Abstract
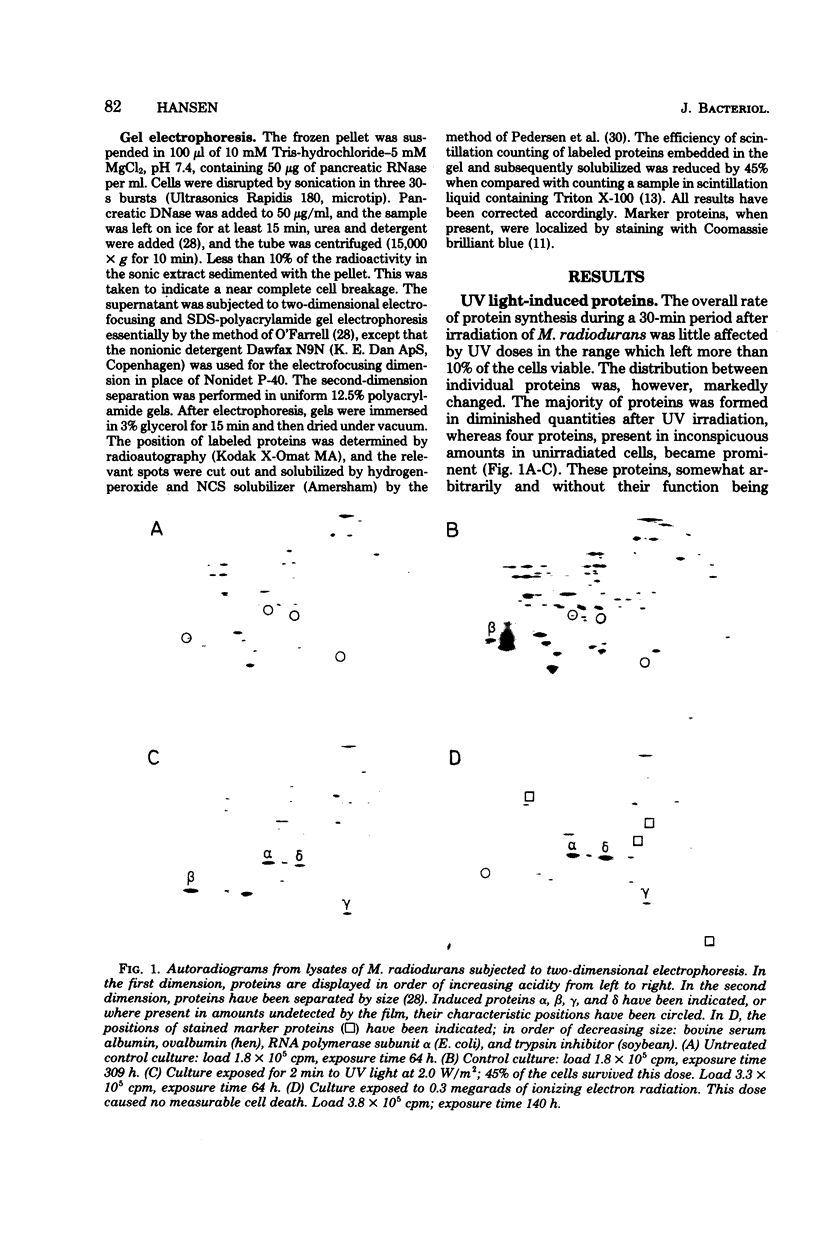
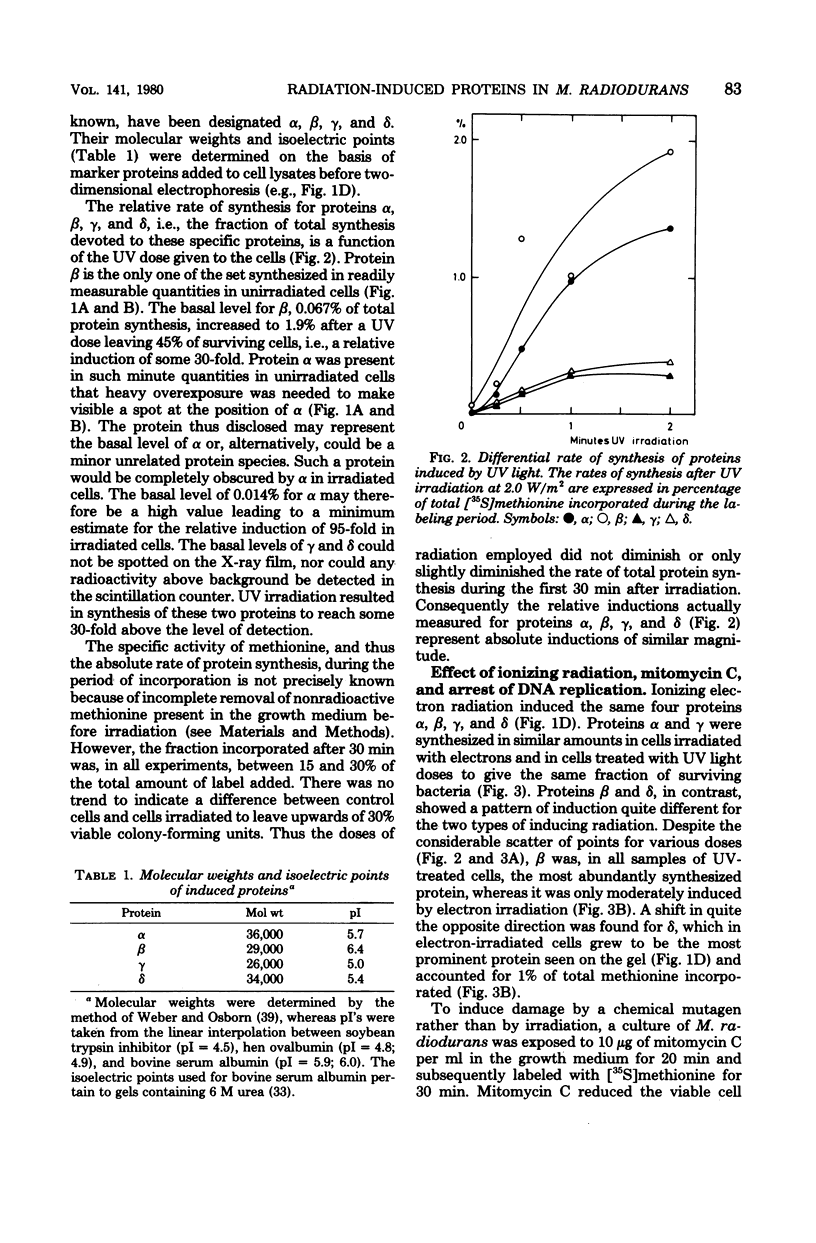
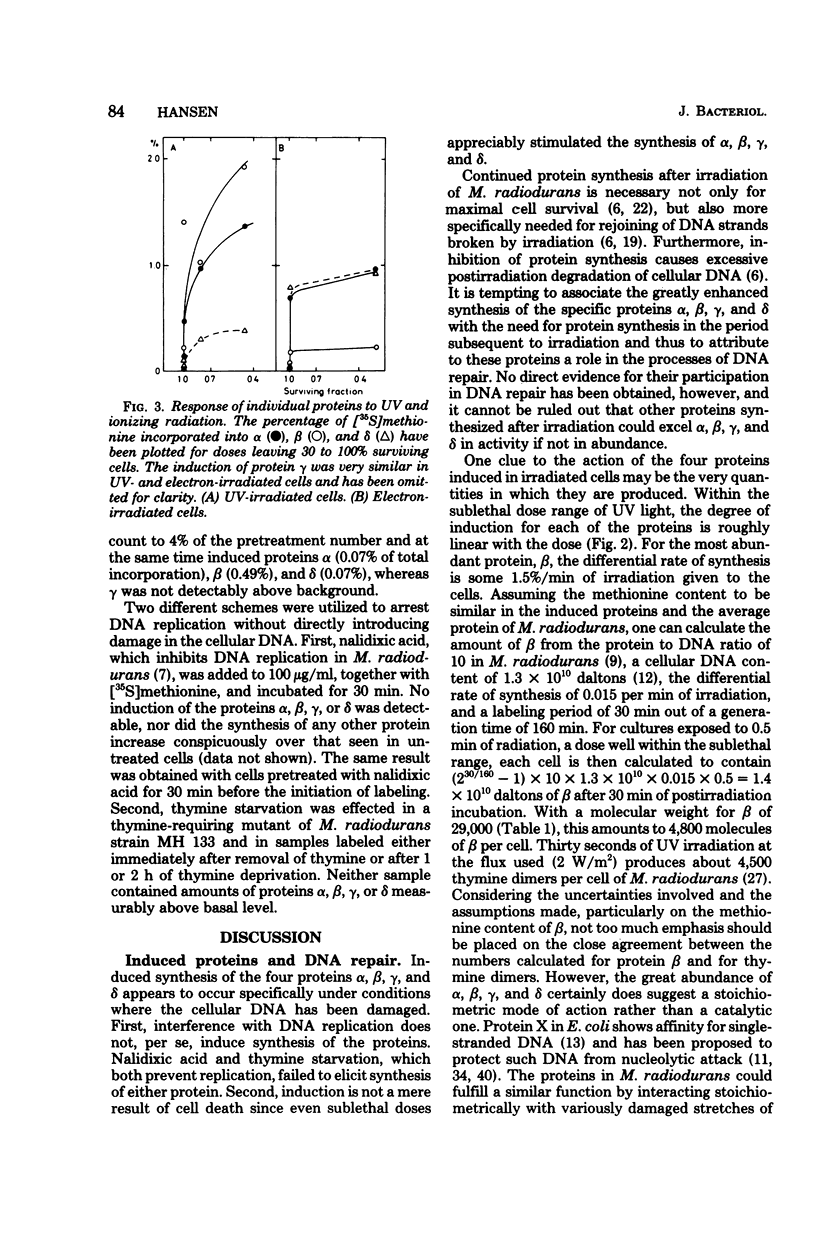
Four proteins, alpha beta, gamma, and delta, preferentially synthesized in ultraviolet light-treated cells of Micrococcus radiodurans, were characterized in terms of their molecular weights and isoelectric points. Within the sublethal-dose range, the differential rate of synthesis for these proteins increased linearly with the inducing UV dose. The degree of induction reached 100-fold, and the most abundant protein beta, amounted to approximately 2% of the total newly synthesized protein after irradiation. Damage caused by ionizing radiation or by treatment with mitomycin C also provoked the synthesis of the four proteins. The proportions between the individual proteins, however, varied strikingly with the damaging agent. In contrast to treatments which introduced damage in the cellular deoxyribonucleic acid, the mere arrest of deoxyribonucleic acid replication, caused by nalidixic acid or by starvation for thymine, failed to elicit the synthesis of either protein. Repair of deoxyribonucleic acid damage requires that a number of versatile and efficient processes by employed. It is proposed that the induced proteins participate in deoxyribonucleic acid repair in M. radiodurans. Mechanisms are discussed which would allow a differentiated cellular response to damages of sufficiently distinctive nature.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boling M. E., Setlow J. K. The resistance of Micrococcus radiodurans to ultraviolet radiation. 3. A repair mechanism. Biochim Biophys Acta. 1966 Jul 20;123(1):26–33. doi: 10.1016/0005-2787(66)90155-9. [DOI] [PubMed] [Google Scholar]
- Bridges B. A. Evidence for a further dark repair process in bacteria. Nat New Biol. 1972 Nov 8;240(97):52–53. doi: 10.1038/newbio240052a0. [DOI] [PubMed] [Google Scholar]
