Abstract
Smooth muscle contraction is initiated primarily by an increase in intracellular Ca2+, Ca(2+)-dependent activation of myosin light chain kinase, and phosphorylation of myosin light chain. In this investigation, we identified pregnancy-associated alterations in myosin light chain phosphorylation, force of contraction, and content of contractile proteins in human myometrium. Steady-state levels of myosin light chain phosphorylation and contractile stress were correlated positively in both tissues, but the myometrial strips from pregnant women developed more stress at any given level of myosin light chain phosphorylation. During spontaneous contractions and during conditions that favor maximal generation of stress, the rate and extent of myosin light chain phosphorylation were attenuated in myometrial strips from pregnant women. The content of myosin and actin per milligram of protein and per tissue cross-sectional area was similar between myometrium of nonpregnant and pregnant women. Although cell size was significantly increased in tissues obtained from pregnant women, the amounts of contractile proteins per cellular cross-sectional area were similar. In addition, myosin light chain kinase and phosphatase activities were similar in the two tissues. The content of caldesmon was significantly increased in myometrium of pregnant women, whereas that of calponin (a smooth muscle-specific protein associated with the thin filaments) was not different. We conclude that adaptations of human myometrium during pregnancy include (a) cellular mechanisms that preclude the development of high levels of myosin light chain phosphorylation during contraction and (b) an increase in the stress generating capacity for any given level of myosin light chain phosphorylation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Conti M. A., Hathaway D. R., Klee C. B. Phosphorylation of smooth muscle myosin light chain kinase by the catalytic subunit of adenosine 3': 5'-monophosphate-dependent protein kinase. J Biol Chem. 1978 Dec 10;253(23):8347–8350. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialojan C., Rüegg J. C., Di Salvo J. Phosphatase-mediated modulation of actin-myosin interaction in bovine aortic actomyosin and skinned porcine carotid artery. Proc Soc Exp Biol Med. 1985 Jan;178(1):36–45. doi: 10.3181/00379727-178-41981. [DOI] [PubMed] [Google Scholar]
- Cavaillé F., Leger J. J. Characterization and comparison of the contractile proteins from human gravid and non-gravid myometrium. Gynecol Obstet Invest. 1983;16(6):341–353. doi: 10.1159/000299293. [DOI] [PubMed] [Google Scholar]
