Abstract
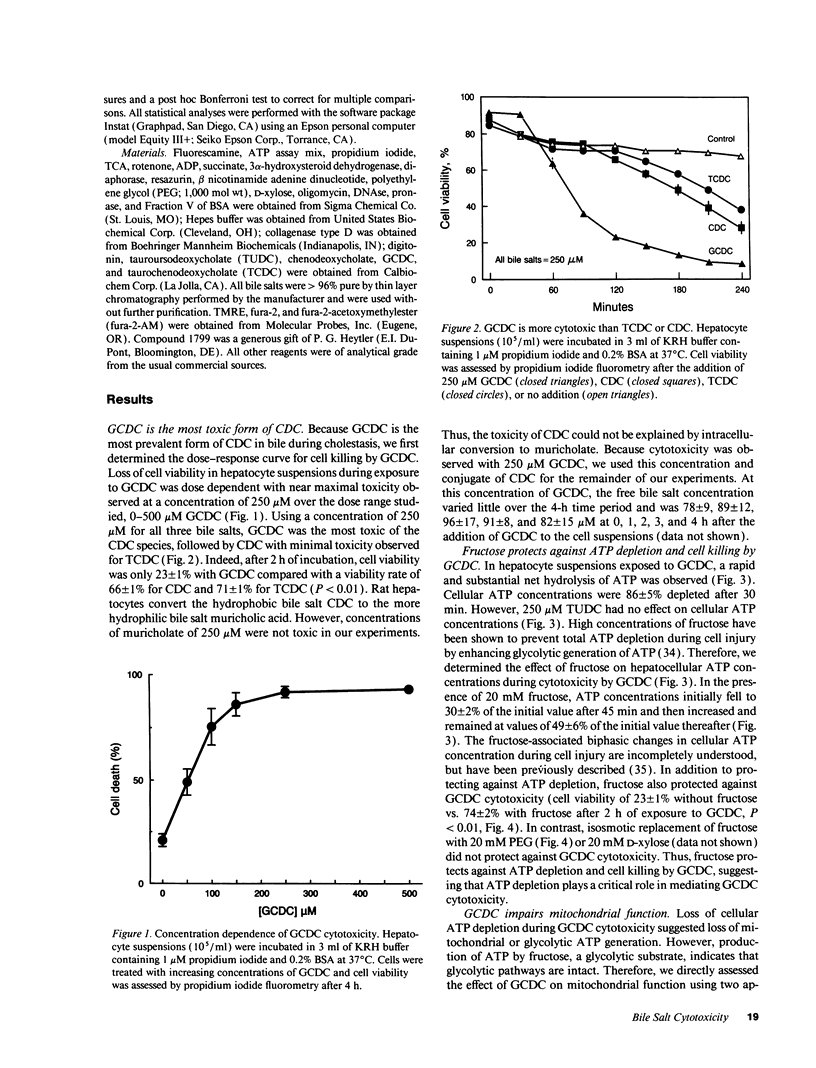
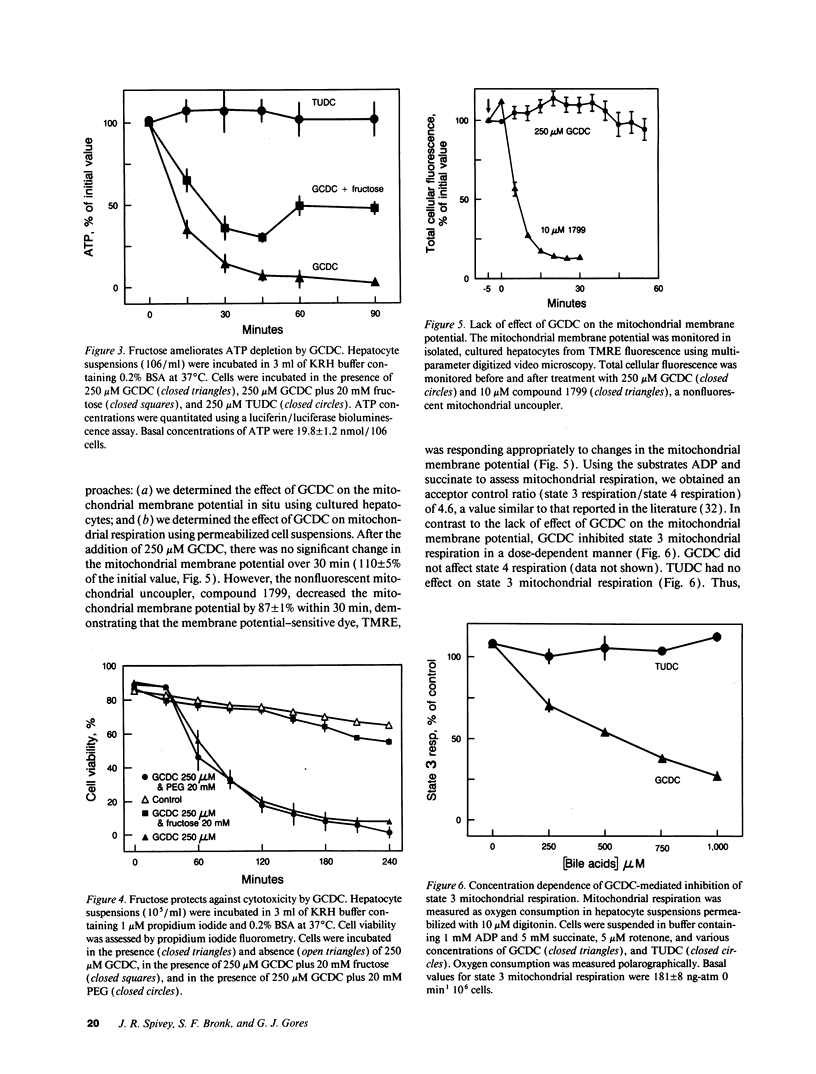
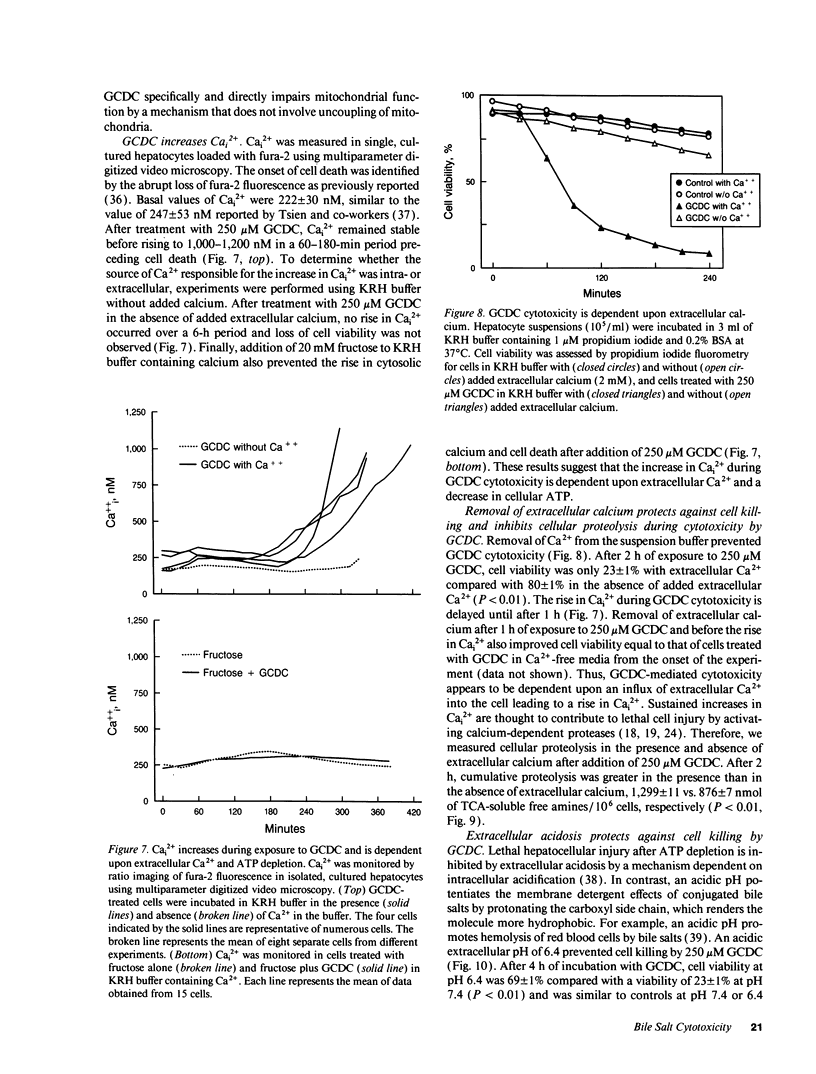
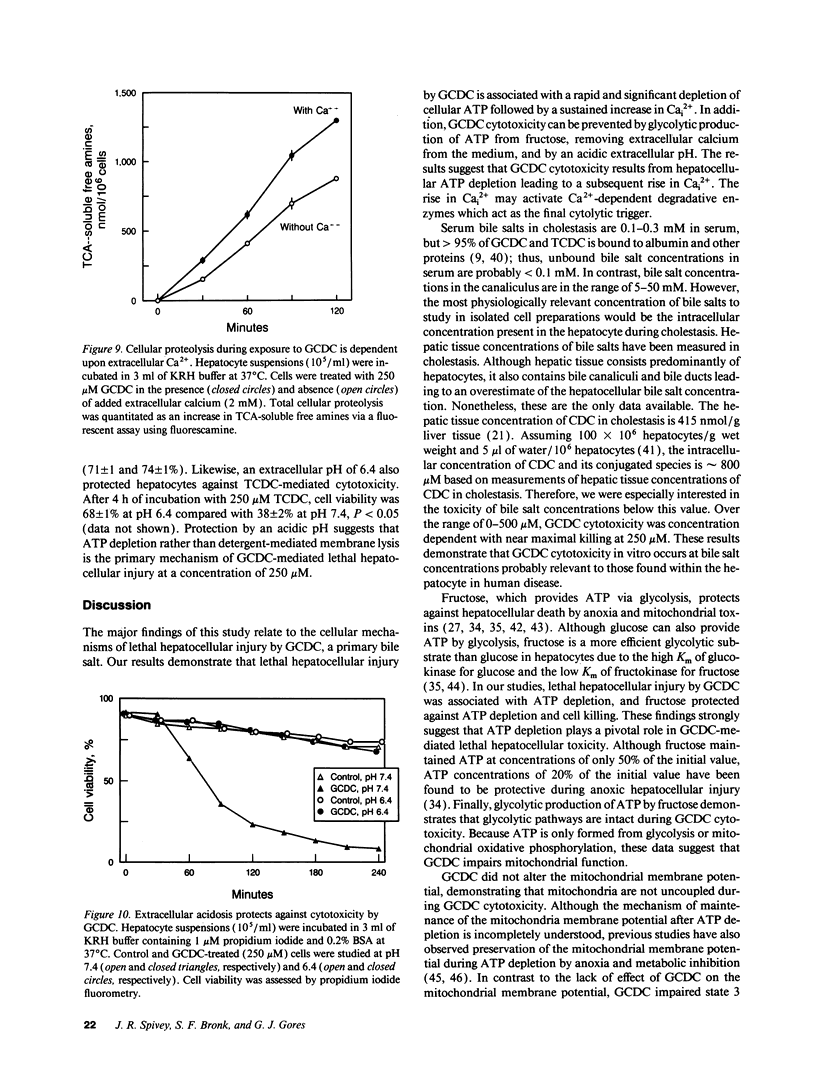
Chenodeoxycholate is toxic to hepatocytes, and accumulation of chenodeoxycholate in the liver during cholestasis may potentiate hepatocellular injury. However, the mechanism of hepatocellular injury by chenodeoxycholate remains obscure. Our aim was to determine the mechanism of cytotoxicity by chenodeoxycholate in rat hepatocytes. At a concentration of 250 microM, glycochenodeoxycholate was more toxic than either chenodeoxycholate or taurochenodeoxycholate. Cellular ATP was 86% depleted within 30 min after addition of glycochenodeoxycholate. Fructose, a glycolytic substrate, maintained ATP concentrations at 50% of the initial value and protected against glycochenodeoxycholate cytotoxicity. ATP depletion in the absence of a glycolytic substrate suggested impairment of mitochondrial function. Indeed, glycochenodeoxycholate inhibited state 3 respiration in digitonin-permeabilized cells in a dose-dependent manner. After ATP depletion, a sustained rise in cytosolic free calcium (Cai2+) was observed. Removal of extracellular Ca2+ abolished the rise in Cai2+, decreased cellular proteolysis, and protected against cell killing by glycochenodeoxycholate. The results suggest that glycochenodeoxycholate cytotoxicity results from ATP depletion followed by a subsequent rise in Cai2+. The rise in Cai2+ leads to an increase in calcium-dependent degradative proteolysis and, ultimately, cell death. We conclude that glycochenodeoxycholate causes a bioenergetic form of lethal cell injury dependent on ATP depletion analogous to the lethal cell injury of anoxia.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson J. J., Shamoo A. E. Anionic detergents as divalent cation ionophores across black lipid membranes. J Membr Biol. 1979 Nov 30;50(3-4):241–255. doi: 10.1007/BF01868891. [DOI] [PubMed] [Google Scholar]