- Burrell A. D., Feldschreiber P., Dean C. J. DNA-membrane association and the repair of double breaks in x-irradiated Micrococcus radiodurans. Biochim Biophys Acta. 1971 Sep 30;247(1):38–53. doi: 10.1016/0005-2787(71)90805-7. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio S. M., Setlow R. B. Enhancement of postreplication repair in Chinese hamster cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2396–2400. doi: 10.1073/pnas.73.7.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C. J., Little J. G., Serianni R. W. The control of post irradiation DNA breakdown in Micrococcus radiodurans. Biochem Biophys Res Commun. 1970 Apr 8;39(1):126–134. doi: 10.1016/0006-291x(70)90767-9. [DOI] [PubMed] [Google Scholar]
- Driedger A. A., Grayston M. J. The effects of nalidixic acid on X-ray-induced DNA degradation and repair in Micrococcus radiodurans. Can J Microbiol. 1971 Apr;17(4):501–505. doi: 10.1139/m71-083. [DOI] [PubMed] [Google Scholar]
- Emmerson P. T., West S. C. Identification of protein X of Escherichia coli as the recA+/tif+ gene product. Mol Gen Genet. 1977 Sep 21;155(1):77–85. doi: 10.1007/BF00268563. [DOI] [PubMed] [Google Scholar]
- Freedman M. L., Bruce A. K. The relationship of radioresistance to balanced growth-rate in Micrococcus radiodurans. Int J Radiat Biol Relat Stud Phys Chem Med. 1971;19(2):111–121. doi: 10.1080/09553007114550161. [DOI] [PubMed] [Google Scholar]
- Gudas L. J., Mount D. W. Identification of the recA (tif) gene product of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5280–5284. doi: 10.1073/pnas.74.12.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. DNA synthesis inhibition and the induction of protein X in Escherichia coli. J Mol Biol. 1976 Mar 15;101(4):459–477. doi: 10.1016/0022-2836(76)90240-0. [DOI] [PubMed] [Google Scholar]
- Hansen M. T., Bennett P. M., von Meyenburg K. Intracistronic polarity during dissociation of translation from transcription in Escherichia coli. J Mol Biol. 1973 Jul 15;77(4):589–604. doi: 10.1016/0022-2836(73)90225-8. [DOI] [PubMed] [Google Scholar]