- Chisholm A. A., Cohen P. The myosin-bound form of protein phosphatase 1 (PP-1M) is the enzyme that dephosphorylates native myosin in skeletal and cardiac muscles. Biochim Biophys Acta. 1988 Sep 16;971(2):163–169. doi: 10.1016/0167-4889(88)90188-7. [DOI] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Csabina S., Mougios V., Bárány M., Bárány K. Characterization of the phosphorylatable myosin light chain in rat uterus. Biochim Biophys Acta. 1986 Jun 23;871(3):311–315. doi: 10.1016/0167-4838(86)90213-x. [DOI] [PubMed] [Google Scholar]
- Dessouky D. A. Myometrical changes in postpartum uterine involution. Am J Obstet Gynecol. 1971 Jun 1;110(3):318–329. doi: 10.1016/0002-9378(71)90721-6. [DOI] [PubMed] [Google Scholar]
- Dokhac L., D'Albis A., Janmot C., Harbon S. Myosin light chain phosphorylation in intact rat uterine smooth muscle. Role of calcium and cyclic AMP. J Muscle Res Cell Motil. 1986 Jun;7(3):259–268. doi: 10.1007/BF01753559. [DOI] [PubMed] [Google Scholar]
- Garfield R. E., Blennerhassett M. G., Miller S. M. Control of myometrial contractility: role and regulation of gap junctions. Oxf Rev Reprod Biol. 1988;10:436–490. [PubMed] [Google Scholar]
- Haeberle J. R., Hathaway D. R., DePaoli-Roach A. A. Dephosphorylation of myosin by the catalytic subunit of a type-2 phosphatase produces relaxation of chemically skinned uterine smooth muscle. J Biol Chem. 1985 Aug 25;260(18):9965–9968. [PubMed] [Google Scholar]
- Haeberle J. R., Hathaway D. R., DePaoli-Roach A. A. Dephosphorylation of myosin by the catalytic subunit of a type-2 phosphatase produces relaxation of chemically skinned uterine smooth muscle. J Biol Chem. 1985 Aug 25;260(18):9965–9968. [PubMed] [Google Scholar]
- Haeberle J. R., Hott J. W., Hathaway D. R. Regulation of isometric force and isotonic shortening velocity by phosphorylation of the 20,000 dalton myosin light chain of rat uterine smooth muscle. Pflugers Arch. 1985 Feb;403(2):215–219. doi: 10.1007/BF00584103. [DOI] [PubMed] [Google Scholar]
- Hai C. M., Murphy R. A. Ca2+, crossbridge phosphorylation, and contraction. Annu Rev Physiol. 1989;51:285–298. doi: 10.1146/annurev.ph.51.030189.001441. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Soderling T. R. Phosphorylation of smooth muscle myosin light chain kinase by Ca2+/calmodulin-dependent protein kinase II: comparative study of the phosphorylation sites. Arch Biochem Biophys. 1990 Apr;278(1):41–45. doi: 10.1016/0003-9861(90)90228-q. [DOI] [PubMed] [Google Scholar]