- Anundi I., King J., Owen D. A., Schneider H., Lemasters J. J., Thurman R. G. Fructose prevents hypoxic cell death in liver. Am J Physiol. 1987 Sep;253(3 Pt 1):G390–G396. doi: 10.1152/ajpgi.1987.253.3.G390. [DOI] [PubMed] [Google Scholar]
- Anundi I., de Groot H. Hypoxic liver cell death: critical Po2 and dependence of viability on glycolysis. Am J Physiol. 1989 Jul;257(1 Pt 1):G58–G64. doi: 10.1152/ajpgi.1989.257.1.G58. [DOI] [PubMed] [Google Scholar]
- Anwer M. S., Engelking L. R., Nolan K., Sullivan D., Zimniak P., Lester R. Hepatotoxic bile acids increase cytosolic Ca++ activity of isolated rat hepatocytes. Hepatology. 1988 Jul-Aug;8(4):887–891. doi: 10.1002/hep.1840080430. [DOI] [PubMed] [Google Scholar]
- Baker P. R., Wilton J. C., Jones C. E., Stenzel D. J., Watson N., Smith G. J. Bile acids influence the growth, oestrogen receptor and oestrogen-regulated proteins of MCF-7 human breast cancer cells. Br J Cancer. 1992 Apr;65(4):566–572. doi: 10.1038/bjc.1992.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuers U., Spengler U., Zwiebel F. M., Pauletzki J., Fischer S., Paumgartner G. Effect of ursodeoxycholic acid on the kinetics of the major hydrophobic bile acids in health and in chronic cholestatic liver disease. Hepatology. 1992 Apr;15(4):603–608. doi: 10.1002/hep.1840150409. [DOI] [PubMed] [Google Scholar]
- Billington D., Evans C. E., Godfrey P. P., Coleman R. Effects of bile salts on the plasma membranes of isolated rat hepatocytes. Biochem J. 1980 May 15;188(2):321–327. doi: 10.1042/bj1880321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronk S. F., Gores G. J. Acidosis protects against lethal oxidative injury of liver sinusoidal endothelial cells. Hepatology. 1991 Jul;14(1):150–157. doi: 10.1002/hep.1840140125. [DOI] [PubMed] [Google Scholar]