- Hansen M. T. Multiplicity of genome equivalents in the radiation-resistant bacterium Micrococcus radiodurans. J Bacteriol. 1978 Apr;134(1):71–75. doi: 10.1128/jb.134.1.71-75.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan P. V., Cerutti P. A. Formation and repair of gamma-ray induced thymine damage in Micrococcus radiodurans. J Mol Biol. 1972 Apr 28;66(1):65–81. doi: 10.1016/s0022-2836(72)80006-8. [DOI] [PubMed] [Google Scholar]
- Holloman W. K., Radding C. M. Recombination promoted by superhelical DNA and the recA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3910–3914. doi: 10.1073/pnas.73.11.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Pardee A. B. Changes of membrane proteins and their relation to deoxyribonucleic acid synthesis and cell division of Escherichia coli. J Biol Chem. 1970 Nov 10;245(21):5813–5819. [PubMed] [Google Scholar]
- Inouye M. Pleiotropic effect of the rec A gene of Escherichia coli: uncoupling of cell division from deoxyribonucleic acid replication. J Bacteriol. 1971 May;106(2):539–542. doi: 10.1128/jb.106.2.539-542.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp D. S., Smith K. C. Chemical nature of chain breaks produced in DNA by x-irradiation in vitro. Radiat Res. 1970 Apr;42(1):34–49. [PubMed] [Google Scholar]
- Kitayama S., Matsuyama A. Mechanism for radiation lethality in M. radiodurans. Int J Radiat Biol Relat Stud Phys Chem Med. 1971;19(1):13–19. doi: 10.1080/09553007114550021. [DOI] [PubMed] [Google Scholar]
- Kitayama S., Matsuyama A. Possibility of the repair of double-strand scissions in Micrococcus radiodurans DNA caused by gamma-rays. Biochem Biophys Res Commun. 1968 Nov 8;33(3):418–422. doi: 10.1016/0006-291x(68)90588-3. [DOI] [PubMed] [Google Scholar]
- Little J. W., Hanawalt P. C. Induction of protein X in Escherichia coli. Mol Gen Genet. 1977 Feb 15;150(3):237–248. doi: 10.1007/BF00268122. [DOI] [PubMed] [Google Scholar]