- Hemric M. E., Chalovich J. M. Effect of caldesmon on the ATPase activity and the binding of smooth and skeletal myosin subfragments to actin. J Biol Chem. 1988 Feb 5;263(4):1878–1885. [PubMed] [Google Scholar]
- Hoar P. E., Pato M. D., Kerrick W. G. Myosin light chain phosphatase. Effect on the activation and relaxation of gizzard smooth muscle skinned fibers. J Biol Chem. 1985 Jul 25;260(15):8760–8764. [PubMed] [Google Scholar]
- Ikebe M., Inagaki M., Kanamaru K., Hidaka H. Phosphorylation of smooth muscle myosin light chain kinase by Ca2+-activated, phospholipid-dependent protein kinase. J Biol Chem. 1985 Apr 25;260(8):4547–4550. [PubMed] [Google Scholar]
- Ikebe M., Reardon S. Binding of caldesmon to smooth muscle myosin. J Biol Chem. 1988 Mar 5;263(7):3055–3058. [PubMed] [Google Scholar]
- Ishihara H., Ozaki H., Sato K., Hori M., Karaki H., Watabe S., Kato Y., Fusetani N., Hashimoto K., Uemura D. Calcium-independent activation of contractile apparatus in smooth muscle by calyculin-A. J Pharmacol Exp Ther. 1989 Jul;250(1):388–396. [PubMed] [Google Scholar]
- Ishikawa R., Kagami O., Hayashi C., Kohama K. The binding of nonmuscle caldesmon from brain to microtubules. Regulations by Ca(2+)-calmodulin and cdc2 kinase. FEBS Lett. 1992 Mar 24;299(1):54–56. doi: 10.1016/0014-5793(92)80099-3. [DOI] [PubMed] [Google Scholar]
- Ito M., Hartshorne D. J. Phosphorylation of myosin as a regulatory mechanism in smooth muscle. Prog Clin Biol Res. 1990;327:57–72. [PubMed] [Google Scholar]
- Izumi H., Ichihara J., Uchiumi Y., Shirakawa K. Gestational changes in mechanical properties of skinned muscle tissues of human myometrium. Am J Obstet Gynecol. 1990 Aug;163(2):638–647. doi: 10.1016/0002-9378(90)91216-y. [DOI] [PubMed] [Google Scholar]
- Kamm K. E., Stull J. T. Myosin phosphorylation, force, and maximal shortening velocity in neurally stimulated tracheal smooth muscle. Am J Physiol. 1985 Sep;249(3 Pt 1):C238–C247. doi: 10.1152/ajpcell.1985.249.3.C238. [DOI] [PubMed] [Google Scholar]
- Kamm K. E., Stull J. T. Regulation of smooth muscle contractile elements by second messengers. Annu Rev Physiol. 1989;51:299–313. doi: 10.1146/annurev.ph.51.030189.001503. [DOI] [PubMed] [Google Scholar]
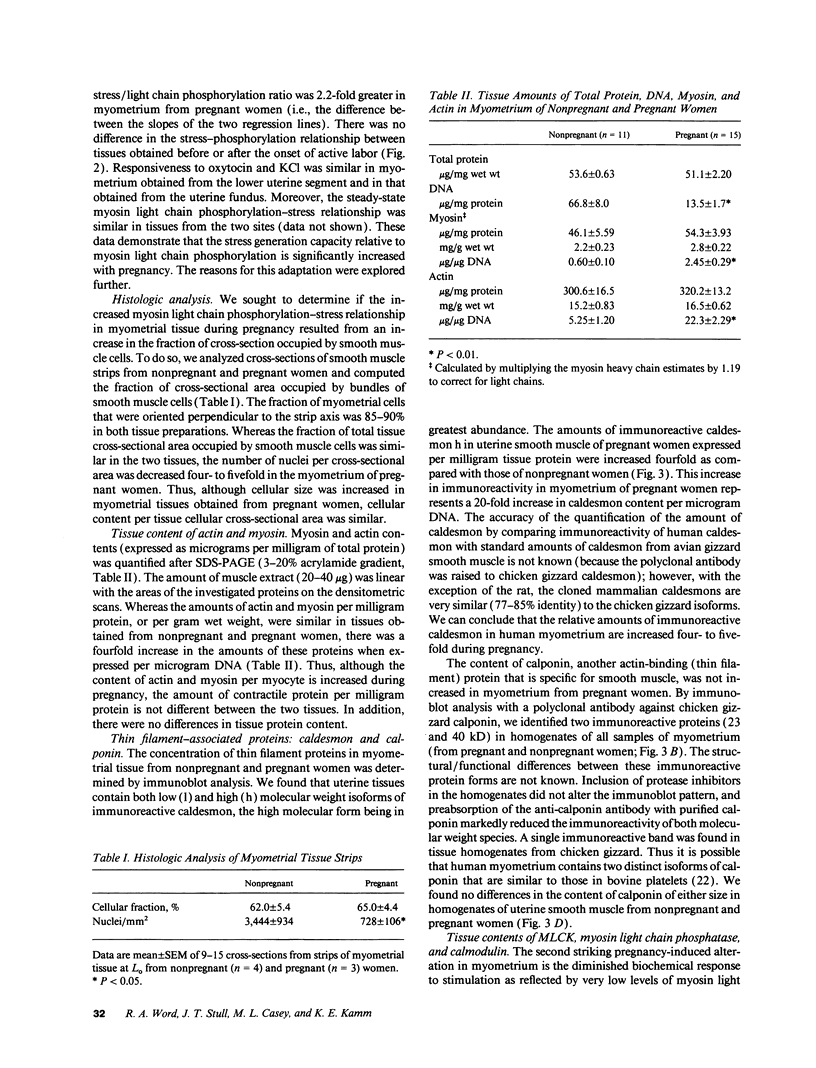
- Kamm K. E., Stull J. T. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- Katsuyama H., Wang C. L., Morgan K. G. Regulation of vascular smooth muscle tone by caldesmon. J Biol Chem. 1992 Jul 25;267(21):14555–14558. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marston S. B., Redwood C. S. Inhibition of actin-tropomyosin activation of myosin MgATPase activity by the smooth muscle regulatory protein caldesmon. J Biol Chem. 1992 Aug 25;267(24):16796–16800. [PubMed] [Google Scholar]
