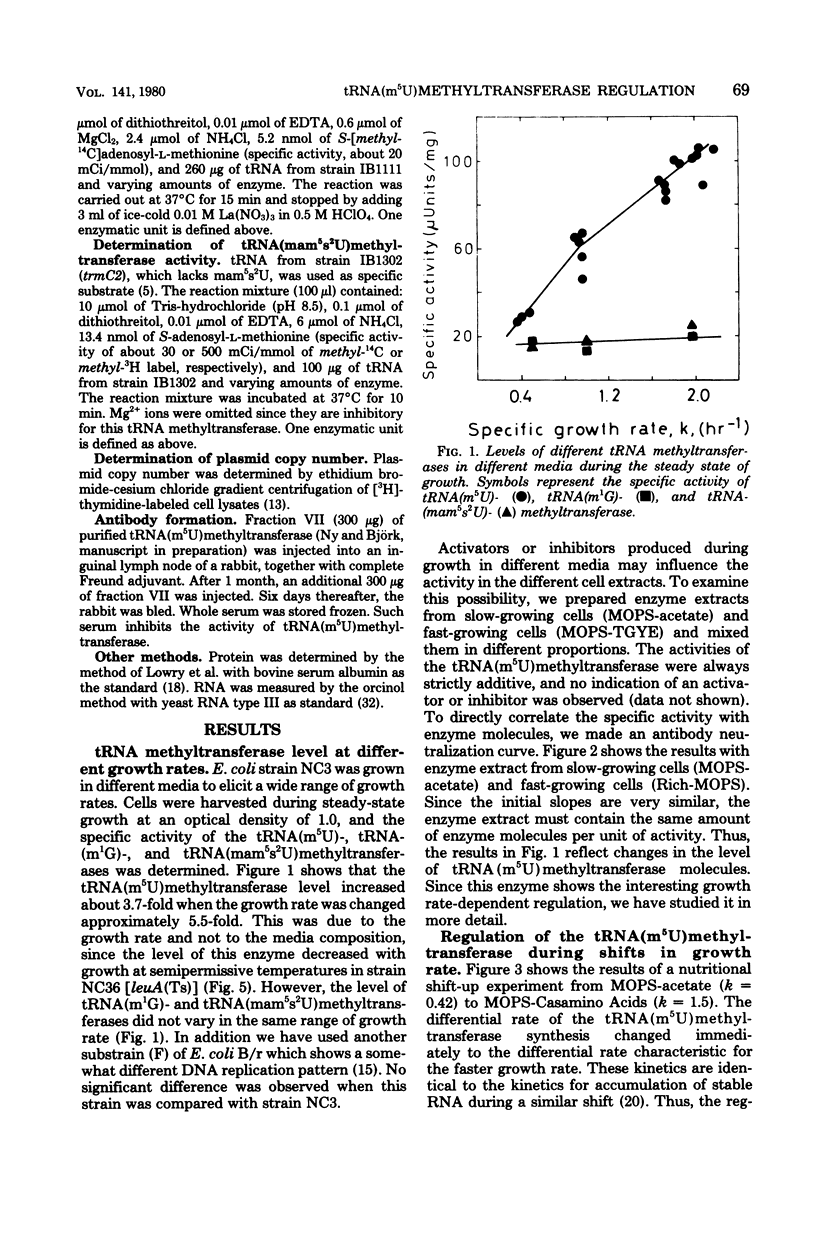
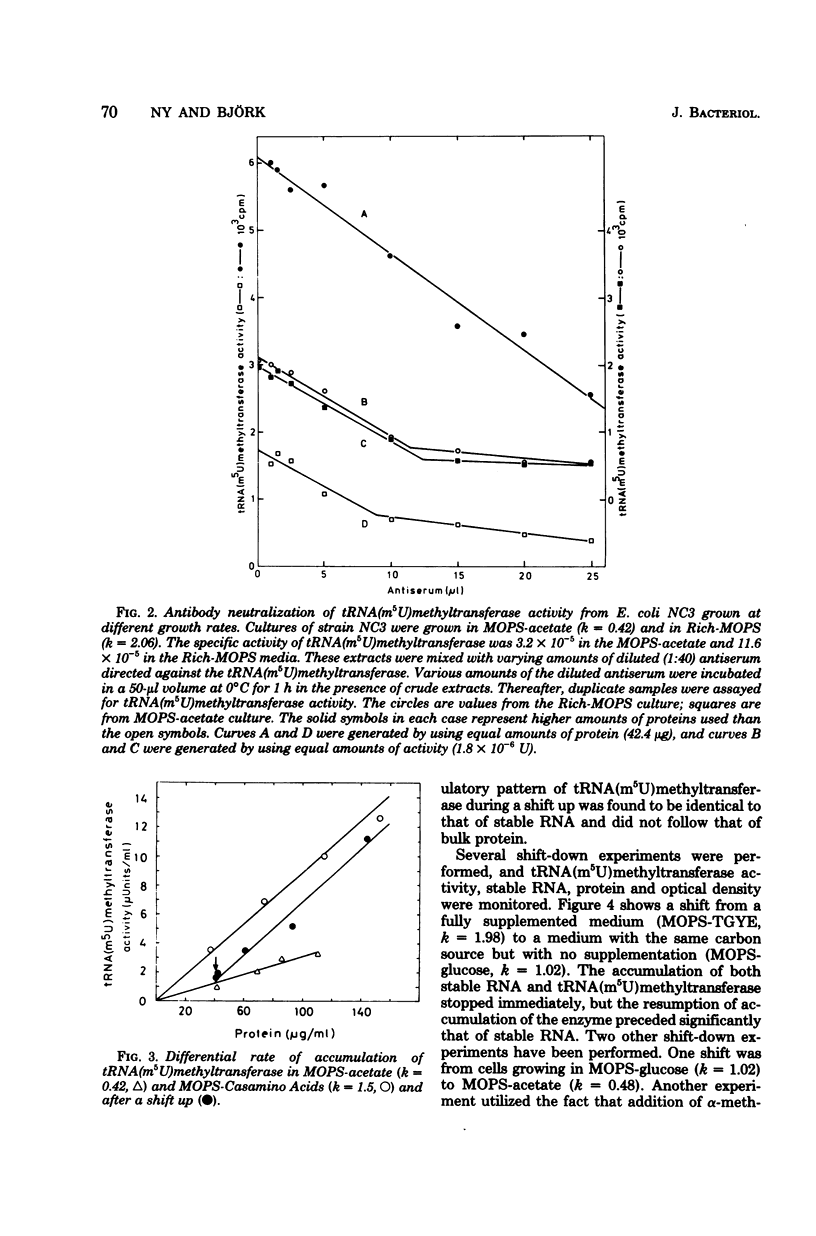
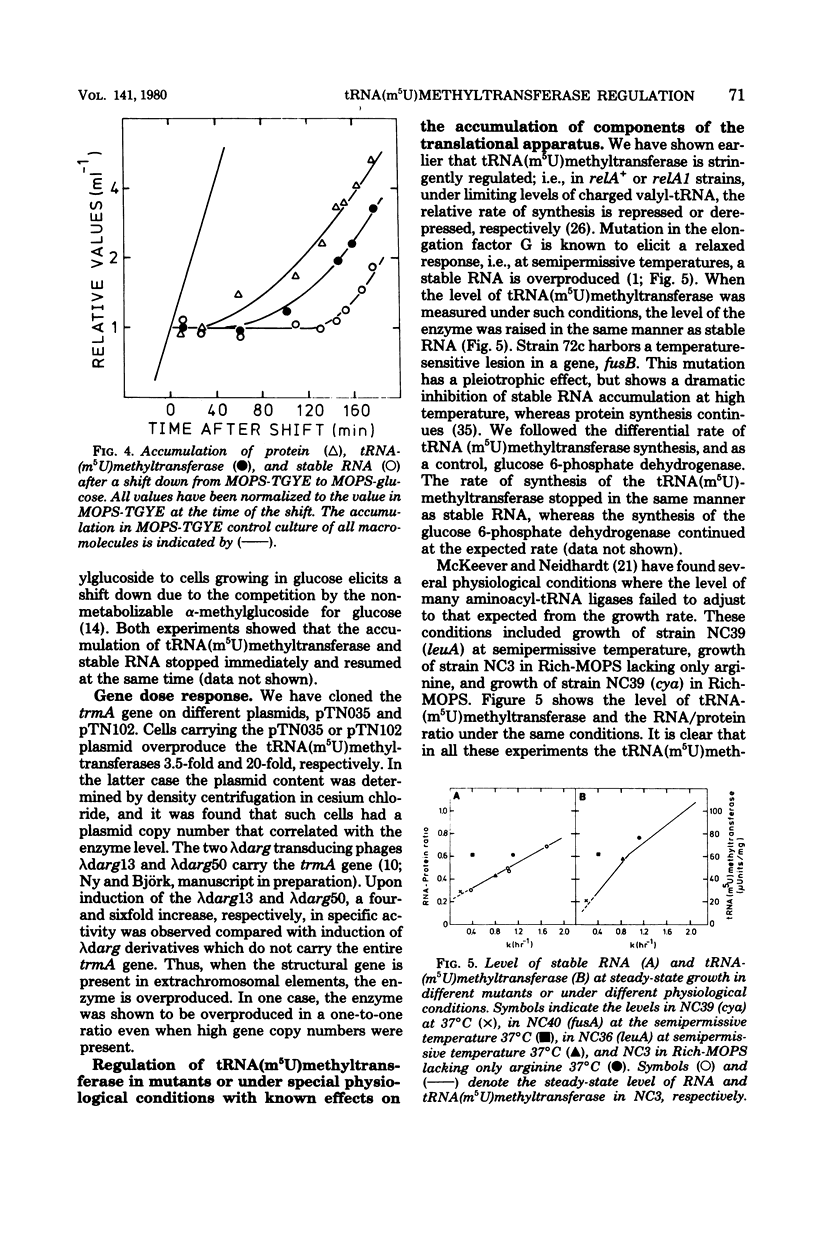
Abstract
Enzymes catalyzing the transfer of methyl groups from S-adenosyl-l-methionine to a precursor transfer ribonucleic acid (tRNA) and forming 5-methyluridine (m5U), 1-methylguanine (m1G), or 5-methylaminomethyl-2-thio-uridine (mam5s2U) are denoted tRNA(m5U)-(EC 2.1.1.35), tRNA(m1G)-(EC 2.1.1.31), and tRNA(mam5s2U)methyltransferase. We have studied the regulation of these tRNA biosynthetic enzymes in Escherichia coli under various physiological conditions and in bacterial mutants known to affect the regulation of components of the translational apparatus. Such studies have revealed that tRNA(m5U)-methyltransferase increases with the growth rate in the same fashion as stable RNA, whereas the activity of two other tRNA methyltransferases remains constant in relation to the growth rate. Thus, these tRNA biosynthetic enzymes were not coordinately regulated. Regulation of both tRNA(m5U)methyltransferase and stable RNA was similar during shift-up and shift-down experiments. This enzyme showed a stringent regulation in relA+ strain (T. Ny and G. R. Björk, J. Bacteriol. 130:635–641, 1977) but also in two temperature-sensitive mutants, fusA and fusB, known to influence the accumulation of guanosine 5′-diphosphate 3′-diphosphate and RNA synthesis at nonpermissive temperatures. The tRNA(m5U)methyltransferase showed a gene dose effect when its structural gene, trmA, was carried on a plasmid or on λ transducing phages. Although the regulation of tRNA-(m5U)methyltransferase was surprisingly coupled to that of stable RNA, this enzyme was expressed at a much lower level.