- Little J. W., Kleid D. G. Escherichia coli protein X is the recA gene product. J Biol Chem. 1977 Sep 25;252(18):6251–6252. [PubMed] [Google Scholar]
- MOSELEY B. E., LASER H. REPAIR OF X-RAY IN MICROCOCCUS RADIODURANS. Proc R Soc Lond B Biol Sci. 1965 Apr 13;162:210–222. doi: 10.1098/rspb.1965.0035. [DOI] [PubMed] [Google Scholar]
- McEntee K. Protein X is the product of the recA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5275–5279. doi: 10.1073/pnas.74.12.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEntee K., Weinstock G. M., Lehman I. R. Initiation of general recombination catalyzed in vitro by the recA protein of Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2615–2619. doi: 10.1073/pnas.76.6.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley B. E., Mattingly A., Copland H. J. Sensitization to radiation by loss of recombination ability in a temperature-sensitive DNA mutant of Micrococcus radiodurans held at its restrictive temperature. J Gen Microbiol. 1972 Sep;72(2):329–338. doi: 10.1099/00221287-72-2-329. [DOI] [PubMed] [Google Scholar]
- Moseley B. E., Williams E. Repair of damage; DNA in bacteria. Adv Microb Physiol. 1977;16:99–156. doi: 10.1016/s0065-2911(08)60048-x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pedersen S., Reeh S. V. Analysis of the proteins synthesized in ultraviolet light-irradiated Escherichia coli following infection with the bacteriophages lambdadrifd 18 and lambdadfus-3. Mol Gen Genet. 1976 Mar 30;144(3):339–343. doi: 10.1007/BF00341733. [DOI] [PubMed] [Google Scholar]
- Radman M. SOS repair hypothesis: phenomenology of an inducible DNA repair which is accompanied by mutagenesis. Basic Life Sci. 1975;5A:355–367. doi: 10.1007/978-1-4684-2895-7_48. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Mount D. W. Inactivation and proteolytic cleavage of phage lambda repressor in vitro in an ATP-dependent reaction. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2283–2287. doi: 10.1073/pnas.74.