- Cobbold P. H., Rink T. J. Fluorescence and bioluminescence measurement of cytoplasmic free calcium. Biochem J. 1987 Dec 1;248(2):313–328. doi: 10.1042/bj2480313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combettes L., Dumont M., Berthon B., Erlinger S., Claret M. Release of calcium from the endoplasmic reticulum by bile acids in rat liver cells. J Biol Chem. 1988 Feb 15;263(5):2299–2303. [PubMed] [Google Scholar]
- Crosignani A., Podda M., Battezzati P. M., Bertolini E., Zuin M., Watson D., Setchell K. D. Changes in bile acid composition in patients with primary biliary cirrhosis induced by ursodeoxycholic acid administration. Hepatology. 1991 Dec;14(6):1000–1007. [PubMed] [Google Scholar]
- Crosignani A., Podda M., Battezzati P. M., Bertolini E., Zuin M., Watson D., Setchell K. D. Changes in bile acid composition in patients with primary biliary cirrhosis induced by ursodeoxycholic acid administration. Hepatology. 1991 Dec;14(6):1000–1007. [PubMed] [Google Scholar]
- Frimmer M., Ziegler K. The transport of bile acids in liver cells. Biochim Biophys Acta. 1988 Feb 24;947(1):75–99. doi: 10.1016/0304-4157(88)90020-2. [DOI] [PubMed] [Google Scholar]
- Galle P. R., Theilmann L., Raedsch R., Otto G., Stiehl A. Ursodeoxycholate reduces hepatotoxicity of bile salts in primary human hepatocytes. Hepatology. 1990 Sep;12(3 Pt 1):486–491. doi: 10.1002/hep.1840120307. [DOI] [PubMed] [Google Scholar]
- Gasbarrini A., Borle A. B., Farghali H., Francavilla A., Van Thiel D. Fructose protects rat hepatocytes from anoxic injury. Effect on intracellular ATP, Ca2+i, Mg2+i, Na+i, and pHi. J Biol Chem. 1992 Apr 15;267(11):7545–7552. [PubMed] [Google Scholar]
- Gores G. J., Nieminen A. L., Fleishman K. E., Dawson T. L., Herman B., Lemasters J. J. Extracellular acidosis delays onset of cell death in ATP-depleted hepatocytes. Am J Physiol. 1988 Sep;255(3 Pt 1):C315–C322. doi: 10.1152/ajpcell.1988.255.3.C315. [DOI] [PubMed] [Google Scholar]
