Abstract
Filamentous fungi have received attention as hosts for heterologous protein production because of their high secretion capability and eukaryotic posttranslational modifications. However, despite these positive attributes, a bottleneck in posttranscriptional processing limits protein yields. The vacuolar protein sorting gene VPS10 encodes a sorting receptor for the recognition and delivery of several yeast vacuolar proteins. Although it can also target recombinant and aberrant proteins for vacuolar degradation, there is limited knowledge of the effect of its disruption on heterologous protein production. In this study, cDNA encoding AoVps10 from the filamentous fungus Aspergillus oryzae was cloned and sequenced. Microscopic observation of the transformant expressing AoVps10 fused with enhanced green fluorescent protein showed that the fusion protein localized at the Golgi and prevacuolar compartments. Moreover, disruption of the Aovps10 gene resulted in missorting and secretion of vacuolar carboxypeptidase AoCpyA into the medium, indicating that AoVps10 is required for sorting of vacuolar proteins to vacuoles. To investigate the extracellular production levels of heterologous proteins, ΔAovps10 mutants expressing either bovine chymosin (CHY) or human lysozyme (HLY) were constructed. Interestingly, the ΔAovps10 mutation increased the maximum extracellular production levels of CHY and HLY by 3- and 2.2-fold, respectively. Western blot analysis of extracellular heterologous proteins also demonstrated an improvement in productivity. These results suggest that AoVps10 plays a role in the regulation of heterologous protein secretion in A. oryzae and may be involved in the vacuolar protein degradation through the Golgi apparatus.
The filamentous fungus Aspergillus oryzae is an important microorganism with a long history of usage in the Japanese food fermentation industry (19). Since it can secrete various and large amounts of enzymes into growth medium (4) and is a GRAS (generally regarded as safe) organism, A. oryzae is also an excellent host for homologous (fungal) and heterologous protein production (36). Moreover, unlike any previously described microbial production/secretion system, such as Escherichia coli (10), proper eukaryotic posttranslational modifications, including glycosylation and protein folding, are expected to occur in A. oryzae. For these reasons, this microorganism is considered one of the most adequate hosts to produce higher eukaryotic proteins. In an attempt to enhance heterologous protein production in A. oryzae, our group previously constructed multiple protease gene disruptants (16, 25, 45), which led to a significant improvement of recombinant bovine chymosin and human lysozyme yields (16, 45).
In general, the production levels of proteins from higher eukaryotes (animals and plants) by A. oryzae are much lower than those of homologous (fungal) proteins (15, 16, 31, 41). It has been shown that the bottleneck in the production of heterologous proteins is not caused by low expression of the heterologous gene but is due to posttranscriptional processes in the secretory pathway (42).
Several homologues of vacuolar protein sorting (VPS) genes, which play important roles in the secretory pathway, have been isolated and characterized (21, 39, 44). Among them, the VPS10 gene codes for a type I transmembrane receptor protein, which is responsible for the recognition and sorting of soluble vacuolar hydrolases, such as carboxypeptidase Y (CPY) and proteinase A (PrA), to the vacuole by cycling between the late-Golgi and prevacuolar compartments (3, 5, 24). In Saccharomyces cerevisiae, however, a vps10 mutant missorts and secretes CPY into the medium (24). The Vps10p receptor has also been implicated in the targeting and delivery of several recombinant proteins from the late-Golgi compartments to vacuoles for degradation by the vacuolar protease complex (11, 12). In our previous work, secreted protein RNase T1 (RntA) fused with enhanced green fluorescent protein (EGFP), RntA-EGFP, was visualized in the vacuoles, as well as in the hyphal tips (27), indicating that a portion of the expressed heterologous proteins was presumably transported into the vacuoles for degradation. These observations suggest that for the improvement of heterologous protein production in A. oryzae, it is necessary to elucidate the machinery which mediates protein trafficking between the Golgi apparatus and vacuoles.
Although several studies have been performed with the aim of improving heterologous protein production in A. oryzae, heterologous protein degradation through the receptor protein Vps10p has not been investigated. Therefore, it was expected that the elucidation of the molecular mechanisms of intracellular protein trafficking to the vacuole could improve the utilization of A. oryzae as a host for heterologous protein production. Here, we report the disruption of a vacuolar protein sorting gene and its effect on production levels of heterologous proteins in A. oryzae. This is the first report demonstrating that disruption of a vacuolar protein sorting gene in filamentous fungi leads to enhanced production levels of heterologous proteins.
MATERIALS AND METHODS
Strains, media, and transformation.
The A. oryzae wild-type strain, RIB40 (23) and a strain with a highly efficient gene-targeting background (niaD− sC− ΔpyrG ΔligD), NSPlD1 (25), were used as a DNA donor and transformation host, respectively. The strains generated in the present study are listed in Table 1. DPY (2% dextrin, 1% polypeptone, 0.5% yeast extract, 0.5% KH2PO4, and 0.05% MgSO4·7H2O [pH 5.5]) and potato dextrose agar (Nissui Pharmaceutical, Tokyo, Japan) media were used for the normal growth and maintenance of all strains. M medium [0.2% NH4Cl, 0.1% (NH4)2SO4, 0.05% KCl, 0.05% NaCl, 0.1% KH2PO4, 0.05% MgSO4·7H2O, 0.002% FeSO4·7H2O, and 2% glucose (pH 5.5)] supplemented with 0.15% methionine (M+Met) was used as a selective medium for disrupting the Aovps10 gene. 5× DPY (pH 5.5) (10% dextrin, 5% polypeptone, 2.5% yeast extract, 0.5% KH2PO4, and 0.05% MgSO4·7H2O [pH 5.5]) was used as a medium for production of CHY. 5× DPY (pH 8.0; 10% dextrin, 5% polypeptone, 2.5% yeast extract, 0.5% KH2PO4, and 0.05% MgSO4·7H2O [pH 8.0]) was used as a medium for production of HLY. Czapek-Dox (CD) medium (0.3% NaNO3, 0.2% KCl, 0.1% KH2PO4, 0.05% MgSO4·7H2O, 0.002% FeSO4·7H2O, 2% glucose [pH 5.5]) supplemented with 0.0015% methionine was used for niaD-based plasmid integration and for microscopic observations. Escherichia coli DH5α [supE44 ΔlacU169 (φ80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1] was used for DNA manipulation. A. oryzae was transformed according to a previously reported method (19).
TABLE 1.