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaman M. R., Williamson A. R. Isoelectric focusing of proteins in the native and denatured states. Anomalous behaviour of plasma albumin. Biochem J. 1971 Mar;122(1):93–99. doi: 10.1042/bj1220093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satta G., Gudas L. J., Pardee A. B. Degradation of Escherichia coli DNA: evidence for limitation in vivo by protein X, the recA gene product. Mol Gen Genet. 1979 Jan 5;168(1):69–80. doi: 10.1007/BF00267935. [DOI] [PubMed] [Google Scholar]
- Shibata T., DasGupta C., Cunningham R. P., Radding C. M. Purified Escherichia coli recA protein catalyzes homologous pairing of superhelical DNA and single-stranded fragments. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1638–1642. doi: 10.1073/pnas.76.4.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompayrac L., Maaloe O. Autorepressor model for control of DNA replication. Nat New Biol. 1973 Jan 31;241(109):133–135. doi: 10.1038/newbio241133a0. [DOI] [PubMed] [Google Scholar]
- Sussman R., Zeev H. B. Proposed mechanism of bacteriophage lambda induction: acquisition of binding sites for lambda repressor by DNA of the host. Proc Natl Acad Sci U S A. 1975 May;72(5):1973–1976. doi: 10.1073/pnas.72.5.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet D. M., Moseley B. E. Accurate repair of ultraviolet-induced damage in Micrococcus radiodurans. Mutat Res. 1974 Jun;23(3):311–318. doi: 10.1016/0027-5107(74)90104-3. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- West S. C., Emmerson P. T. Induction of protein synthesis in Escherichia coli following UV- or gamma-irradiation, mitomycin C treatment or tif Expression. Mol Gen Genet. 1977 Feb 28;151(1):57–67. doi: 10.1007/BF00446913. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcel A., Burgi E. On the structure of the folded chromosome of Escherichia coli. J Mol Biol. 1972 Nov 14;71(2):127–147. doi: 10.1016/0022-2836(72)90342-7. [DOI] [PubMed] [Google Scholar]



