Abstract
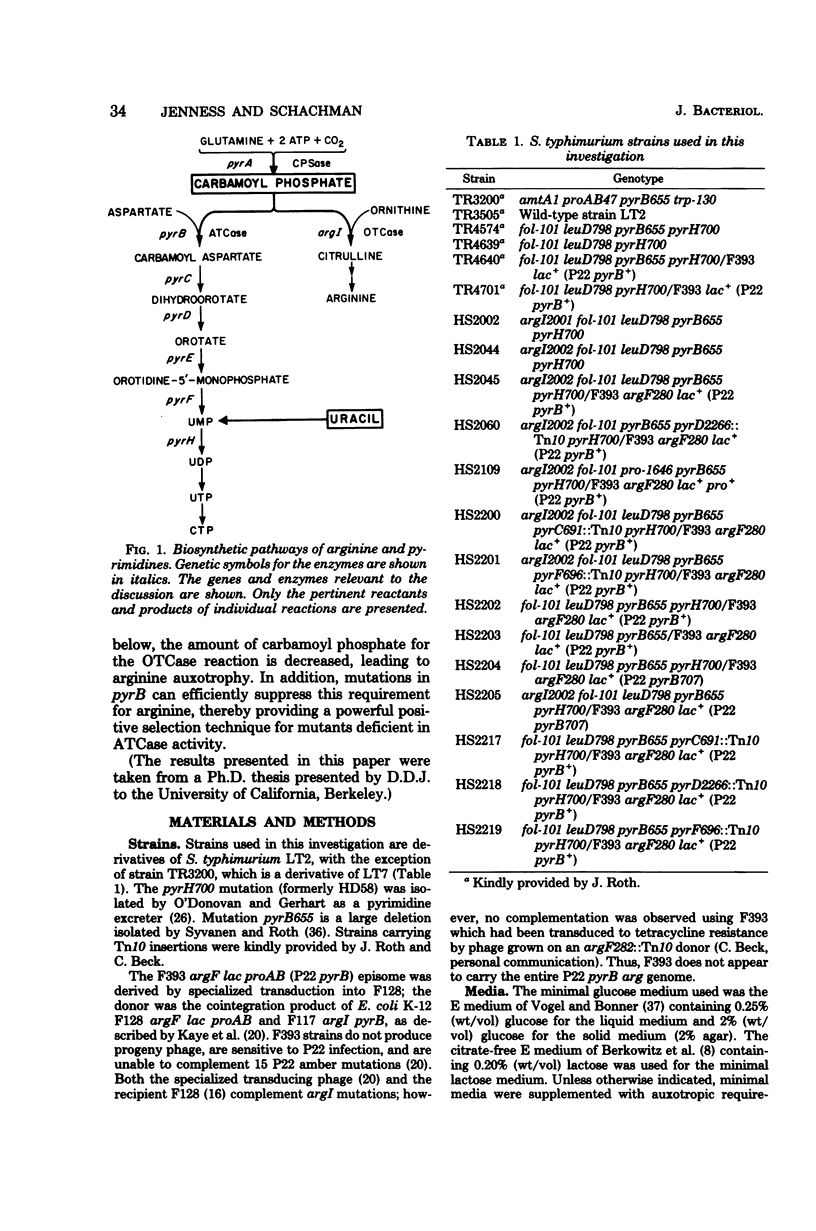
Salmonella typhimurium strains which produce high constitutive levels of aspartate transcarbamoylase due to the pyrH700 mutation were found to grow more slowly in minimal medium than pyrH+ controls. The addition of arginine or citrulline but not ornithine restored normal growth rates. This requirement for arginine was completely suppressed by pyrB mutations and partially suppressed by pyrC and pyrD mutations. No suppression was observed with mutants at the pyrF locus. Introduction of leaky mutation argI2002 resulted in a more extreme arginine requirement and accentuated suppression by pyrB mutations. Suppression by the pyrC and pyrD mutations was reduced as a result of the incorporation of the leaky argI2002 allele. These results indicate that in pyrH700 strains carbamoyl phosphate is preferentially directed toward the formation of intermediates in the pyrimidine biosynthetic pathway. Arginine auxotrophy results from the reduced availability of carbamoyl phosphate for the biosynthesis of arginine. Suppression of this arginine dependence for growth is used as a convenient positive selection technique for pyrB mutations.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abd-el-Al A., Ingraham J. L. Cold sensitivity and other phenotypes resulting from mutation in pyrA gene. J Biol Chem. 1969 Aug 10;244(15):4039–4045. [PubMed] [Google Scholar]
- Abd-el-Al A., Ingraham J. L. Control of carbamyl phosphate synthesis in Salmonella typhimurium. J Biol Chem. 1969 Aug 10;244(15):4033–4038. [PubMed] [Google Scholar]
- Abdelal A. T., Ingraham J. L. Carbamylphosphate synthetase from Salmonella typhimurium. Regulations, subunit composition, and function of the subunits. J Biol Chem. 1975 Jun 25;250(12):4410–4417. [PubMed] [Google Scholar]
- Abdelal A. T., Kennedy E. H., Nainan O. Ornithine transcarbamylase from Salmonella typhimurium: purification, subunit composition, kinetic analysis, and immunological cross-reactivity. J Bacteriol. 1977 Mar;129(3):1387–1396. doi: 10.1128/jb.129.3.1387-1396.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P. M., Meister A. Control of Escherichia coli carbamyl phosphate synthetase by purine and pyrimidine nucleotides. Biochemistry. 1966 Oct;5(10):3164–3169. doi: 10.1021/bi00874a013. [DOI] [PubMed] [Google Scholar]
- BECKWITH J. R., PARDEE A. B., AUSTRIAN R., JACOB F. Coordination of the synthesis of the enzymes in the pyrimidine pathway of E. coli. J Mol Biol. 1962 Dec;5:618–634. doi: 10.1016/s0022-2836(62)80090-4. [DOI] [PubMed] [Google Scholar]
- BRESCH C. Genetical studies on bacteriophage TI. Ann Inst Pasteur (Paris) 1953 Jan;84(1):157–163. [PubMed] [Google Scholar]
- Bach M. L., Lacroute F. Direct selective techniques for the isolation of pyrimidine auxotrophs in yeast. Mol Gen Genet. 1972;115(2):126–130. doi: 10.1007/BF00277292. [DOI] [PubMed] [Google Scholar]