Strains used in this study
| Name | Parental strain | Genotype | Source or reference |
|---|---|---|---|
| RIB40 | Wild-type | 23 | |
| niaD300 | RIB40 | niaD− | 29 |
| NSPlD1 | NSR-ΔlD2 | niaD−sC−adeA− ΔargB::adeA− ΔligD::argB ΔpyrG::adeA | 25 |
| VTG1 | niaD300 | niaD− pnVTG [PAovps10::Aovps10 cDNA-egfp::TamyB::niaD] | This study |
| NSlDv10 | NSPlD1 | niaD−sC−adeA− ΔargB::adeA− ΔligD::argB ΔpyrG::adeA ΔAovps10::pyrG | This study |
| NSlD1 | NSPlD1 | niaD−sC−adeA− ΔargB::adeA− ΔligD::argB ΔpyrG::adeA pgEpG [pyrG] | This study |
| SlD-CG | NSlD1 | niaD−sC−adeA− ΔargB::adeA− ΔligD::argB ΔpyrG::adeA pgEpG [pyrG] pCGFP [PAocpyA::AocpyA-egfp::Tnos3′::niaD] | This study |
| SlDv10-CG | NSlDv10 | niaD−sC−adeA− ΔargB::adeA− ΔligD::argB ΔpyrG::adeA ΔAovps10::pyrG pCGFP [PAocpyA::AocpyA-egfp::Tnos3′::niaD] | This study |
| SlD1 | NSlD1 | niaD−sC−adeA− ΔargB::adeA− ΔligD::argB ΔpyrG::adeA pgEpG [pyrG] pgDN [niaD] | This study |
| SlDv10-1∼2 | NSlDv10 | niaD−sC−adeA− ΔargB::adeA− ΔligD::argB ΔpyrG::adeA ΔAovps10::pyrG pgDN [niaD] | This study |
| SlDv10-VTG1∼2 | NSlDv10 | niaD−sC−adeA− ΔargB::adeA− ΔligD::argB ΔpyrG::adeA ΔAovps10::pyrG pnVTG [PAovps10::Aovps10 cDNA-egfp::TamyB::niaD] | This study |
| SlD-AKC1 | NSlD1 | niaD−::pgAKCN [PamyB::amyB-kex2-CHY::TamyB::niaD] sC−adeA− ΔargB::adeA− ΔligD::argB ΔpyrG::adeA pgEpG [pyrG] | This study |
| SlDv10-AKC3∼4 | NSlDv10 | niaD−::pgAKCN [PamyB::amyB-kex2-CHY::TamyB::niaD] sC−adeA− ΔargB::adeA− ΔligD::argB ΔpyrG::adeA ΔAovps10::pyrG | This study |
| SlD-HLY1 | NSlD1 | niaD−::pgAFLN [PamyB::amyB-kex2-HLY::TamyB::niaD] sC−adeA− ΔargB::adeA− ΔligD::argB ΔpyrG::adeA pgEpG [pyrG] | This study |
| SlDv10-HLY1∼2 | NSlDv10 | niaD−::pgAFLN [PamyB::amyB-kex2-HLY::TamyB::niaD] sC−adeA− ΔargB::adeA− ΔligD::argB ΔpyrG::adeA ΔAovps10::pyrG | This study |
Molecular techniques for DNA manipulation.
The BP and LR recombination reactions for all plasmid construction using the MultiSite Gateway system were performed as instructed by the manufacturer (Invitrogen, Carlsbad, CA) (22). The PCR for cloning was performed with a 50-μl mixture containing 1× PCR buffer with Mg2+, deoxynucleoside triphosphate (dNTP) solution (0.2 mM each), primers (0.2 μM each), 1.25 U of PrimeSTAR HS DNA polymerase (TaKaRa, Otsu, Japan), and 100 ng of DNA. The thermocycling program consisted of an initial heating (98°C, 2 min), followed by 30 cycles of denaturation at (98°C, 10 s), annealing (58°C, 15 s), and extension at (72°C, 1 min/kb). The PCR for verification of the Aovps10 gene disruption was performed with a 20-μl mixture containing 1× PCR buffer with Mg2+, dNTP solution (0.2 mM each), primers (0.5 μM each), 0.5 U of ExTaq DNA polymerase (TaKaRa), and 10 ng of DNA. The thermal cycling parameters consisted of an initial heating (98°C, 2 min), followed by 30 cycles of denaturation at (98°C, 10 s), annealing (58°C, 30 s), and extension at (72°C, 6 min). All of the primers used in the present study are listed in Table 2.
TABLE 2.
Primers used in this study
| Function | Primer | Sequence (5′-3′)a |
|---|---|---|
| Amplification of Aovps10 cDNA for cloning | VPS10-cDNA-PF | CGGTCATGATTTATCGATGGC |
| VPS10-cDNA-RG | TCAAGCCTCGTCATCTTCATC | |
| Amplification of Aovps10 cDNA for expression plasmid construction | VPS10-cDNA-PF | CGGTCATGATTTATCGATGGC |
| VPS10-cDNA-RG-I | AGCCTCGTCATCTTCATCCAA | |
| Amplification of Aovps10 native promoter region for expression of plasmid construction | VPS10up-F | GAACTTCTCATCAAACAGCACCC |
| VPS10up-R | GACCGCTACTCTTGAAACATTTGAAAC | |
| Amplification of AocpyA gene for expression of plasmid construction | CPY-1458F | GGGGATATCCGCTGGTGCTGCAGACTTGA |
| CPY1678R | GGGGATATCGAACCATTCACCACCCAACCAG | |
| Disruption of Aovps10 gene | aB4-5v10_F | GGGGACAACTTTGTATAGAAAAGTTGCATGGACTACTCGCTTTGCGATA |
| aB1r-5v10_R | GGGGACTGCTTTTTTGTACAAACTTGCAACGCACACAAAGGCTTACGTT | |
| aB2r-v10_F | GGGGACAGCTTTCTTGTACAAAGTGGGATCGATACTGGATGGTGTGCT | |
| f-v10+p_R | CACTATAGATCAACGCACACAAAGGCTTACGTT | |
| f-v10+p_F | GTGTGCGTTGATCTATAGTGTACGGTTGTGGGC | |
| aB3-v10_R | GGGGACAACTTTGTATAATAAAGTTGCTTCAGGCGCTGTTAAACATGTC | |
| Verification of Aovps10 gene disruption/ verification PCR | Aovps10up-1527bp-F | GGATTCGGACCTGAGTTCTGATA |
| Aovps10down-4671bp-R | TAAAATCATGGATACGGCGGAGC | |
| Southern blot probe | Aovps10up-1500bp-F | CATGGACTACTCGCTTTGCGATAA |
| Aovps10up-413bp-R | ATCTCAACCATGGGGTGAACATC |
The attB sequences for cloning with MultiSite Gateway system are underlined. The nucleotide sequences for fusion PCR are indicated in boldface.
Cloning of Aovps10 cDNA and sequencing.
For the cloning of Aovps10 cDNA, a DNA fragment containing the Aovps10 cDNA was amplified by reverse transcription-PCR. Total RNA was extracted by using an RNeasy midikit (Qiagen), which was then subjected to mRNA purification by using an Oligotex-dT30<Super> kit (TaKaRa). Total cDNA was synthesized by SuperScript reverse transcriptase (Invitrogen) and was used as a template for PCR with the two primers VPS10-cDNA-PF and VPS10-cDNA-RG and ligated to pT7Blue, generating pVPTcD. cDNA sequences of AoVps10-encoding genes were determined by ABI Prism 310NT genetic analyzer (Applied Biosystems, Foster City, CA).
Construction of the Aovps10 gene disruptant.
The 1.4-kb upstream flanking region of the Aovps10 open reading frame (ORF) was amplified with the primers aB4-5v10_F and aB1r-5v10_R using genomic DNA as a template and then inserted into pDONRP4-P1R by the BP recombination reaction, generating the 5′ entry clone, pg5′v10. The 0.3-kb upstream and 1.4-kb downstream flanking regions of the gene were amplified with the primer pairs aB2r-v10_F/f-v10+p_R and f-v10+p_F/aB3-v10_R, respectively. The two fragments were fused with the primers aB2r-v10_F and aB3-v10_R and inserted into pDONRP2R-P3 by the BP recombination reaction, generating the 3′ entry clone, pg3′v10+p. The obtained 5′ and 3′ entry clones, together with the center entry clone plasmid, pgEpG, were combined for the LR recombination reaction with the destination vector, pDESTR4-R3, generating pgΔv10pG. The gene disruption fragment for Aovps10 was amplified with the primers aB4-5v10_F and aB3-v10_R using pgΔv10pG as a template and was introduced into the NSPlD1 strain (niaD− sC− ΔpyrG ΔligD). M+Met medium was used for selection of transformants. For verification of the Aovps10 gene disruption, genomic DNA was used as a template for PCR analysis using the primers Aovps10up-1527bp-F and Aovps10down-4671bp-R.