- Gores G. J., Nieminen A. L., Wray B. E., Herman B., Lemasters J. J. Intracellular pH during "chemical hypoxia" in cultured rat hepatocytes. Protection by intracellular acidosis against the onset of cell death. J Clin Invest. 1989 Feb;83(2):386–396. doi: 10.1172/JCI113896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greim H., Czygan P., Schaffner F., Popper H. Determination of bile acids in needle biopsies of human liver. Biochem Med. 1973 Oct;8(2):280–286. doi: 10.1016/0006-2944(73)90032-x. [DOI] [PubMed] [Google Scholar]
- Groskreutz J. L., Bronk S. F., Gores G. J. Ruthenium red delays the onset of cell death during oxidative stress of rat hepatocytes. Gastroenterology. 1992 Mar;102(3):1030–1038. doi: 10.1016/0016-5085(92)90193-3. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Ilani A., Granoth R. The pH dependence of the hemolytic potency of bile salts. Biochim Biophys Acta. 1990 Aug 24;1027(2):199–204. doi: 10.1016/0005-2736(90)90085-3. [DOI] [PubMed] [Google Scholar]
- Jewell S. A., Bellomo G., Thor H., Orrenius S., Smith M. Bleb formation in hepatocytes during drug metabolism is caused by disturbances in thiol and calcium ion homeostasis. Science. 1982 Sep 24;217(4566):1257–1259. doi: 10.1126/science.7112127. [DOI] [PubMed] [Google Scholar]
- Kawanishi T., Blank L. M., Harootunian A. T., Smith M. T., Tsien R. Y. Ca2+ oscillations induced by hormonal stimulation of individual fura-2-loaded hepatocytes. J Biol Chem. 1989 Aug 5;264(22):12859–12866. [PubMed] [Google Scholar]
- Kimura T. [Cytotoxicity of bile acids on cultured cells (author's transl)]. Nihon Shokakibyo Gakkai Zasshi. 1980 Feb;77(2):185–194. [PubMed] [Google Scholar]
- Krähenbühl S., Stucki J., Reichen J. Reduced activity of the electron transport chain in liver mitochondria isolated from rats with secondary biliary cirrhosis. Hepatology. 1992 Jun;15(6):1160–1166. doi: 10.1002/hep.1840150630. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Frank S., Vanderklish P., Arai A., Lynch G. Inhibition of proteolysis protects hippocampal neurons from ischemia. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7233–7237. doi: 10.1073/pnas.88.16.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasters J. J., DiGuiseppi J., Nieminen A. L., Herman B. Blebbing, free Ca2+ and mitochondrial membrane potential preceding cell death in hepatocytes. Nature. 1987 Jan 1;325(6099):78–81. doi: 10.1038/325078a0. [DOI] [PubMed] [Google Scholar]
