wavelengths, respectively.
Then, 10 nM Esc(1-18) was added to the wells containing infected or uninfected worms, and the increase in fluorescence was measured until the fluorescent signal reached a constant value (approximately 40 min). Infected nematodes not treated with the peptide, but in the presence of peptide solvent (20% ethanol) at a final concentration of 0.3%, were used for comparison. Nematode survival was checked at the end of the experiments, and 10% lethality was observed in both peptide-treated and non-peptide-treated animals.
RESULTS
In vitro studies. (i) Antibacterial activity.
The antibacterial activities of temporin-1Tb, -1Tf, and -1Tl, Esc(1-18), and Esc(1-21) (Table 1) against the MDR clinical isolate of P. aeruginosa were assessed using the microdilution broth assay (Table 2).
TABLE 1.
Amino acid sequences and net charge of frog skin peptides
| Peptide | Sequencea | Net charge at neutral pH |
|---|---|---|
| Esc(1-18) | H2N-GIFSKLAGKKLKNLLISG-CONH2 | +5 |
| Esc(1-21) | H2N-GIFSKLAGKKIKNLLISGLKG-CONH2 | +6 |
| Temporin-1Tb | H2N-LLPIVGNLLKSLL-CONH2 | +2 |
| Temporin-1Tf | H2N-FLPLIGKVLSGIL-CONH2 | +2 |
| Temporin-1Tl | H2N-FVQWFSKFLGRIL-CONH2 | +3 |
Amino acids are indicated using the single-letter code.
As reported in Table 2, only temporin-1Tl, Esc(1-18), and Esc(1-21) were found to be active, with MIC values of 64, 8, and 4 μM, respectively. We then analyzed the killing activities of the peptides after 90 min of incubation with bacteria in both MHB and a physiological solution (PBS), as described in Materials and Methods. In MHB, Esc(1-18) and Esc(1-21) induced a reduction in the number of viable cells of ≥3 log10 CFU/ml (99.9% mortality) at peptide concentrations 8-fold and 4-fold higher than their MICs, respectively (Table 2). Bactericidal activities above 64 μM were observed for all the other CAMPs. Interestingly, when the peptides were tested in PBS (containing 150 mM sodium salt compared to 100 mM sodium salt of MHB [21]), the bactericidal concentration of Esc(1-18) or Esc(1-21) was found to be 2-fold higher or equal to the corresponding MIC, respectively (Table 2). In contrast, Tl exhibited a bactericidal effect at a peptide concentration 4-fold lower than its MIC.
These results suggest that esculentin peptides and temporin-1Tl are able to display antibacterial activity at high ionic strengths of the incubation medium. One explanation for their reduced efficacy in MHB compared with PBS is that it is due to their binding to complex carbohydrates/proteins of MHB, thus decreasing the availability of active peptide molecules. Alternatively, MHB components can bind to the surfaces of Pseudomonas cells and make it more difficult for the peptide to reach and permeate the target microbial membrane (see below).
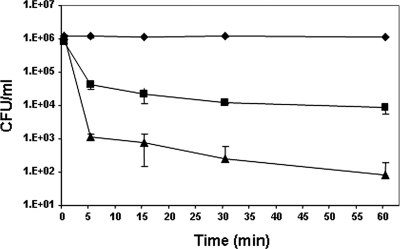
The killing kinetics of the most promising peptides, Esc(1-18) and Esc(1-21), was also investigated at their MICs in PBS. Both peptides gave rise to a significant reduction in the number of living bacteria [∼95 × 104 CFU/ml and 99.8 × 104 CFU/ml were killed by Esc(1-18) and Esc(1-21), respectively] within the first 5 min (Fig. 1).
FIG. 1.
Time-kill curves of P. aeruginosa by esculentin fragments. Bacteria (1 × 106 CFU/ml) were grown in MHB at 37°C, diluted in PBS, and incubated with Esc(1-18) (▪) and Esc(1-21) (▴) at the MIC (8 and 4 μM, respectively). Controls (⧫) were bacteria incubated in the presence of peptide solvent (20% ethanol) at a final concentration of 0.6%. The data are the means ± standard deviations (SD) of three independent experiments.
(ii) IM permeation.