Expression plasmid construction.
The plasmid encoding AoVps10-EGFP was constructed as follows. The ORF of the Aovps10 gene was PCR amplified with the primers VPS10-cDNA-PF and VPS10-cDNA-RG-I using pVPTcD as a template. The amplified Aovps10 cDNA fragment was phosphorylated and inserted into SmaI-digested pgEHH (22), generating pgVTcD (Invitrogen; for generation of the center entry clone). The promoter region of the Aovps10 gene was amplified with the primers VPS10up-F and VPS10up-R using genomic DNA as a template. The amplified Aovps10 promoter fragment was phosphorylated and inserted into SmaI-digested pg5′Pp (22), generating 5′ entry clone, pgVTPr. The generated center entry clone (pgVTcD), 5′ entry clone (pgVTPr), and 3′ entry clone (pg3′E) (22) were mixed with the destination vector (pgDN) (22) for the LR recombination reaction. The resulting plasmid pnVTG contained the Aovps10 cDNA-egfp fusion gene under the control of the Aovps10 native promoter, along with the niaD marker.
The AoCpyA-EGFP expression plasmid was constructed by amplifying the AocpyA gene, including the promoter region, using the primers CPY-1458F and CPY1678R and genomic DNA of A. oryzae RIB40 strain as a template. The amplified fragment was digested with EcoRI and EcoRV and then ligated with EcoRI/EcoRV-digested pBluescript II SK(+), creating pCG1. The fragment of the egfp gene with nos3′ terminator (∼1 kb) excised from pBEGFP-F (26) by the EcoRI was inserted into the EcoRI site of pCG1, generating pCG2. The 4.3-kb fragment containing AocpyA-egfp with nos3′ terminator was excised by EcoRV and SmaI and then ligated with the HindII/SmaI-digested/blunted pNR10 containing the niaD marker gene to generate pCGFP.
The expression plasmids for bovine chymosin (CHY) and human lysozyme (HLY) used in the present study were constructed as described previously (32, 45).
Southern blot analysis.
The Aovps10 gene disruptants and strains expressing heterologous proteins were analyzed by Southern blot analysis. Briefly, after electrophoresis, the digested genomic DNAs were transferred onto the Hybond N+ membrane (GE Healthcare, Buckinghamshire, United Kingdom). The enhanced chemiluminescence (ECL) direct nucleic acid labeling and detection system (GE Healthcare) and a LAS-4000miniEPUV luminescent image analyzer (Fuji Photo Film, Tokyo, Japan) were used for detection.
Western blot analysis.
CHY-expressing transformants were cultured in 20 ml of 5× DPY (pH 5.5) medium at 30°C for 4 days. Portions (4 μl) of the culture supernatant were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred onto a cellulose nitrate membrane Immobilon-NC (Millipore, Bedford, MA) by using a semidry blotting system (Nihon Eido, Tokyo, Japan). The membrane was immunostained using a polyclonal rabbit serum against CHY (Nordic Immunological Laboratories, Tilburg, Netherlands) and anti-rabbit immunoglobulin G labeled with horseradish peroxidase (Vector Laboratories, Peterborough, United Kingdom), and the bands were visualized by using an ECL Advance Western blotting detection kit (GE Healthcare).
Fluorescence microscopy.
For fluorescence microscopy, we used an Olympus system microscope model BX52 (Olympus, Tokyo, Japan) equipped with an UPlanApo 100× objective lens (1.35 numerical aperture) (Olympus). A GFP filter (495/520-nm excitation, 510-nm dichroic, 530/535-nm emission; Chroma Technologies, Brattleboro, VT) was used for observing EGFP fluorescence. Confocal microscopy was performed with an IX71 inverted microscope (Olympus) equipped with a 100× Neofluor objective lens (1.40 numerical aperture); 488-nm (Furukawa Electric, Japan) and 561-nm (Melles Griot) semiconductor lasers; GFP, DsRed, and DualView filters (Nippon Roper, Chiba, Japan); a CSU22 confocal scanning system (Yokogawa Electronics, Tokyo, Japan); and an Andor iXon cooled digital charge-coupled device camera (Andor Technology PLC, Belfast, United Kingdom). Images were analyzed with the Andor iQ software (Andor Technology PLC). Approximately 105 conidia were inoculated in 100 μl of liquid medium, followed by incubation on cover glasses for fluorescence microscopy or in glass-based dishes (Asahi Techno Glass, Chiba, Japan) for confocal laser microscopy. FM4-64 (Molecular Probes, Eugene, OR) was prepared as a 1.6 mM solution in dimethyl sulfoxide. Approximately 18 h after inoculation, the cultures were transferred into a medium containing 8 μM FM4-64 and incubated for 2 min at room temperature. After incubation, FM4-64-containing medium was replaced with fresh dye-free medium, and the samples were examined.
Measurement of EGFP fluorescence in the medium.
Extracellular EGFP fluorescence was measured by using a SAFIRE multifunctional microplate reader with excitation and emission wavelengths of 488 and 510 nm, respectively (Tecan Group Ltd., Mannedorf, Switzerland).
Protease activity assay.
The total extracellular protease activity in the culture medium (DPY [pH 5.5]) of each A. oryzae strain was analyzed by using a Calbiochem protease assay kit (EMD Bioscience, San Diego, CA), in which a fluorescein thiocarbamoyl-labeled casein (FTC-casein) was used as the protease substrate. The total protease activity of each sample was then determined from the obtained absorbance at 492 nm of the proteolytically cleaved small FTC-peptides in the reaction supernatant, which were collected by precipitating the uncleaved FTC-casein with trichloroacetic acid (3.6% [wt/vol]) treatment.
Measurement of CHY production yield.
Approximately 2 × 105 conidia of the CHY-expressing transformant were inoculated into 20 ml of 5× DPY (pH 5.5) medium and then cultured at 30°C for 3 to 6 days. The CHY activity was measured by the method described previously by Yoon et al. (45).
HLY activity assay.
Approximately 106 conidia of the HLY-expressing transformant were inoculated and cultured in 100 ml of 5× DPY (pH 8.0) medium at 30°C. Lysozyme activity was measured as described by Morsky and Aine (30). The culture supernatant (20 μl) was mixed with 80 μl of a 150-μg/ml suspension of Micrococcus lysodeikticus ATCC 4698 lyophilized cells (Sigma) in 50 mM phosphate buffer (pH 6.2). The decrease in absorbance at 450 nm of the mixture, which was due to lysis of the bacterial cells, was monitored at room temperature. One unit was defined as the activity that reduced the absorbance value by 0.001 per min.
RESULTS
Cloning and sequence analysis of cDNA encoding A. oryzae AoVps10.
The A. oryzae genome database DOGAN (http://www.bio.nite.go.jp/dogan/MicroTop?GENOME_ID=ao) was first searched for the gene homologous to the S. cerevisiae vacuolar sorting receptor gene (VPS10), and a gene (ID AO090010000769) was identified and designated Aovps10. The predicted amino acid sequence of Aovps10 was aligned and compared to using the CLUSTAL W (http://align.genome.jp/) program. Comparative sequence analysis between the nucleotide sequence of the Aovps10 gene and the cDNA revealed that the AoVps10 sequence encoded for 1,488 amino acids and included one intron of 63 bp in the coding region. Furthermore, a strong sequence similarity was found among Vps10p from other yeasts. For example, Vps10p from S. cerevisiae and S. pombe shared 29% identity (see Fig. S1 in the supplemental material).