- Berkowitz D., Hushon J. M., Whitfield H. J., Jr, Roth J., Ames B. N. Procedure for identifying nonsense mutations. J Bacteriol. 1968 Jul;96(1):215–220. doi: 10.1128/jb.96.1.215-220.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Galván M., Martuscelli J. Biochemical and genetic characterization of a carbamyl phosphate synthetase mutant of Escherichia coli K12. J Gen Microbiol. 1976 May;94(1):142–148. doi: 10.1099/00221287-94-1-142. [DOI] [PubMed] [Google Scholar]
- Davies G. E., Vanaman T. C., Stark G. R. Aspartate transcarbamylase. Stereospecific restrictions on the binding site for L-aspartate. J Biol Chem. 1970 Mar 10;245(5):1175–1179. [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. ASPARTATE TRANSCARBAMYLASE, AN ENZYME DESIGNED FOR FEEDBACK INHIBITION. Fed Proc. 1964 May-Jun;23:727–735. [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. The enzymology of control by feedback inhibition. J Biol Chem. 1962 Mar;237:891–896. [PubMed] [Google Scholar]
- Hoppe I., Roth J. Specialized transducing phages derived from salmonella phage P22. Genetics. 1974 Apr;76(4):633–654. doi: 10.1093/genetics/76.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraham J. L., Neuhard J. Cold-sensitive mutants of Salmonella typhimurium defective in uridine monophosphate kinase (pyrH). J Biol Chem. 1972 Oct 10;247(19):6259–6265. [PubMed] [Google Scholar]
- Justesen J., Neuhard J. pyrR identical to pyrH in Salmonella typhimurium: control of expression of the pyr genes. J Bacteriol. 1975 Sep;123(3):851–854. doi: 10.1128/jb.123.3.851-854.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye R., Barravecchio J., Roth J. Isolation of P22 specialized transducing phage followong F'-episome fusion. Genetics. 1974 Apr;76(4):655–667. doi: 10.1093/genetics/76.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelln R. A., O'Donovan G. A. Isolation and partial characterization of an argR mutant of Salmonella typhimurium. J Bacteriol. 1976 Nov;128(2):528–535. doi: 10.1128/jb.128.2.528-535.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIEBERMAN I., KORNBERG A. Enzymatic synthesis and breakdown of a pyrimidine, orotic acid. I. Dihydroortic acid, ureidosuccinic acid, and 5-carboxymethylhydantoin. J Biol Chem. 1954 Apr;207(2):911–924. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Legrain C., Stalon V., Glansdorff N., Gigot D., Piéard A., Crabeel M. Structural and regulatory mutations allowing utilization of citrulline or carbamoylaspartate as a source of carbamoylphosphate in Escherichia coli K-12. J Bacteriol. 1976 Oct;128(1):39–48. doi: 10.1128/jb.128.1.39-48.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain C., Stalon V. Ornithine carbamoyltransferase from Escherichia coli W. Purification, structure and steady-state kinetic analysis. Eur J Biochem. 1976 Mar 16;63(1):289–301. doi: 10.1111/j.1432-1033.1976.tb10230.x. [DOI] [PubMed] [Google Scholar]