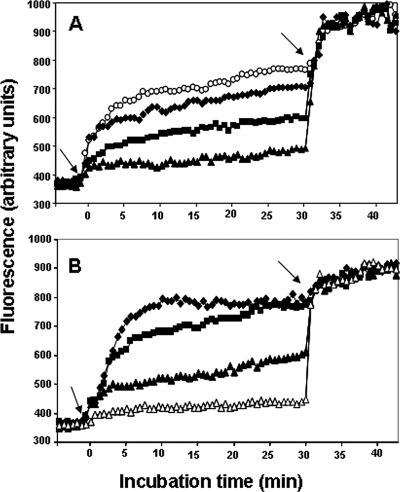
To obtain insight into the modes of action of these two peptides on P. aeruginosa, we studied the ability of Esc(1-18) (Fig. 2 A) and Esc(1-21) (Fig. 2B) to alter the permeability of the bacterial IM by monitoring the intracellular influx of Sytox Green upon addition of peptide (concentrations ranged from 2× MIC to 1/4× MIC). Sytox Green is a cationic dye that is excluded by intact membranes, but not by those having lesions large enough to allow its entrance (27). Its fluorescence dramatically increases when it is bound to intracellular nucleic acids. The data indicated that the peptides were able to increase the permeability of the plasma membrane in a concentration-dependent manner (Fig. 2) and with kinetics similar to those of bacterial killing (Fig. 1). In particular, Esc(1-21) almost completely permeabilized the membrane at a concentration 2-fold higher than its MIC (8 μM). This is reflected by a slight change in the fluorescence intensity upon addition of EDTA-Triton to fully solubilize the membrane (Fig. 2, arrows at 30 min).
FIG. 2.
Effect of esculentin fragments on the IM permeation of P. aeruginosa. Cells (1 × 105) were incubated in 100 μl of PBS with 1 μM Sytox Green. Once basal fluorescence reached a constant value, the peptide was added (left arrows; t = 0), and changes in fluorescence were monitored (λexc = 485 nm; λems = 535 nm) and plotted as arbitrary units. Maximal membrane permeation (right arrows; t = 30 min) was obtained after the addition of 1 mM EDTA plus 0.5% Triton X-100. The data points represent the means of triplicate samples, with SD values not exceeding 2.5% from a single experiment, representative of three different experiments. Peptide concentrations used for Esc(1-18) (A) and Esc(1-21) (B) were as follows: 1 μM (▵), 2 μM (▴), 4 μM (▪), 8 μM (⧫), and 16 μM (○). The fluorescence values of controls (bacteria without peptide) were subtracted from each sample.
(iii) Hemolytic activity.
According to previous papers (3, 33), temporin-1Tl was found to be a highly hemolytic peptide, causing lysis of red blood cells, from 2 to 100%, at peptide concentrations ranging from 2 to 64 μM. In contrast, temporin-1Tb and -1Tf gave rise to ∼5 and 12% hemolysis at 32 and 64 μM, respectively, but they were devoid of toxic effects at the lower peptide concentration. Importantly, similar behavior was also observed with both esculentin fragments, but they were practically nonhemolytic up to 64 μM.
In vivo studies. (i) Effects of the peptides on the survival of infected nematodes.
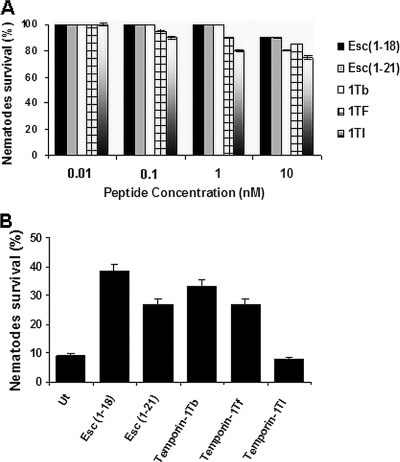
A toxicity study was initially conducted to determine the in vivo lethal doses of the peptides against uninfected nematodes (Fig. 3 A). Accordingly, peptides at sublethal concentrations were tested for their effects on the viability of P. aeruginosa-infected C. elegans. Interestingly, as shown in Fig. 3B, both temporin-1Tb and Esc(1-18) increased worm survival by approximately 4-fold after 40 h of peptide treatment at 0.3 nM in liquid medium. Note that temporin-1Tb lacked anti-Pseudomonas activity in vitro (Table 2). In contrast, temporin-1Tl did not promote the survival of the infected worms at 0.01 nM (Fig. 3B) and in fact killed C. elegans when used at a higher dosage (Fig. 3A), in agreement with its in vitro toxicity to erythrocytes.