AoVps10 was found to contain a typical N-terminal signal peptide of 23 amino acid residues and two 23-amino-acid transmembrane domains near the C-terminal region, as determined by the web-based search programs SignalP 3.0 (http://www.cbs.dtu.dk/services/SignalP/) and TMHMM 2.0 (http://www.cbs.dtu.dk/services/TMHMM/). Moreover, Asp box motifs (Ser/Thr-X-Asp-X-Gly-X-Thr-Trp/Phe) (17, 37) and two highly conserved residues of a cysteine-rich motif (13, 35) were present in both luminal domains 1 and 2 (see Fig. S1 in the supplemental material). The characteristics of these conserved amino acid sequences of AoVps10 agreed with known properties of Vps10p from S. cerevisiae and S. pombe. Thus, based on the observed similarities in the DNA and protein sequences, AoVps10 likely shares the identical functional properties as other sorting receptors for vacuolar proteins in A. oryzae.
Localization analysis of AoVps10-EGFP in A. oryzae.
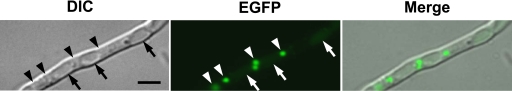
To determine the localization of AoVps10 in A. oryzae, a strain ectopically expressing Aovps10 cDNA-egfp, was constructed. Examination of the AoVps10-EGFP-expressing strain (VTG1) by fluorescence microscopy revealed that AoVps10 localized as small punctate structures in the vicinity of vacuoles (Fig. 1). This localization pattern was similar to those observed in yeasts, such as S. cerevisiae, suggesting that Vps10p is a receptor that sorts several different vacuolar proteins by cycling between late-Golgi and prevacuolar compartments (3, 5, 24). Thus, we assumed that the dot-like structures observed near the vacuoles were prevacuolar compartments, and the structures distant from these vacuoles were late-Golgi, which is in agreement with the systematic observation of SNARE localization in A. oryzae (20, 38).
FIG. 1.
Localization of AoVps10-EGFP. The strain expressing AoVps10-EGFP (VTG1) was cultivated in CD medium adjusted to pH 5.5 at 30°C for 18 h on a cover glass and observed by DIC and fluorescence microscopy. The arrows and arrowheads in the panel indicate vacuoles and dot-like structures, respectively. Bar, 5 μm.
Disruption of the Aovps10 gene.
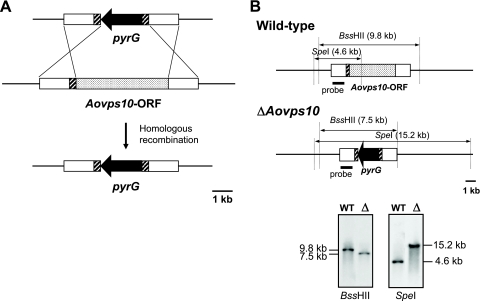
To generate a strain of A. oryzae unable to express Aovps10, the 1.4-kb flanking regions of the Aovps10 ORF were cloned and ligated with the pyrG gene (Fig. 2 A). In this construct, the 3′ end of the upstream flanking region (∼0.3 kb) was fused with the downstream flanking region in order to flank the pyrG marker gene with ∼0.3-kb directed repeats (Fig. 2A, hatched box). After the disrupted fragment of the Aovps10 gene was introduced into the NSPlD1 strain and successful homologous recombination was confirmed by PCR and Southern blot analyses, five of the six transformants, representing an efficiency of 83%, contained a disruption of the Aovps10 gene (Fig. 2B). Examination of the growth and morphological characteristics did not reveal any significant abnormalities in the Aovps10 gene disruptant (data not shown). As a result, it was confirmed that an Aovps10 gene disruptant, called NSlDv10, was successfully generated.
FIG. 2.
Disruption of the Aovps10 gene in the A. oryzae strain NSPlD1 (niaD− sC− ΔpyrG ΔligD). (A) Schematic model of the approach used to disrupt the Aovps10 gene using the pyrG marker gene. The open boxes (1.4 kb) represent the flanking regions of the Aovps10 gene. The 0.3-kb upstream flanking region of the Aovps10 gene (hatched boxes) was attached at the 5′ end of the downstream flanking region to introduce direct repeat sequences. (B) Southern blot analysis of the Aovps10 gene disruptants. After isolated genomic DNA was digested with BssHII and SpeI, separated by gel electrophoresis, and subjected to Southern blot analysis, a clone was identified which exhibited the expected banding pattern for disruption of the Aovps10 gene. “WT” and “Δ” represent the wild-type strain (NSPlD1) and gene disruptant (NSlDv10), respectively.
Functional analysis of AoVps10 in A. oryzae.
Using the S. cerevisiae CPY gene, which encodes vacuolar carboxypeptidase Y, to search the A. oryzae genome database DOGAN allowed the identification of one homologous gene designated AocpyA (ID AO090103000332). The predicted amino acid sequence of the AocpyA gene was compared to the sequences of vacuolar carboxypeptidases (CPYs) from a filamentous fungus and several yeast species and demonstrated high similarities (see Fig. S2 in the supplemental material). Mature AoCpyA showed 90, 65, and 58% identities to CPYs from A. nidulans (33), S. cerevisiae (43), and S. pombe (40), respectively.
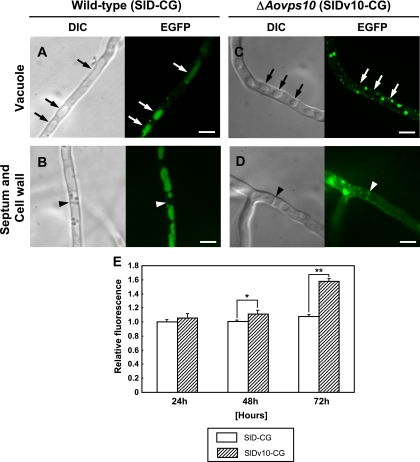
To examine the effect of the Aovps10 gene disruption on the localization of carboxypeptidase Y (AoCpyA) in A. oryzae, the SlDv10-CG strain, which expresses the AocpyA-egfp fusion gene in the NSlDv10 background, was generated. Hyphae of the ΔAovps10 mutant were observed by both differential interference contrast (DIC) and confocal laser microscopy (Fig. 3 A to D). For wild-type strain SlD-CG, large, elongated, vacuolelike structures identified by DIC microscopy were found to contain high levels of EGFP fluorescence during confocal microscopic analysis (Fig. 3A), although EGFP fluorescence was not present in septa or cell walls (Fig. 3B). In contrast, the ΔAovps10 strain localized the AoCpyA-EGFP fusion protein to septa and cell walls as opposed to vacuoles (Fig. 3C and D). Moreover, these observations were consistent with the known septal localization patterns of secretory hydrolytic enzymes fused with (E)GFP, such as the RNase of A. oryzae (27) and the glucoamylase of A. niger (9). The dispersed, round structures observed in the ΔAovps10 strain were attributed to accumulation of the fusion protein at unknown organelles (Fig. 3C and D). To determine whether the small punctuate structures are endosomes, the ΔAovps10 strain was stained with FM4-64, a lipophilic styryl dye used as an endocytic marker. The dye labels cortical punctuate structures, as well as the membranes of circular, hollow organelles, and intermediate and large size vacuoles (34). The dispersed, round structures observed in the ΔAovps10 strain did not colocalize to endocytic organelles, suggesting that the unknown organelles were not involved in the endocytic pathway in A. oryzae (see Fig. S3 in the supplemental material). This result (Fig. 3A to D) indicates that in the Aovps10 gene disruptant, intracellular trafficking of AoCpyA to vacuoles was reduced and, thus, its localization in vacuoles was not observed. The localization to septa and cell walls was attributed to AoCpyA, which was no longer transported to the vacuoles by disruption of the Aovps10 gene and was instead missorted and secreted into the medium. Taken together, these results suggest that AoVps10 is a vacuolar protein sorting receptor that is required for targeting and delivery of vacuolar enzyme AoCpyA to the vacuole in A. oryzae.