- Maenz D. D., Forsyth G. W. Calcium ionophore activity of intestinal secretory compounds. An in vitro porcine model for the effects of bile acids, hydroxy-fatty acids and dioctyl sulfosuccinate. Digestion. 1984;30(3):138–150. doi: 10.1159/000199098. [DOI] [PubMed] [Google Scholar]
- Mashige F., Imai K., Osuga T. A simple and sensitive assay of total serum bile acids. Clin Chim Acta. 1976 Jul 1;70(1):79–86. doi: 10.1016/0009-8981(76)90007-3. [DOI] [PubMed] [Google Scholar]
- Mekhjian H. S., Phillips S. F. Perfusion of the canine colon with unconjugated bile acids. Effect on water and electrolyte transport, morphology, and bile acid absorption. Gastroenterology. 1970 Jul;59(1):120–129. [PubMed] [Google Scholar]
- Miyazaki K., Nakayama F., Koga A. Effect of chenodeoxycholic and ursodeoxycholic acids on isolated adult human hepatocytes. Dig Dis Sci. 1984 Dec;29(12):1123–1130. doi: 10.1007/BF01317087. [DOI] [PubMed] [Google Scholar]
- Moreadith R. W., Fiskum G. Isolation of mitochondria from ascites tumor cells permeabilized with digitonin. Anal Biochem. 1984 Mar;137(2):360–367. doi: 10.1016/0003-2697(84)90098-8. [DOI] [PubMed] [Google Scholar]
- Nicotera P., Hartzell P., Baldi C., Svensson S. A., Bellomo G., Orrenius S. Cystamine induces toxicity in hepatocytes through the elevation of cytosolic Ca2+ and the stimulation of a nonlysosomal proteolytic system. J Biol Chem. 1986 Nov 5;261(31):14628–14635. [PubMed] [Google Scholar]
- Nicotera P., Thor H., Orrenius S. Cytosolic-free Ca2+ and cell killing in hepatoma 1c1c7 cells exposed to chemical anoxia. FASEB J. 1989 Jan;3(1):59–64. doi: 10.1096/fasebj.3.1.2910738. [DOI] [PubMed] [Google Scholar]