- O'Donovan G. A., Gerhart J. C. Isolation and partial characterization of regulatory mutants of the pyrimidine pathway in Salmonella typhimurium. J Bacteriol. 1972 Mar;109(3):1085–1096. doi: 10.1128/jb.109.3.1085-1096.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan G. A., Holoubek H., Gerhart J. C. Regulatory properties of intergeneric hybrids of aspartate transcarbamylase. Nat New Biol. 1972 Aug 30;238(87):264–266. doi: 10.1038/newbio238264a0. [DOI] [PubMed] [Google Scholar]
- PARDEE A. B., YATES R. A. Pyrimidine biosynthesis in Escherichia coli. J Biol Chem. 1956 Aug;221(2):743–756. [PubMed] [Google Scholar]
- Piérard A. Control of the activity of Escherichia coli carbamoyl phosphate synthetase by antagonistic allosteric effectors. Science. 1966 Dec 23;154(3756):1572–1573. doi: 10.1126/science.154.3756.1572. [DOI] [PubMed] [Google Scholar]
- Piérard A., Glansdorff N., Gigot D., Crabeel M., Halleux P., Thiry L. Repression of Escherichia coli carbamoylphosphate synthase: relationships with enzyme synthesis in the arginine and pyrimidine pathways. J Bacteriol. 1976 Jul;127(1):291–301. doi: 10.1128/jb.127.1.291-301.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. W., Modebe M. O., Stark G. R. Aspartate transcarbamylase. Kinetic studies of the catalytic subunit. J Biol Chem. 1969 Apr 10;244(7):1846–1859. [PubMed] [Google Scholar]
- Schachman H. K. Anatomy and physiology of a regulatory enzyme-aspartate transcarbamylase. Harvey Lect. 1974;68:67–113. [PubMed] [Google Scholar]
- Schwartz M., Neuhard J. Control of expression of the pyr genes in Salmonella typhimurium: effects of variations in uridine and cytidine nucleotide pools. J Bacteriol. 1975 Mar;121(3):814–822. doi: 10.1128/jb.121.3.814-822.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson H., Somerville R. L. Expression of the tryptophan operon in merodiploids of Escherichia coli. I. Gene dosage, gene position and marker effects. Mol Gen Genet. 1971;111(4):342–351. doi: 10.1007/BF00569786. [DOI] [PubMed] [Google Scholar]
- Syvanen J. M., Roth J. R. Structural genes for catalytic and regulatory subunits of aspartate transcarbamylase. J Mol Biol. 1973 May 25;76(3):363–378. doi: 10.1016/0022-2836(73)90510-x. [DOI] [PubMed] [Google Scholar]
- Syvanen J. M., Roth J. R. Structural genes for ornithine transcarbamylase in Salmonella typhimurium and Escherichia coli K-12. J Bacteriol. 1972 Apr;110(1):66–70. doi: 10.1128/jb.110.1.66-70.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Williams J. C., O'Donovan G. A. Repression of enzyme synthesis of the pyrimidine pathway in Salmonella typhimurium. J Bacteriol. 1973 Sep;115(3):1071–1076. doi: 10.1128/jb.115.3.1071-1076.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack J. E., O'Donovan G. A. Orotic acid excretion in some wild-type strains of Escherichia coli K-12. J Bacteriol. 1978 Nov;136(2):825–827. doi: 10.1128/jb.136.2.825-827.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Demerec M. Genetic analysis of pyrimidine mutants of Salmonella typhimurium. Genetics. 1965 Sep;52(3):643–651. doi: 10.1093/genetics/52.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]