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherly A. G. Temperature-sensitive relaxed Phenotype in a stringent strain of Escherichia coli. J Bacteriol. 1973 Jan;113(1):178–182. doi: 10.1128/jb.113.1.178-182.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avital S., Elson D. A convenient procedure for preparing transfer ribonucleic acid from Escherichia coli. Biochim Biophys Acta. 1969 Apr 22;179(2):297–307. doi: 10.1016/0005-2787(69)90038-0. [DOI] [PubMed] [Google Scholar]
- Björk G. R., Isaksson L. A. Isolation of mutants of Escherichia coli lac king 5-methyluracil in transfer ribonucleic acid or 1-methylguanine in ribosomal RNA. J Mol Biol. 1970 Jul 14;51(1):83–100. doi: 10.1016/0022-2836(70)90272-x. [DOI] [PubMed] [Google Scholar]
- Björk G. R., Kjellin-Stråby K. Escherichia coli mutants with defects in the biosynthesis of 5-methylaminomethyl-2-thio-uridine or 1-methylguanosine in their tRNA. J Bacteriol. 1978 Feb;133(2):508–517. doi: 10.1128/jb.133.2.508-517.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassio D., Mathien Y., Waller J. P. Enhanced level and metabolic regulation of methionyl-transfer ribonucleic acid synthetase in different strains of Escherichia coli K-12. J Bacteriol. 1975 Aug;123(2):580–588. doi: 10.1128/jb.123.2.580-588.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler M. G., Pritchard R. H. The effect of gene concentration and relative gene dosage on gene output in Escherichia coli. Mol Gen Genet. 1975;138(2):127–141. doi: 10.1007/BF02428117. [DOI] [PubMed] [Google Scholar]