FIG. 3.
In vivo toxicity assay of frog skin CAMPs on C. elegans worms and their efficacies on nematodes infected with an MDR strain of P. aeruginosa. (A) Adult C. elegans worms were incubated with different concentrations of CAMPs, as described in Materials and Methods. Nematode survival was monitored 48 h after peptide addition and is expressed as a percentage with respect to non-peptide-treated worms. The results are the means of two independent experiments; the error bars indicate SD. (B) Nematode infection was carried out for 6 h as described in Materials and Methods. C. elegans survival was monitored after 40 h of exposure to peptides in a liquid medium assay. Peptides were used at the following concentrations: Esc(1-18), 0.3 nM; Esc(1-21), 0.3 nM; temporin-1Tb, 0.3 nM; temporin-1Tf, 0.01 nM; and temporin-1Tl, 0.01 nM. Infected animals not treated with the peptides (Ut) were included for comparison. Control animals not exposed to the pathogen and fed with E. coli OP50 exhibited 100% viability. The results represent the means of at least three independent experiments; the error bars indicate SD.
(ii) Effects of the peptides on the bacterial colonization of the nematode intestinal tract.
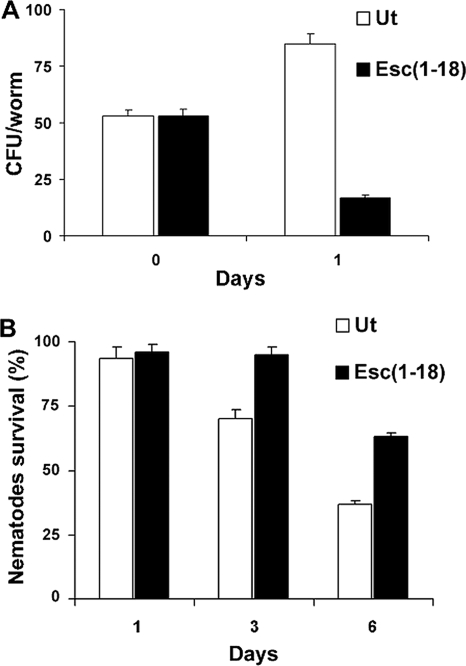
We examined the effects of both Esc(1-18) and temporin-1Tb on the colonization of Pseudomonas in the intestinal lumen of the nematode. After 6 h of infection, an average of 50 viable Pseudomonas cells were recovered from each worm (Fig. 4 A). Then, a group of infected nematodes was transferred to E. coli OP50-supplemented agar plates and incubated or not with Esc(1-18). Surprisingly, at 24-h post-peptide addition, the bacterial counts dropped 5-fold with respect to untreated infected worms (Fig. 4A). Almost identical results were obtained when Esc(1-18) was replaced by temporin-1Tb, and therefore, they are not shown.
FIG. 4.
Effect of Esc(1-18) on the number of live Pseudomonas cells within the worm gut and the animal's survival. Adult worms were infected with the MDR strain of P. aeruginosa for 6 h and then transferred to NGM agar plates supplemented with E. coli OP50, to which a solution of 0.5 nM Esc(1-18) was directly added. (A) CFU were determined in the guts of 10 live worms removed from the plates before (0 days) and after 1 day of peptide treatment. Infected nematodes not treated with the peptide (Ut) were included for comparison. (B) Survival of infected C. elegans upon peptide treatment was analyzed at the indicated time points in comparison to untreated infected nematodes (Ut). Control worms not exposed to the pathogen and fed with E. coli OP50 showed 100% viability at each time point. The reported values represent the means of at least three independent experiments; the error bars indicate SD.