FIG. 3.
Mislocalization of AoCpyA-EGFP in the A. oryzae Aovps10 gene disruptant NSlDv10. The strains SlD-CG (A and B) and SlDv10-CG (C and D), which both expressed AoCpyA-EGFP, were cultivated in CD medium adjusted to pH 5.5 at 30°C for 20 h and observed by DIC and confocal laser microscopy. The arrows and arrowheads indicate vacuoles (top panels) and septa (lower panels), respectively. Bars, 5 μm. (E) Total amount of extracellular EGFP fluorescence in the culture medium of strains SlD-CG and SlDv10-CG expressing AoCpyA-EGFP. After culture in 20 ml of DPY medium (pH 5.5) at 30°C for 24 h to 72 h, the total relative amounts of each cell culture supernatant were measured and compared. Three replicate experiments were performed, and the average values and standard deviations were determined (*, P < 0.05; **, P < 0.001 [Student t test]).
To investigate the total amount of missorted AoCpyA caused by the Aovps10 gene disruption, the culture medium for mutant strain SlDv10-CG, which expresses the AocpyA-egfp fusion gene, was measured for EGFP fluorescence. Since this measurement can be used to estimate the total amount of an EGFP-fused target protein, a microtiter plate assay was used as a fast, simple, and reliable method for the quantification of EGFP in the medium. After culturing for 24 h, no significant changes in the relative amount of EGFP fluorescence were observed between the ΔAovps10 and wild-type (SlD-CG) strains. However, after 48 h, the relative amount of EGFP fluorescence for strain SlDv10-CG was significantly higher than that of SlD-CG (Fig. 3E). This result is in agreement with the DIC and fluorescence microscopic analyses shown in Fig. 3A to D.
Complementation analysis by expressing the Aovps10-egfp fusion gene.
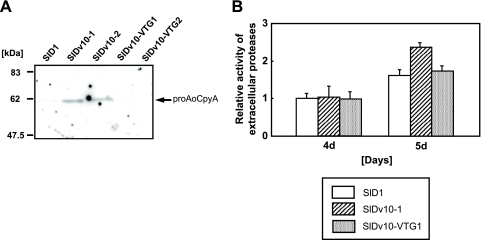
To confirm whether AoVps10-EGFP is functional in ΔAovps10 mutant, the SlDv10-VTG strains, which express the AocpyA-egfp fusion gene in the NSlDv10 background, were generated. Western blot analysis with anti-AoCpyA antibody revealed the presence of proAoCpyA in the culture supernatant of the Aovps10 gene disruptants (SlDv10-1∼2). In contrast, the Aovps10-egfp fusion gene complemented strains SlDv10-VTG1 and SlDv10-VTG2 could not secrete proAoCpyA into the culture medium (Fig. 4 A). Furthermore, total extracellular protease activity test also showed that missorting and secretion of proAoCpyA into the culture medium could be restored ca. 90% by the complementation of Aovps10-egfp fusion gene (Fig. 4B), suggesting that AoVps10-EGFP is functional in ΔAovps10 mutant.
FIG. 4.
Complementation analysis by expressing the Aovps10-egfp fusion gene. (A) Western blot analysis of the culture supernatant of the Aovps10-egfp fusion gene complemented strains. The control (SlD1), SlDv10, and SlDv10-VTG strains expressing AoVps10-EGFP were cultivated in 20 ml of DPY (pH 5.5) medium at 30°C for 2 days. Culture supernatants (20 μl) were subjected to Western blot analysis. The predicted bands of ∼62 kDa in size were confirmed by using the anti-AoCpyA antibody. (B) Relative extracellular protease activity in the Aovps10-egfp fusion gene complemented strains. The control (SlD1), SlDv10, and SlDv10-VTG strains expressing AoVps10-EGFP were cultivated in 20 ml of DPY (pH 5.5) medium at 30°C for 4 to 5 days. The total extracellular protease activity was measured with each culture medium of the strain using FTC-casein as the protease substrate. Three replicate experiments were performed, and the values of the average and standard deviations are represented.
Effect of the Aovps10 gene disruption on heterologous protein production in A. oryzae.
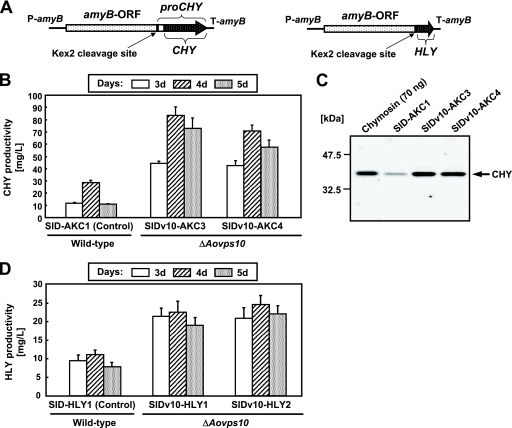
In order to investigate whether the production levels of heterologous proteins were improved in the Aovps10 gene disruptant NSlDv10, we constructed strains expressing CHY, as a model protein. The initial translation product of CHY, prochymosin, contains a 42-amino-acid N-terminal sequence which is automatically cleaved at low pH to yield active chymosin (7, 8). A. oryzae α-amylase (AmyB) was used as the carrier protein for fusion with CHY since such fusions have been shown to aid the successful production of heterologous proteins (15, 16, 31). A plasmid for CHY production was constructed in which the gene expression construct consisted of the amyB promoter and structural gene, followed by the region of the CHY gene that contains the prosequence. In addition, the Kex2 cleavage site (-Lys-Arg-) was included upstream of prochymosin (Fig. 5 A, left). The plasmid for CHY expression was introduced into the pyrG marker gene-complemented strain (NSlD1) and the Aovps10 gene disruptant (NSlDv10) using the niaD gene as the selectable marker. The transformants possessing a single copy of the plasmid, which was integrated homologously at the niaD locus were identified by Southern blot analysis (data not shown).
FIG. 5.
Extracellular production of heterologous proteins by the Aovps10 gene disruptant. (A) Schematic structures of either the CHY- or HLY-expressing cassette. The proCHY gene was fused with the A. oryzae α-amylase gene (amyB) and the Kex2 cleavage site (-Lys-Arg-) was inserted upstream of proCHY (left). The HLY gene was fused with the amyB gene. The Kex2 cleavage site was inserted at the boundary of two genes (right). (B) Time course analysis for extracellular production of CHY by the Aovps10 gene disruptant. Approximately 2 × 105 conidia of the control (SlD-AKC1), SlDv10-AKC3, and SlDv10-AKC4 strains expressing CHY were cultivated in 20 ml of 5× DPY (pH 5.5) medium at 30°C for 3 to 5 days. The amount of CHY produced by each strain was calculated by determining the time required for a skimmed milk solution to clot using culture supernatant and comparing this value to a standard curve generated with purified bovine CHY (Sigma). (C) Western blot analysis of the culture supernatant of the CHY-expressing strains. The control (SlD-AKC1), SlDv10-AKC3, and SlDv10-AKC4 strains expressing CHY were cultivated in 20 ml of 5× DPY (pH 5.5) medium at 30°C for 4 days. Culture supernatants (4 μl) were subjected to Western blot analysis. Bands of ∼35.4 kDa were detected using the anti-CHY antibody. “Chymosin” refers to purified bovine chymosin (∼70 ng; Sigma). (D) Extracellular production of HLY by the Aovps10 gene disruptant. The HLY-expressing transformants were cultivated in 100 ml of 5× DPY (pH 8.0) liquid medium at 30°C for 3 to 5 days. The production yields of HLY were calculated on the basis of lysozyme activities.