- Cooper S. Cell division and DNA replication following a shift to a richer medium. J Mol Biol. 1969 Jul 14;43(1):1–11. doi: 10.1016/0022-2836(69)90074-6. [DOI] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Crabeel M., Charlier D., Glansdorff N. Studies on the bipolar argECBH operon of E. coli: characterization of restriction endonuclease fragments obtained from gammadargECBH transducing phages and a ColE1 argECBH plasmid. Mol Gen Genet. 1977 Mar 7;151(2):161–168. doi: 10.1007/BF00338690. [DOI] [PubMed] [Google Scholar]
- Dennis P. P. In vivo stability, maturation and relative differential synthesis rates of individual ribosomal proteins in Escherichia coli B/r. J Mol Biol. 1974 Sep 5;88(1):25–41. doi: 10.1016/0022-2836(74)90293-9. [DOI] [PubMed] [Google Scholar]
- Geyl D., Böck A. Synthesis of ribosomal proteins in merodiploid strains and in minicells of Escherichia coli. Mol Gen Genet. 1977 Sep 9;154(3):327–334. doi: 10.1007/BF00571290. [DOI] [PubMed] [Google Scholar]
- Grinsted J., Saunders J. R., Ingram L. C., Sykes R. B., Richmond M. H. Properties of a R factor which originated in Pseudomonas aeruginosa 1822. J Bacteriol. 1972 May;110(2):529–537. doi: 10.1128/jb.110.2.529-537.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M. T., Pato M. L., Molin S., Fill N. P., von Meyenburg K. Simple downshift and resulting lack of correlation between ppGpp pool size and ribonucleic acid accumulation. J Bacteriol. 1975 May;122(2):585–591. doi: 10.1128/jb.122.2.585-591.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter C. E., Pierucci O. DNA synthesis during the division cycle of three substrains of Escherichia coli B/r. J Mol Biol. 1976 Apr 15;102(3):477–486. doi: 10.1016/0022-2836(76)90329-6. [DOI] [PubMed] [Google Scholar]