Note that although the killing of Pseudomonas occurred within 1 day of treatment, the peptide's ability to increase worm survival became evident only at 3 days post-peptide administration, and the number of living animals was double that of untreated nematodes within 6 days (Fig. 4B). Note also that in these experiments, the peptide was used on agar medium plates. In comparison, in liquid medium, the peptide had higher solubility and greater diffusibility (Fig. 3). Such a difference could be responsible for the delayed effect of the peptide on the animal survival rate when tested in an agar medium assay.
To further visualize the reduction of bacterial colonization in the nematode intestinal tract, C. elegans was infected with a reference strain of P. aeruginosa (ATCC 15692) transformed with the plasmid pSMC21 (see Materials and Methods), resulting in the appearance of green bacteria under a fluorescence microscope. We used this specific reference strain owing to the difficulty of identifying GFP-transformed MDR Pseudomonas cells by means of antibiotic selection plates. Note that the MICs of Esc(1-18) and temporin-1Tb against P. aeruginosa ATCC 15692 were the same as those obtained for the clinical isolate (8 μM and >64 μM, respectively).
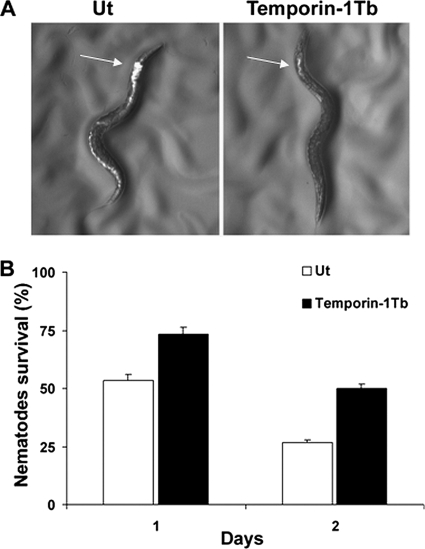
As shown in Fig. 5 A, Pseudomonas cells were spread throughout the digestive tract of the animal, with higher accumulation at the anterior intestine (Fig. 5A, left). In contrast, a significant reduction in the fluorescence intensity was visualized throughout the gut of the peptide-treated nematodes (Fig. 5A, right), due to the killing of GFP-expressing Pseudomonas. This effect was detected already after the first 90 min of incubation with temporin-1Tb (Fig. 5A) or Esc(1-18) (data not shown) and was followed by increased worm viability (Fig. 5B).
FIG. 5.
Effect of temporin-1Tb on the colonization of P. aeruginosa ATCC 15692 within the nematode gut and the animal's survival. Nematodes were infected with the GFP-expressing P. aeruginosa strain ATCC 15692 for 16 h to increase the number of intestinal bacteria, as described in Materials and Methods. (A) Fluorescence photomicrographs of a representative nematode after 90 min of treatment with 0.5 nM temporin-1Tb. A representative non-peptide-treated animal (Ut) was included for comparison. The arrows indicate the anterior intestine. (B) Survival of infected nematodes after 1 and 2 days of treatment with temporin-1Tb at 0.5 nM in an agar medium assay in comparison with untreated infected nematodes (Ut). The reported values represent the means of at least three independent experiments; the error bars indicate SD. Control animals not exposed to the pathogen and fed with E. coli OP50 exhibited 100% viability at each time point. Similar data were obtained when temporin-1Tb was replaced by Esc(1-18) and therefore are not shown.
(iii) Permeation of the bacterial membrane induced by Esc(1-18) in living infected worms.
Finally, to determine whether the capacity of Esc(1-18) to alter the bacterial membrane permeability was also preserved under in vivo conditions, nematodes infected with the clinical isolate of P. aeruginosa were exposed to 1 μM Sytox Green, which is not toxic to the animal (9, 18).
As seen in Fig. 6, a significant rise in fluorescence intensity was observed within 40 min post-peptide addition (black bars), compared with untreated infected worms (white bars). Importantly, no fluorescence increase could be detected in peptide-treated uninfected nematodes (gray bars). This excludes the possible membrane permeabilization of C. elegans intestinal cells (or other cells) by Esc(1-18). The following findings further support the notion that the intracellular Sytox Green uptake is not due to the peptide's ability to permeate the membranes of E. coli cells that might remain within the nematode gut. (i) When C. elegans was fed GFP-expressing E. coli OP50, no green-fluorescence-labeled bacteria were found in the digestive systems of the nematodes after 16 h. In addition, no viable E. coli cells could be found in the worm lysates. (ii) When CFU counting in whole lysates of Pseudomonas-infected nematodes was performed before peptide was added, identical numbers of bacteria (approximately 1,000 cells per worm) were recovered from both LB and ampicillin selection agar plates (where only P. aeruginosa was able to grow). This rules out the possible presence of E. coli cells within the gut of the infected worm and supports the notion that Esc(1-18) is able to induce injury to the IM of Pseudomonas cells within the animal.