The amount of CHY produced by each strain was then determined by the milk-clotting activity of the culture supernatant, as described above (Fig. 5B). Comparative time course analysis revealed that the production yields by the Aovps10 gene disruptants SlDv10-AKC3 and SlDv10-AKC4 were 83.1 and 70.3 mg/liter, respectively, representing 3- and 2.5-fold higher production levels than the control strain SlD-AKC1 (28.7 mg/liter) (Fig. 5B). The culture supernatant of the Aovps10 gene disruptants was also examined by Western blot analysis with anti-CHY antibody, which confirmed the presence of mature CHY, as indicated by positively stained bands of ∼35.4 kDa (Fig. 5C). Moreover, Western blot analysis indicated that the amount of CHY produced by the Aovps10 gene disruptant was higher than that of the control strain.
To determine whether the extracellular production levels of other heterologous proteins were increased in the Aovps10 gene disruptant, we next generated HLY-producing strains from NSlDv10 by introducing the HLY expression plasmid (Fig. 5A, right). Transformation was performed with the A. oryzae niaD gene as the selectable marker. Southern blot analysis demonstrated that a single copy of the plasmid was homologously integrated at the niaD locus in the obtained transformant (data not shown).
The disruptant of Aovps10 gene was analyzed for extracellular HLY production (Fig. 5D). Disruption of the Aovps10 gene positively affected the production of HLY. Comparative time course analysis showed that the production of HLY peaked on the 4 days when the strains were cultivated in the 5× DPY medium (pH 8.0). The maximum extracellular HLY production yields from SlDv10-HLY1 and SlDv10-HLY2 were 22.6 and 24.6 mg/liter, respectively, which were 2- and 2.2-fold higher than the levels in the control strain (SlD-HLY1; 11.1 mg/liter) (Fig. 5D). These results demonstrate that disruption of the vacuolar protein sorting receptor gene Aovps10 in A. oryzae is very effective for increasing the extracellular production levels of not only CHY but HLY.
To date, the relationships between the vacuolar protein sorting gene disruption and homologous protein production are still unknown in the filamentous fungi. Thus, in order to investigate the effect of Aovps10 gene disruption on homologous protein production in A. oryzae, we measured the activities of α-amylase, an endogenous protein secreted in large amounts. As a result, the activities of the Aovps10 gene disruptant NSlDv10 were comparable to those of the control strain NSlD1 (data not shown), suggesting that AoVps10 receptor did not significantly affect the homologous protein production in A. oryzae.
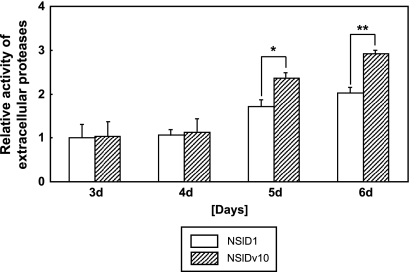
Despite the improvement in heterologous protein yields, a slight decrease in secretion was observed in the late growth phase of the ΔAovps10 mutant after 5 days of culture (Fig. 5B and D). In order to examine whether endopeptidase or exopeptidase activity influenced the yields of recombinant protein produced in the Aovps10 gene disruptant, an assay to measure total extracellular protease activity was conducted (Fig. 6). This analysis confirmed that the total protease activity in the late growth phase (5 to 6 days) was greater than in the middle growth phase (3 to 4 days). Furthermore, the relative activity of extracellular proteases in the Aovps10 gene disruptant (NSlDv10) was slightly higher than that of the wild-type strain (NSlD1), which may have been due to the missorting and accumulation of vacuolar proteases in the medium caused by the Aovps10 gene disruption. Taken together, these results suggest that in order to achieve high total yields of heterologous protein in A. oryzae, it is also necessary to limit the total protease activity.
FIG. 6.
Time course analysis for the relative extracellular protease activity in the Aovps10 gene disruptant. The control (NSlD1) and the NSlDv10 strains were cultivated in 20 ml of 5× DPY (pH 5.5) medium at 30°C for 3 to 6 days. The total extracellular protease activity was measured with each culture medium of the strain using FTC-casein as the protease substrate. Three replicate experiments were performed, and the values of the average and standard deviations are represented (*, P < 0.05; **, P < 0.001 [Student t test]).
DISCUSSION
In the present study, we isolated a cDNA encoding AoVps10 from the filamentous fungus A. oryzae for the first time. The predicted amino acid sequence analysis of the Aovps10 ORF showed a high similarity to vacuolar protein sorting receptors from several microorganisms (see Fig. S1 in the supplemental material), suggesting that the vacuolar protein sorting pathway is highly conserved in the filamentous fungi.
We investigated the effect of the Aovps10 gene disruption in A. oryzae on heterologous protein production and also elucidated the molecular mechanism of intracellular protein trafficking to vacuoles by AoVps10. As shown in Fig. 1, the localization pattern of AoVps10 suggests that Vps10p is a receptor that sorts several different vacuolar proteins by cycling between late-Golgi and prevacuolar compartments. Moreover, functional analysis of AoVps10 revealed that it is a vacuolar protein sorting receptor required for targeting and delivery of AoCpyA to vacuoles in A. oryzae (Fig. 3). Interestingly, it was also confirmed that either recombinant CHY or HLY production in a single Aovps10 gene disruptant resulted in 3- and 2.2-fold higher yields than the wild-type strain, respectively (Fig. 5), which suggests that increased protein production levels are due to defects in the vacuolar protein trafficking pathway between the Golgi apparatus and vacuoles. The remarkable increase in CHY production by only a single gene disruption is very intriguing since it was comparable to the yield of a quintuple protease gene disruptant (84.4 mg/liter) (45). Although very recently the vps10 gene deletion was found to be effective for the production of recombinant human growth hormone (r-hGH) in the fission yeast S. pombe (14), this is the first report of targeted disruption of a vacuolar protein sorting gene in a filamentous fungus which could dramatically enhance the production levels of heterologous proteins.
Previously, we used DNA microarrays to monitor the expression of 134 protease genes for screening of the disruption target (18). In the course of monitoring global gene expression, several protease genes, including tppA, pepE, nptB, dppIV, and dppV, were identified which were elevated in the late growth phase (5 to 6 days) (18) and were subsequently disrupted to examine their effect on heterologous protein degradation (45). Based on these studies, it is expected that disruption of the vacuolar protein sorting gene Aovps10, in addition to these protease genes, might synergistically improve heterologous protein productivity and yields in A. oryzae. Meanwhile, alternatively, unexpected proteolytic degradation by the activities of certain kinds of missorted and secreted vacuolar proteases into the culture medium might also occur, since AoVps10 receptor is responsible for the recognition and sorting of some vacuolar proteases to the vacuole. These gene disruption combinations should provide new insights into molecular breeding approaches for filamentous fungi.
We measured the activities of α-amylase and could not observe any significant differences between the wild-type strain and the ΔAovps10 mutants. α-Amylase is not a vacuolar hydrolase but one of the most efficiently and abundantly secreted endogenous enzyme in A. oryzae. Therefore, it is suggested that α-amylase is independent to AoVps10 receptor.
The protein quality control process is a mechanism by which newly synthesized proteins in the endoplasmic reticulum (ER) are monitored and assisted to ensure that proper folding and assembly occur (6). This process also detects misfolded or aberrant proteins and removes them from the secretory pathway. Another mechanism for removing abnormal proteins from the secretory pathway has been identified that involves routing targeted proteins from the Golgi to the vacuolar system for degradation (2). Although delivery of incorrectly assembled membrane complexes for lysosomal degradation has been described in mammalian cells (1, 28), it seems likely that a Golgi-based quality control process might play a more prominent role in yeast (12, 46) and the filamentous fungus A. oryzae for removing both misfolded and aberrantly assembled proteins.