- Nieminen A. L., Dawson T. L., Gores G. J., Kawanishi T., Herman B., Lemasters J. J. Protection by acidotic pH and fructose against lethal injury to rat hepatocytes from mitochondrial inhibitors, ionophores and oxidant chemicals. Biochem Biophys Res Commun. 1990 Mar 16;167(2):600–606. doi: 10.1016/0006-291x(90)92067-a. [DOI] [PubMed] [Google Scholar]
- Nieminen A. L., Gores G. J., Dawson T. L., Herman B., Lemasters J. J. Toxic injury from mercuric chloride in rat hepatocytes. J Biol Chem. 1990 Feb 5;265(4):2399–2408. [PubMed] [Google Scholar]
- Oelberg D. G., Dubinsky W. P., Sackman J. W., Wang L. B., Adcock E. W., Lester R. Bile salts induce calcium uptake in vitro by human erythrocytes. Hepatology. 1987 Mar-Apr;7(2):245–252. doi: 10.1002/hep.1840070207. [DOI] [PubMed] [Google Scholar]
- Pacifici R. E., Davies K. J. Protein degradation as an index of oxidative stress. Methods Enzymol. 1990;186:485–502. doi: 10.1016/0076-6879(90)86143-j. [DOI] [PubMed] [Google Scholar]
- Poupon R. E., Balkau B., Eschwège E., Poupon R. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. UDCA-PBC Study Group. N Engl J Med. 1991 May 30;324(22):1548–1554. doi: 10.1056/NEJM199105303242204. [DOI] [PubMed] [Google Scholar]
- Quist R. G., Ton-Nu H. T., Lillienau J., Hofmann A. F., Barrett K. E. Activation of mast cells by bile acids. Gastroenterology. 1991 Aug;101(2):446–456. doi: 10.1016/0016-5085(91)90024-f. [DOI] [PubMed] [Google Scholar]
- Roda A., Cappelleri G., Aldini R., Roda E., Barbara L. Quantitative aspects of the interaction of bile acids with human serum albumin. J Lipid Res. 1982 Mar;23(3):490–495. [PubMed] [Google Scholar]
- Scharschmidt B. F., Keeffe E. B., Vessey D. A., Blankenship N. M., Ockner R. K. In vitro effect of bile salts on rat liver plasma membrane, lipid fluidity, and ATPase activity. Hepatology. 1981 Mar-Apr;1(2):137–145. doi: 10.1002/hep.1840010209. [DOI] [PubMed] [Google Scholar]