FIG. 6.
Effect of Esc(1-18) on the membrane permeation of the MDR strain of P. aeruginosa within nematodes. Infected nematodes were transferred to a 24-well microtiter plate (200 worms per well), each well containing 300 μl of K medium, and incubated with 1 μM Sytox Green as described in Materials and Methods. Once basal fluorescence reached a constant value (time zero), 10 nM Esc(1-18) was added, and changes in fluorescence intensity were monitored (λexc = 485 nm; λems = 535 nm) until the fluorescent signal reached a constant value (approximately 40 min). The increase in fluorescence reflected damage at the IM level of Pseudomonas cells. Infected nematodes not treated with the peptide, as well as peptide-treated uninfected worms, were used for comparison. The reported values represent the means of two independent experiments; the error bars indicate SD.
DISCUSSION
Over the past decade, extensive efforts have been made to overcome the emergence of antimicrobial resistance and to discover new antibiotics. An alarming expansion in the number of hospital-acquired infections owing to MDR Gram-negative bacteria has also occurred, but only a limited number of effective drugs are currently being produced (19); nevertheless, studies on naturally occurring CAMPs have increased considerably in recent years. CAMPs usually have a net positive charge at neutral pH and fold into amphipathic structures in a hydrophobic environment (11). These properties allow them to interact with the negatively charged components of the microbial surface, such as the lipopolysaccharide in Gram-negative bacteria, and to be inserted into the anionic cytoplasmic membrane, considered to be a major target for the actions of most CAMPs, including those from amphibian skin (23, 25, 35).
A key aspect to take into account when developing new drugs is their effectiveness in vivo and their lack of toxicity. To address these points, we evaluated the abilities of several CAMPs from frog skin to promote the survival of bacterium-infected C. elegans, a known predictive model for studying microbial pathogenesis in mammals (2, 15). To this end, the nematode was infected with a clinical isolate of P. aeruginosa resistant to almost all available antibiotics.
Our studies have pointed out that, with the exception of temporin-1Tl, all the tested amphibian CAMPs reduced the lethality of infected nematodes. Among them, temporin-1Tb and Esc(1-18) were the most active molecules (Fig. 3B), although temporin-1Tb was not active in MHB and PBS (Table 2). Note that the behavior of a molecule can differ in vivo compared with in vitro, where the number of bacteria used and the environmental conditions do not always correspond to those in animals.
The decreasing number of live Pseudomonas cells within the nematode gut after peptide treatment (Fig. 4 and 5) confirms that the peptide can affect the pathogen's viability in vivo.
In addition, the finding that Esc(1-18) can alter the membrane permeability of Pseudomonas in the worm intestine (Fig. 6) suggests that the mode of action used by this peptide to promote worm survival is based on its direct effect on pathogen growth, rather than on making bacteria avirulent and therefore harmless. However, at this stage, we cannot exclude the possibility that the reduction of Pseudomonas colonization could be due to an enhanced host immune reaction, as well.
It is important to emphasize that crucial mechanisms underlying innate immunity have been highly conserved during evolution, from nematodes to mammals (1, 15, 20). Note also that the C. elegans genome has been fully sequenced and annotated (http://www.wormbase.org). Even if C. elegans cannot replace mammals, it certainly provides an advanced step in selecting antimicrobial molecules in a whole living organism.
Hence, as stated by Chamilos and colleagues (4), we retain this invertebrate minihost model as the best available compromise between the complexity of a multicellular organism and the possibility to perform, at low cost and in a short time, large-scale in vivo studies on the toxicity and antimicrobial properties of peptide antibiotics for a more comprehensive analysis of their effects on the host-pathogen interactions.