In conclusion, we have demonstrated in the present study that the vacuolar protein sorting pathway is highly conserved in the filamentous fungus A. oryzae, with AoVps10 playing a role in the regulation of the heterologous protein secretory pathway by its involvement in vacuolar protein degradation through the Golgi apparatus. In future studies, transcriptomic analysis by DNA microarrays to identify genes affecting heterologous protein production, together with the determination of ligand-binding domains for recombinant protein interactions, will reveal important details about the specific role of AoVps10 in heterologous protein production.
Supplementary Material
Acknowledgments
We thank Toshiko Tanaka for experimental help and Chikara Tokunaga for providing plasmid pCGFP.
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and by the Program for the Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN) of Japan.
Footnotes
Published ahead of print on 9 July 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Armstrong, J., S. Patel, and P. Riddle. 1990. Lysosomal sorting mutants of coronavirus E1 protein, a Golgi membrane protein. J. Cell Sci. 95:191-197. [DOI] [PubMed] [Google Scholar]
- 2.Arvan, P., X. Zhao, J. Ramos-Castaneda, and A. Chang. 2002. Secretory pathway quality control in the Golgi, plasmalemmal, and endosomal systems. Traffic 3:771-780. [DOI] [PubMed] [Google Scholar]
- 3.Cereghino, J. L., E. G. Marcusson, and S. D. Emr. 1995. The cytoplasmic tail domain of the vacuolar protein sorting receptor Vps10p and a subset of VPS gene products regulate receptor stability, function, and localization. Mol. Biol. Cell 6:1089-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christensen, T., H. Woeldike, F. Boel, S. B. Mortensen, K. Hjortshoej, L. Thim, and M. Hansen. 1988. High-level expression of recombinant genes in Aspergillus oryzae. Biotechnology 6:1419-1422. [Google Scholar]
- 5.Cooper, A. A., and T. H. Stevens. 1996. Vps10p cycles between the late-Golgi and prevacuolar compartments in its function as the sorting receptor for multiple yeast vacuolar hydrolases. J. Cell Biol. 133:529-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellgard, L., M. Molinari, and A. Helenius. 1999. Setting the standards: quality control in the secretory pathway. Science 286:1882-1888. [DOI] [PubMed] [Google Scholar]
- 7.Foltmann, B. 1970. Prochymosin and chymosin (prorennin and rennin). Methods Enzymol. 19:421-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foltmann, B. 1971. The biochemistry of prorennin (prochymosin) and rennin (chymosin), p. 217-254. In H. A. McKenzie (ed.), The milk proteins, vol. 2. Academic Press, Inc., New York, NY. [Google Scholar]
- 9.Gordon, C. L., V. Khalaj, A. F. Ram, D. B. Archer, J. L. Brookman, A. P. Trinci, D. J. Jeenes, J. H. Doonan, B. Wells, P. J. Punt, C. A. van den Hondel, and G. D. Robson. 2000. Glucoamylase::green fluorescent protein fusions to monitor protein secretion in Aspergillus niger. Microbiology 146:415-426. [DOI] [PubMed] [Google Scholar]
- 10.Gottesman, S. 1990. Minimizing proteolysis in Escherichia coli: genetic solutions. Methods Enzymol. 185:119-129. [DOI] [PubMed] [Google Scholar]
- 11.Holkeri, H., and M. Makarow. 1998. Different degradation pathways for heterologous glycoproteins in yeast. FEBS Lett. 429:162-166. [DOI] [PubMed] [Google Scholar]
- 12.Hong, E., A. R. Davidson, and C. A. Kaiser. 1996. A pathway for targeting soluble misfolded proteins to the yeast vacuole. J. Cell Biol. 135:623-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horazdovsky, B. F., D. B. DeWald, and S. D. Emr. 1995. Protein transport to the yeast vacuole. Curr. Opin. Cell Biol. 7:544-551. [DOI] [PubMed] [Google Scholar]
- 14.Idiris, A., H. Tohda, M. Sasaki, K. Okada, H. Kumagai, Y. Giga-Hama, and K. Takegawa. 2009. Enhanced protein secretion from multiprotease-deficient fission yeast by modification of its vacuolar protein sorting pathway. Appl. Microbiol. Biotechnol. 85:667-677. [DOI] [PubMed] [Google Scholar]
- 15.Ito, K., T. Asakura, Y. Morita, K. Nakajima, A. Koizumi, A. Shimizu-Ibuka, K. Masuda, M. Ishiguro, T. Terada, J. Maruyama, K. Kitamoto, T. Misaka, and K. Abe. 2007. Microbial production of sensory-active miraculin. Biochem. Biophys. Res. Commun. 360:407-411. [DOI] [PubMed] [Google Scholar]
- 16.Jin, F. J., T. Watanabe, P. R. Juvvadi, J. Maruyama, M. Arioka, and K. Kitamoto. 2007. Double disruption of the proteinase genes, tppA and pepE, increases the production level of human lysozyme by Aspergillus oryzae. Appl. Microbiol. Biotechnol. 76:1059-1068. [DOI] [PubMed] [Google Scholar]
- 17.Jørgensen, M. U., S. D. Emr, and J. R. Winther. 1999. Ligand recognition and domain structure of Vps10p, a vacuolar protein sorting receptor in Saccharomyces cerevisiae. Eur. J. Biochem. 260:461-469. [DOI] [PubMed] [Google Scholar]
- 18.Kimura, S., J. Maruyama, M. Takeuchi, and K. Kitamoto. 2008. Monitoring global gene expression of proteases and improvement of human lysozyme production in the nptB gene disruptant of Aspergillus oryzae. Biosci. Biotechnol. Biochem. 72:499-505. [DOI] [PubMed] [Google Scholar]
- 19.Kitamoto, K. 2002. Molecular biology of the Koji molds. Adv. Appl. Microbiol. 51:129-153. [DOI] [PubMed] [Google Scholar]
- 20.Kuratsu, M., A. Taura, J. Y. Shoji, S. Kikuchi, M. Arioka, and K. Kitamoto. 2007. Systematic analysis of SNARE localization in the filamentous fungus Aspergillus oryzae. Fungal Genet. Biol. 44:1310-1323. [DOI] [PubMed] [Google Scholar]
- 21.Lemmon, S. K., and L. M. Traub. 2000. Sorting in the endosomal system in yeast and animal cells. Curr. Opin. Cell Biol. 12:457-466. [DOI] [PubMed] [Google Scholar]
- 22.Mabashi, Y., T. Kikuma, J. Maruyama, M. Arioka, and K. Kitamoto. 2006. Development of a versatile expression plasmid construction system for Aspergillus oryzae and its application to visualization of mitochondria. Biosci. Biotechnol. Biochem. 70:1882-1889. [DOI] [PubMed] [Google Scholar]