- Ikemura T., Nomura M. Expression of spacer tRNA genes in ribosomal RNA transcription units carried by hybrid Col E1 plasmids in E. coli. Cell. 1977 Aug;11(4):779–793. doi: 10.1016/0092-8674(77)90291-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Littauer U. Z., Inouye H. Regulation of tRNA. Annu Rev Biochem. 1973;42:439–470. doi: 10.1146/annurev.bi.42.070173.002255. [DOI] [PubMed] [Google Scholar]
- McKeever W. G., Neidhardt F. C. Growth rate modulation of four aminoacyl-transfer ribonucleic acid synthetases in enteric bacteria. J Bacteriol. 1976 May;126(2):634–645. doi: 10.1128/jb.126.2.634-645.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Pedersen S., Reeh S. Chemical measurement of steady-state levels of ten aminoacyl-transfer ribonucleic acid synthetases in Escherichia coli. J Bacteriol. 1977 Jan;129(1):378–387. doi: 10.1128/jb.129.1.378-387.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neihardt F. C., Parker J., McKeever W. G. Function and regulation of aminoacyl-tRNA synthetases in prokaryotic and eukaryotic cells. Annu Rev Microbiol. 1975;29:215–250. doi: 10.1146/annurev.mi.29.100175.001243. [DOI] [PubMed] [Google Scholar]
- Nierlich D. P. Regulation of bacterial growth, RNA, and protein synthesis. Annu Rev Microbiol. 1978;32:393–432. doi: 10.1146/annurev.mi.32.100178.002141. [DOI] [PubMed] [Google Scholar]
- Ny T., Björk G. R. Stringent regulation of the synthesis of a transfer ribonucleic acid biosynthetic enzyme: transfer ribonucleic acid(m5U)methyltransferase from Escherichia coli. J Bacteriol. 1977 May;130(2):635–641. doi: 10.1128/jb.130.2.635-641.