In summary, this paper emphasizes two major findings. (i) Frog skin peptides, such as temporin-1Tb and -1Tf and esculentin fragments, are endowed with antibacterial activity in animal models infected with an MDR nosocomial pathogen, such as P. aeruginosa. Importantly, these peptides can display in vivo anti-Pseudomonas activity, regardless of their inactivity (in the case of temporin-1Tb and -1Tf) under the in vitro experimental conditions.
(ii) The most active peptide, Esc(1-18), is able to alter the membrane permeability of an MDR strain of Pseudomonas, both in vitro and in vivo.
Importantly, to the best of our knowledge, this is the first study demonstrating permeation of a bacterial membrane inside living species as a possible mode of action by an antimicrobial peptide.
In addition, besides shedding light on a plausible mode of action in vivo of amphibian CAMPs, our data suggest that esculentin and temporin peptides can serve as attractive molecules for the development of new therapeutic strategies to fight life-threatening infectious diseases.
Acknowledgments
This work was supported by grants from Sapienza Università di Roma, Italian Ministero dell'Istruzione, Università e Ricerca (PRIN 2008), and Istituto di Biologia e Patologia Molecolari of the National Research Council.
We thank Anna Rita Blanco, at SIFI, Catania, Italy, for providing the clinical isolate of P. aeruginosa; Emanuela Santopietro for contributing to the infection experiments; and the Caenorhabditis Genetics Center (University of Minnesota) for the N2 strain used in this study.
Footnotes
Published ahead of print on 6 July 2010.
REFERENCES
- 1.Ausubel, F. M. 2005. Are innate immune signaling pathways in plants and animals conserved? Nat. Immunol. 6:973-979. [DOI] [PubMed] [Google Scholar]
- 2.Bhavsar, A. P., and E. D. Brown. 2006. The worm turns for antimicrobial discovery. Nat. Biotechnol. 24:1098-1100. [DOI] [PubMed] [Google Scholar]
- 3.Carotenuto, A., S. Malfi, M. R. Saviello, P. Campiglia, I. Gomez-Monterrey, M. L. Mangoni, L. M. Gaddi, E. Novellino, and P. Grieco. 2008. A different molecular mechanism underlying antimicrobial and hemolytic actions of temporins A and L. J. Med. Chem. 51:2354-2362. [DOI] [PubMed] [Google Scholar]
- 4.Chamilos, G., M. S. Lionakis, R. E. Lewis, and D. P. Kontoyiannis. 2007. Role of mini-host models in the study of medically important fungi. Lancet Infect. Dis. 7:42-55. [DOI] [PubMed] [Google Scholar]
- 5.Cirioni, O., C. Silvestri, R. Ghiselli, F. Orlando, A. Riva, F. Mocchegiani, L. Chiodi, S. Castelletti, E. Gabrielli, V. Saba, G. Scalise, and A. Giacometti. 2008. Protective effects of the combination of alpha-helical antimicrobial peptides and rifampicin in three rat models of Pseudomonas aeruginosa infection. J. Antimicrob. Chemother. 62:1332-1338. [DOI] [PubMed] [Google Scholar]
- 6.Conlon, J. M. 2008. Reflections on a systematic nomenclature for antimicrobial peptides from the skins of frogs of the family Ranidae. Peptides 29:1815-1819. [DOI] [PubMed] [Google Scholar]
- 7.Dohar, J. E., P. A. Hebda, R. Veeh, M. Awad, J. W. Costerton, J. Hayes, and G. D. Ehrlich. 2005. Mucosal biofilm formation on middle-ear mucosa in a nonhuman primate model of chronic suppurative otitis media. Laryngoscope 115:1469-1472. [DOI] [PubMed] [Google Scholar]
- 8.Garsin, D. A., C. D. Sifri, E. Mylonakis, X. Qin, K. V. Singh, B. E. Murray, S. B. Calderwood, and F. M. Ausubel. 2001. A simple model host for identifying Gram-positive virulence factors. Proc. Natl. Acad. Sci. U. S. A. 98:10892-10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gill, M. S., A. Olsen, J. N. Sampayo, and G. J. Lithgow. 2003. An automated high-throughput assay for survival of the nematode Caenorhabditis elegans. Free Radic. Biol. Med. 35:558-565. [DOI] [PubMed] [Google Scholar]