- 23.Machida, M., K. Asai, M. Sano, T. Tanaka, T. Kumagai, G. Terai, K. Kusumoto, T. Arima, O. Akita, Y. Kashiwagi, K. Abe, K. Gomi, H. Horiuchi, K. Kitamoto, T. Kobayashi, M. Takeuchi, D. W. Denning, J. E. Galagan, W. C. Nierman, J. Yu, D. B. Archer, J. W. Bennett, D. Bhatnagar, T. E. Cleveland, N. D. Fedorova, O. Gotoh, H. Horikawa, A. Hosoyama, M. Ichinomiya, R. Igarashi, K. Iwashita, P. R. Juvvadi, M. Kato, Y. Kato, T. Kin, A. Kokubun, H. Maeda, N. Maeyama, J. Maruyama, H. Nagasaki, T. Nakajima, K. Oda, K. Okada, I. Paulsen, K. Sakamoto, T. Sawano, M. Takahashi, K. Takase, Y. Terabayashi, J. R. Wortman, O. Yamada, Y. Yamagata, H. Anazawa, Y. Hata, Y. Koide, T. Komori, Y. Koyama, T. Minetoki, S. Suharnan, A. Tanaka, K. Isono, S. Kuhara, N. Ogasawara, and H. Kikuchi. 2005. Genome sequencing and analysis of Aspergillus oryzae. Nature 438:1157-1161. [DOI] [PubMed] [Google Scholar]
- 24.Marcusson, E. G., B. F. Horazdovsky, J. L. Cereghino, E. Gharakhanian, and S. D. Emr. 1994. The sorting receptor for yeast vacuolar carboxypeptidase Y is encoded by the VPS10 gene. Cell 77:579-586. [DOI] [PubMed] [Google Scholar]
- 25.Maruyama, J., and K. Kitamoto. 2008. Multiple gene disruptions by marker recycling with highly efficient gene-targeting background (ΔligD) in Aspergillus oryzae. Biotechnol. Lett. 30:1811-1817. [DOI] [PubMed] [Google Scholar]
- 26.Maruyama, J., H. Nakajima, and K. Kitamoto. 2001. Visualization of nuclei in Aspergillus oryzae with EGFP and analysis of the number of nuclei in each conidium by FACS. Biosci. Biotechnol. Biochem. 65:1504-1510. [DOI] [PubMed] [Google Scholar]
- 27.Masai, K., J. Maruyama, H. Nakajima, and K. Kitamoto. 2003. In vivo visualization of the distribution of a secretory protein in Aspergillus oryzae hyphae using the RntA-EGFP fusion protein. Biosci. Biotechnol. Biochem. 67:455-459. [DOI] [PubMed] [Google Scholar]
- 28.Minami, Y., A. M. Weissman, L. E. Samelson, and R. D. Klausner. 1987. Building a multichain receptor: synthesis, degradation, and assembly of the T-cell antigen receptor. Proc. Natl. Acad. Sci. U. S. A. 84:2688-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minetoki, T., Y. Nunokawa, K. Gomi, K. Kitamoto, C. Kumagai, and G. Tamura. 1996. Deletion analysis of promoter elements of the Aspergillus oryzae agdA gene encoding alpha-glucosidase. Curr. Genet. 30:432-438. [DOI] [PubMed] [Google Scholar]
- 30.Morsky, P., and E. Aine. 1983. Determination of lysozyme in tears by immunoturbidimetric and optimized kinetic bacteriolytic methods. Clin. Chim. Acta 129:201-209. [DOI] [PubMed] [Google Scholar]
- 31.Nakajima, K., T. Asakura, J. Maruyama, Y. Morita, H. Oike, A. Shimizu-Ibuka, T. Misaka, H. Sorimachi, S. Arai, K. Kitamoto, and K. Abe. 2006. Extracellular production of neoculin, a sweet-tasting heterodimeric protein with taste-modifying activity, by Aspergillus oryzae. Appl. Environ. Microbiol. 72:3716-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nemoto, T., T. Watanabe, Y. Mizogami, J. Maruyama, and K. Kitamoto. 2009. Isolation of Aspergillus oryzae mutants for heterologous protein production from a double proteinase gene disruptant. Appl. Microbiol. Biotechnol. 82:1105-1114. [DOI] [PubMed] [Google Scholar]
- 33.Ohsumi, K., Y. Matsuda, H. Nakajima, and K. Kitamoto. 2001. Cloning and characterization of the cpyA gene encoding intracellular carboxypeptidase from Aspergillus nidulans. Biosci. Biotechnol. Biochem. 65:1175-1180. [DOI] [PubMed] [Google Scholar]
- 34.Peñalva, M. A. 2005. Tracing the endocytic pathway of Aspergillus nidulans with FM4-64. Fungal Genet. Biol. 42:963-975. [DOI] [PubMed] [Google Scholar]
- 35.Petersen, C. M., M. S. Nielsen, A. Nykjaer, L. Jacobsen, N. Tommerup, H. H. Rasmussen, H. Roigaard, J. Gliemann, P. Madsen, and S. K. Moestrup. 1997. Molecular identification of a novel candidate sorting receptor purified from human brain by receptor-associated protein affinity chromatography. J. Biol. Chem. 272:3599-3605. [DOI] [PubMed] [Google Scholar]
- 36.Punt, P. J., N. van Biezen, A. Conesa, A. Albers, J. Mangnus, and C. van den Hondel. 2002. Filamentous fungi as cell factories for heterologous protein production. Trends Biotechnol. 20:200-206. [DOI] [PubMed] [Google Scholar]
- 37.Roggentin, P., B. Rothe, J. B. Kaper, J. Galen, L. Lawrisuk, E. R. Vimr, and R. Schauer. 1989. Conserved sequences in bacterial and viral sialidases. Glycoconj. J. 6:349-353. [DOI] [PubMed] [Google Scholar]
- 38.Shoji, J., M. Arioka, and K. Kitamoto. 2006. Vacuolar membrane dynamics in the filamentous fungus Aspergillus oryzae. Eukaryot. Cell 5:411-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki, T., N. Oiso, R. Gautam, E. K. Novak, J. J. Panthier, P. G. Suprabha, T. Vida, R. T. Swank, and R. A. Spritz. 2003. The mouse organellar biogenesis mutant buff results from a mutation in Vps33a, a homologue of yeast vps33 and Drosophila carnation. Proc. Natl. Acad. Sci. U. S. A. 100:1146-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tabuchi, M., D. Iwaihara, Y. Ohtani, N. Ohuchi, J.-I. Sakurai, T. Morita, S. Iwahara, and K. Takegawa. 1997. Vacuolar protein sorting in fission yeast: cloning, biosynthesis, transport, and processing of carboxypeptidase Y from Schizosaccharomyces pombe. J. Bacteriol. 179:4179-4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsuchiya, K., T. Nagashima, Y. Yamamoto, K. Gomi, K. Kitamoto, and C. Kumagai. 1994. High-level secretion of calf chymosin using a glucoamylase prochymosin fusion gene in Aspergillus oryzae. Biosci. Biotechnol. Biochem. 58:895-899. [DOI] [PubMed] [Google Scholar]
- 42.Tsuchiya, K., S. Tada, K. Gomi, K. Kitamoto, C. Kumagai, Y. Jigami, and G. Tamura. 1992. High-level expression of the synthetic human lysozyme gene in Aspergillus oryzae. Appl. Microbiol. Biotechnol. 39:738-743. [DOI] [PubMed] [Google Scholar]
- 43.Valls, L. A., C. P. Hunter, J. H. Rothman, and Y. H. Stevens. 1987. Protein sorting in yeast; the localization determinant of yeast vacuolar carboxypeptidase Y resides in the propeptide. Cell 48:887-897. [DOI] [PubMed] [Google Scholar]
- 44.Welters, P. 1996. Vacuolar targeting: proteins of the transport machinery. Plant Physiol. Biochem. 34:229-235. [Google Scholar]
- 45.Yoon, J., S. Kimura, J. Maruyama, and K. Kitamoto. 2009. Construction of quintuple protease gene disruptant for heterologous protein production in Aspergillus oryzae. Appl. Microbiol. Biotechnol. 82:691-701. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, B., A. Chang, T. B. Kjeldsen, and P. Arvan. 2001. Intracellular retention of newly synthesized insulin in yeast is caused by endoproteolytic processing in the Golgi complex. J. Cell Biol. 153:1187-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.