- Schmucker D. L., Ohta M., Kanai S., Sato Y., Kitani K. Hepatic injury induced by bile salts: correlation between biochemical and morphological events. Hepatology. 1990 Nov;12(5):1216–1221. doi: 10.1002/hep.1840120523. [DOI] [PubMed] [Google Scholar]
- Schubert R., Schmidt K. H. Structural changes in vesicle membranes and mixed micelles of various lipid compositions after binding of different bile salts. Biochemistry. 1988 Nov 29;27(24):8787–8794. doi: 10.1021/bi00424a015. [DOI] [PubMed] [Google Scholar]
- Schölmerich J., Becher M. S., Schmidt K., Schubert R., Kremer B., Feldhaus S., Gerok W. Influence of hydroxylation and conjugation of bile salts on their membrane-damaging properties--studies on isolated hepatocytes and lipid membrane vesicles. Hepatology. 1984 Jul-Aug;4(4):661–666. doi: 10.1002/hep.1840040416. [DOI] [PubMed] [Google Scholar]
- Sols A., Salas M., Viñuela E. Induced biosynthesis of liver glucokinase. Adv Enzyme Regul. 1964;2:177–188. doi: 10.1016/s0065-2571(64)80012-1. [DOI] [PubMed] [Google Scholar]
- Trump B. F., Berezesky I. K. The role of calcium in cell injury and repair: a hypothesis. Surv Synth Pathol Res. 1985;4(3):248–256. doi: 10.1159/000156978. [DOI] [PubMed] [Google Scholar]
- Trump B. F., Berezesky I. K. The role of cytosolic Ca2+ in cell injury, necrosis and apoptosis. Curr Opin Cell Biol. 1992 Apr;4(2):227–232. doi: 10.1016/0955-0674(92)90037-d. [DOI] [PubMed] [Google Scholar]
- Yamazaki K., LaRusso N. F. The liver and intracellular digestion: how liver cells eat! Hepatology. 1989 Nov;10(5):877–886. doi: 10.1002/hep.1840100522. [DOI] [PubMed] [Google Scholar]
- Yu Q. C., Marzella L. Response of autophagic protein degradation to physiologic and pathologic stimuli in rat hepatocyte monolayer cultures. Lab Invest. 1988 Jun;58(6):643–652. [PubMed] [Google Scholar]
- Zimniak P., Little J. M., Radominska A., Oelberg D. G., Anwer M. S., Lester R. Taurine-conjugated bile acids act as Ca2+ ionophores. Biochemistry. 1991 Sep 3;30(35):8598–8604. doi: 10.1021/bi00099a015. [DOI] [PubMed] [Google Scholar]