- 10.Gooderham, W. J., M. Bains, J. B. McPhee, I. Wiegand, and R. E. Hancock. 2008. Induction by cationic antimicrobial peptides and involvement in intrinsic polymyxin and antimicrobial peptide resistance, biofilm formation, and swarming motility of PsrA in Pseudomonas aeruginosa. J. Bacteriol. 190:5624-5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hancock, R. E. 1997. Peptide antibiotics. Lancet 349:418-422. [DOI] [PubMed] [Google Scholar]
- 12.Hancock, R. E., and D. P. Speert. 2000. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and impact on treatment. Drug Resist. Updat. 3:247-255. [DOI] [PubMed] [Google Scholar]
- 13.Hassett, D. J., J. Cuppoletti, B. Trapnell, S. V. Lymar, J. J. Rowe, S. S. Yoon, G. M. Hilliard, K. Parvatiyar, M. C. Kamani, D. J. Wozniak, S. H. Hwang, T. R. McDermott, and U. A. Ochsner. 2002. Anaerobic metabolism and quorum sensing by Pseudomonas aeruginosa biofilms in chronically infected cystic fibrosis airways: rethinking antibiotic treatment strategies and drug targets. Adv. Drug Deliv. Rev. 54:1425-1443. [DOI] [PubMed] [Google Scholar]
- 14.Huang, L. C., R. L. Redfern, S. Narayanan, R. Y. Reins, and A. M. McDermott. 2007. In vitro activity of human beta-defensin 2 against Pseudomonas aeruginosa in the presence of tear fluid. Antimicrob. Agents Chemother. 51:3853-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irazoqui, J. E., J. M. Urbach, and F. M. Ausubel. 2010. Evolution of host innate defence: insights from Caenorhabditis elegans and primitive invertebrates. Nat. Rev. Immunol. 10:47-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Islas-Rodrìguez, A. E., L. Marcellini, B. Orioni, D. Barra, L. Stella, and M. L. Mangoni. 2009. Esculentin 1-21: a linear antimicrobial peptide from frog skin with inhibitory effect on bovine mastitis-causing bacteria. J. Pept. Sci. 15:607-614. [DOI] [PubMed] [Google Scholar]
- 17.Jenssen, H., P. Hamill, and R. E. Hancock. 2006. Peptide antimicrobial agents. Clin. Microbiol. Rev. 19:491-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kampkötter, A., T. Pielarski, R. Rohrig, C. Timpel, Y. Chovolou, W. Watjen, and R. Kahl. 2007. The ginkgo biloba extract EGb761 reduces stress sensitivity, ROS accumulation and expression of catalase and glutathione S-transferase 4 in Caenorhabditis elegans. Pharmacol. Res. 55:139-147. [DOI] [PubMed] [Google Scholar]
- 19.Kaul, D. R., C. D. Collins, and R. C. Hyzy. 2008. New developments in antimicrobial use in sepsis. Curr. Pharm. Des. 14:1912-1920. [DOI] [PubMed] [Google Scholar]
- 20.Kim, D. H., and F. M. Ausubel. 2005. Evolutionary perspectives on innate immunity from the study of Caenorhabditis elegans. Curr. Opin. Immunol. 17:4-10. [DOI] [PubMed] [Google Scholar]
- 21.Li, X. Z., K. Poole, and H. Nikaido. 2003. Contributions of MexAB-OprM and an EmrE homolog to intrinsic resistance of Pseudomonas aeruginosa to aminoglycosides and dyes. Antimicrob. Agents Chemother. 47:27-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maisetta, G., G. Batoni, S. Esin, W. Florio, D. Bottai, F. Favilli, and M. Campa. 2006. In vitro bactericidal activity of human beta-defensin 3 against multidrug-resistant nosocomial strains. Antimicrob. Agents Chemother. 50:806-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mangoni, M. L. 2006. Temporins, anti-infective peptides with expanding properties. Cell. Mol. Life Sci. 63:1060-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mangoni, M. L., D. Fiocco, G. Mignogna, D. Barra, and M. Simmaco. 2003. Functional characterisation of the 1-18 fragment of esculentin-1b, an antimicrobial peptide from Rana esculenta. Peptides 24:1771-1777. [DOI] [PubMed] [Google Scholar]