- Matsui K., Higashi K., Yoshimura T., Ito M., Miyamoto E. Myosin light chain kinase and cyclic AMP-dependent protein kinase activities and calmodulin levels in placental and non-placental regions of the rabbit myometrium. J Endocrinol. 1986 Apr;109(1):97–100. doi: 10.1677/joe.0.1090097. [DOI] [PubMed] [Google Scholar]
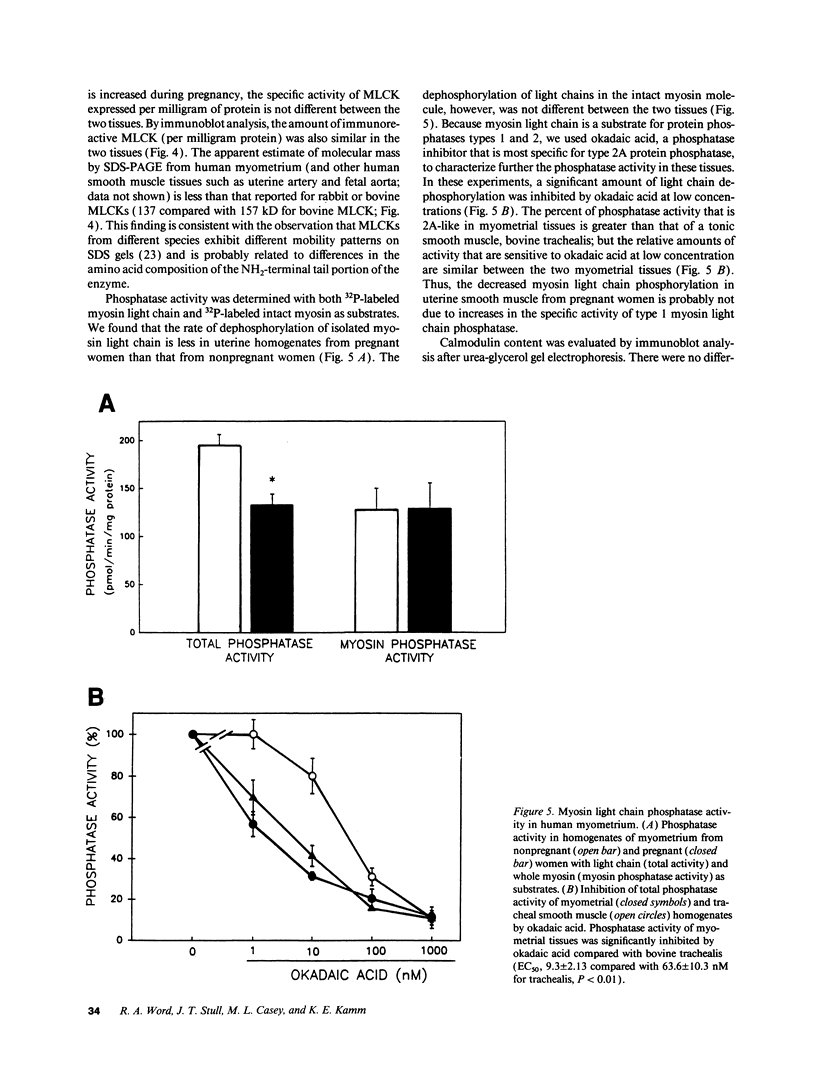
- Moody C., Lehman W., Craig R. Caldesmon and the structure of smooth muscle thin filaments: electron microscopy of isolated thin filaments. J Muscle Res Cell Motil. 1990 Apr;11(2):176–185. doi: 10.1007/BF01766496. [DOI] [PubMed] [Google Scholar]
- Murphy R. A. Filament organization and contractile function in vertebrate smooth muscle. Annu Rev Physiol. 1979;41:737–748. doi: 10.1146/annurev.ph.41.030179.003513. [DOI] [PubMed] [Google Scholar]
- Nishikawa M., Shirakawa S., Adelstein R. S. Phosphorylation of smooth muscle myosin light chain kinase by protein kinase C. Comparative study of the phosphorylated sites. J Biol Chem. 1985 Jul 25;260(15):8978–8983. [PubMed] [Google Scholar]
- Pato M. D., Kerc E. Comparison of the properties of the protein phosphatases from avian and mammalian smooth muscles: purification and characterization of rabbit uterine smooth muscle phosphatases. Arch Biochem Biophys. 1990 Jan;276(1):116–124. doi: 10.1016/0003-9861(90)90017-s. [DOI] [PubMed] [Google Scholar]
- Pato M. D., Kerc E. Purification and characterization of a smooth muscle myosin phosphatase from turkey gizzards. J Biol Chem. 1985 Oct 5;260(22):12359–12366. [PubMed] [Google Scholar]
- Persechini A., Kamm K. E., Stull J. T. Different phosphorylated forms of myosin in contracting tracheal smooth muscle. J Biol Chem. 1986 May 15;261(14):6293–6299. [PubMed] [Google Scholar]
- Pinter K., Marston S. B. Phosphorylation of vascular smooth muscle caldesmon by endogenous kinase. FEBS Lett. 1992 Jul 6;305(3):192–196. doi: 10.1016/0014-5793(92)80665-4. [DOI] [PubMed] [Google Scholar]
- Richardson M. R., Taylor D. A., Casey M. L., MacDonald P. C., Stull J. T. Biochemical markers of contraction in human myometrial smooth muscle cells in culture. In Vitro Cell Dev Biol. 1987 Jan;23(1):21–28. doi: 10.1007/BF02623489. [DOI] [PubMed] [Google Scholar]
- Rubenstein P. A., Spudich J. A. Actin microheterogeneity in chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Jan;74(1):120–123. doi: 10.1073/pnas.74.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue K., Kanda K., Tanaka T., Ueki N. Caldesmon: a common actin-linked regulatory protein in the smooth muscle and nonmuscle contractile system. J Cell Biochem. 1988 Jul;37(3):317–325. doi: 10.1002/jcb.240370306. [DOI] [PubMed] [Google Scholar]