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson M. O., Isaksson L. A. Analysis of rpsD mutations in Escherichia coli. III. Effects of rpsD mutations on expression of some ribosomal protein genes. Mol Gen Genet. 1979 Feb 1;169(3):271–278. doi: 10.1007/BF00382273. [DOI] [PubMed] [Google Scholar]
- Parker J., Flashner M., Mckeever W. G., Neidhardt F. C. Metabolic regulation of the arginyl and valyl transfer ribonucleic acid synthetases in bacteria. J Biol Chem. 1974 Feb 25;249(4):1044–1053. [PubMed] [Google Scholar]
- Pedersen S., Bloch P. L., Reeh S., Neidhardt F. C. Patterns of protein synthesis in E. coli: a catalog of the amount of 140 individual proteins at different growth rates. Cell. 1978 May;14(1):179–190. doi: 10.1016/0092-8674(78)90312-4. [DOI] [PubMed] [Google Scholar]
- Piepersberg A., Hennecke H., Engelhard M., Nass G., Böck A. Cross-reactivity of phenylalanyl-transfer ribonucleic acid ligases from different microorganisms. J Bacteriol. 1975 Dec;124(3):1482–1488. doi: 10.1128/jb.124.3.1482-1488.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A., RajBhandary U. L. Transfer RNA: molecular structure, sequence, and properties. Annu Rev Biochem. 1976;45:805–860. doi: 10.1146/annurev.bi.45.070176.004105. [DOI] [PubMed] [Google Scholar]
- Smith J. D. Transcription and processing of transfer RNA precursors. Prog Nucleic Acid Res Mol Biol. 1976;16:25–73. doi: 10.1016/s0079-6603(08)60755-2. [DOI] [PubMed] [Google Scholar]
- Takata R., Isaksson L. A. The temperature sensitive mutant 72c. II. Accumulation at high temperature of ppGpp and pppGpp in the presence of protein synthesis. Mol Gen Genet. 1978 Apr 25;161(1):15–21. doi: 10.1007/BF00266610. [DOI] [PubMed] [Google Scholar]