- 25.Mangoni, M. L., N. Grovale, A. Giorgi, G. Mignogna, M. Simmaco, and D. Barra. 2000. Structure-function relationships in bombinins H, antimicrobial peptides from Bombina skin secretions. Peptides 21:1673-1679. [DOI] [PubMed] [Google Scholar]
- 26.Mangoni, M. L., G. Maisetta, M. Di Luca, L. M. Gaddi, S. Esin, W. Florio, F. L. Brancatisano, D. Barra, M. Campa, and G. Batoni. 2008. Comparative analysis of the bactericidal activities of amphibian peptide analogues against multidrug-resistant nosocomial bacterial strains. Antimicrob. Agents Chemother. 52:85-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcellini, L., M. Borro, G. Gentile, A. C. Rinaldi, L. Stella, P. Aimola, D. Barra, and M. L. Mangoni. 2009. Esculentin-1b(1-18)—a membrane-active antimicrobial peptide that synergizes with antibiotics and modifies the expression level of a limited number of proteins in Escherichia coli. FEBS J. 276:5647-5664. [DOI] [PubMed] [Google Scholar]
- 28.Montie, T. C., D. Doyle-Huntzinger, R. C. Craven, and I. A. Holder. 1982. Loss of virulence associated with absence of flagellum in an isogenic mutant of Pseudomonas aeruginosa in the burned-mouse model. Infect. Immun. 38:1296-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mookherjee, N., and R. E. Hancock. 2007. Cationic host defence peptides: innate immune regulatory peptides as a novel approach for treating infections. Cell. Mol. Life Sci. 64:922-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moy, T. I., A. R. Ball, Z. Anklesaria, G. Casadei, K. Lewis, and F. M. Ausubel. 2006. Identification of novel antimicrobials using a live-animal infection model. Proc. Natl. Acad. Sci. U. S. A. 103:10414-10419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mylonakis, E., and A. Aballay. 2005. Worms and flies as genetically tractable animal models to study host-pathogen interactions. Infect. Immun. 73:3833-3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navon-Venezia, S., R. Feder, L. Gaidukov, Y. Carmeli, and A. Mor. 2002. Antibacterial properties of dermaseptin S4 derivatives with in vivo activity. Antimicrob. Agents Chemother. 46:689-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rinaldi, A. C., M. L. Mangoni, A. Rufo, C. Luzi, D. Barra, H. Zhao, P. K. Kinnunen, A. Bozzi, A. Di Giulio, and M. Simmaco. 2002. Temporin L: antimicrobial, haemolytic and cytotoxic activities, and effects on membrane permeabilization in lipid vesicles. Biochem. J. 368:91-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rowe, S. M., S. Miller, and E. J. Sorscher. 2005. Cystic fibrosis. N. Engl. J. Med. 352:1992-2001. [DOI] [PubMed] [Google Scholar]
- 35.Shai, Y. 2002. Mode of action of membrane active antimicrobial peptides. Biopolymers 66:236-248. [DOI] [PubMed] [Google Scholar]
- 36.Shapira, M., B. J. Hamlin, J. Rong, K. Chen, M. Ronen, and M. W. Tan. 2006. A conserved role for a GATA transcription factor in regulating epithelial innate immune responses. Proc. Natl. Acad. Sci. U. S. A. 103:14086-14091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sifri, C. D., J. Begun, and F. M. Ausubel. 2005. The worm has turned—microbial virulence modeled in Caenorhabditis elegans. Trends Microbiol. 13:119-127. [DOI] [PubMed] [Google Scholar]
- 38.Sifri, C. D., J. Begun, F. M. Ausubel, and S. B. Calderwood. 2003. Caenorhabditis elegans as a model host for Staphylococcus aureus pathogenesis. Infect. Immun. 71:2208-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stiernagel, T. 11 February 2006, posting date. Maintenance of C. elegans, WormBook, ed. The C. elegans Research Community, WormBook. doi/ 10.1895/wormbook.1.101.1. http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- 40.Zhang, L., and T. J. Falla. 2009. Host defense peptides for use as potential therapeutics. Curr. Opin. Invest. Drugs 10:164-171. [PubMed] [Google Scholar]