- Soloff M. S., Alexandrova M., Fernstrom M. J. Oxytocin receptors: triggers for parturition and lactation? Science. 1979 Jun 22;204(4399):1313–1315. doi: 10.1126/science.221972. [DOI] [PubMed] [Google Scholar]
- Sparrow M. P., Mohammad M. A., Arner A., Hellstrand P., Rüegg J. C. Myosin composition and functional properties of smooth muscle from the uterus of pregnant and non-pregnant rats. Pflugers Arch. 1988 Oct;412(6):624–633. doi: 10.1007/BF00583764. [DOI] [PubMed] [Google Scholar]
- Stull J. T., Hsu L. C., Tansey M. G., Kamm K. E. Myosin light chain kinase phosphorylation in tracheal smooth muscle. J Biol Chem. 1990 Sep 25;265(27):16683–16690. [PubMed] [Google Scholar]
- Takahashi K., Hiwada K., Kokubu T. Vascular smooth muscle calponin. A novel troponin T-like protein. Hypertension. 1988 Jun;11(6 Pt 2):620–626. doi: 10.1161/01.hyp.11.6.620. [DOI] [PubMed] [Google Scholar]
- Takai A., Troschka M., Mieskes G., Somlyo A. V. Protein phosphatase composition in the smooth muscle of guinea-pig ileum studied with okadaic acid and inhibitor 2. Biochem J. 1989 Sep 1;262(2):617–623. doi: 10.1042/bj2620617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey M. G., Word R. A., Hidaka H., Singer H. A., Schworer C. M., Kamm K. E., Stull J. T. Phosphorylation of myosin light chain kinase by the multifunctional calmodulin-dependent protein kinase II in smooth muscle cells. J Biol Chem. 1992 Jun 25;267(18):12511–12516. [PubMed] [Google Scholar]
- Winder S. J., Walsh M. P. Smooth muscle calponin. Inhibition of actomyosin MgATPase and regulation by phosphorylation. J Biol Chem. 1990 Jun 15;265(17):10148–10155. [PubMed] [Google Scholar]
- Word R. A., Casey M. L., Kamm K. E., Stull J. T. Effects of cGMP on [Ca2+]i, myosin light chain phosphorylation, and contraction in human myometrium. Am J Physiol. 1991 Apr;260(4 Pt 1):C861–C867. doi: 10.1152/ajpcell.1991.260.4.C861. [DOI] [PubMed] [Google Scholar]
- Word R. A., Kamm K. E., Casey M. L. Contractile effects of prostaglandins, oxytocin, and endothelin-1 in human myometrium in vitro: refractoriness of myometrial tissue of pregnant women to prostaglandins E2 and F2 alpha. J Clin Endocrinol Metab. 1992 Oct;75(4):1027–1032. doi: 10.1210/jcem.75.4.1400867. [DOI] [PubMed] [Google Scholar]
- Yamashiro S., Matsumura F. Mitosis-specific phosphorylation of caldesmon: possible molecular mechanism of cell rounding during mitosis. Bioessays. 1991 Nov;13(11):563–568. doi: 10.1002/bies.950131103. [DOI] [PubMed] [Google Scholar]