Abstract
We have previously demonstrated that the phosphatidylinositol-3 kinase (PI3K)/Akt signaling is essential for pancreatic regeneration after partial pancreatectomy in mice. In the present study, we examined a role of PI3K/Akt signaling for pancreatic duct cell differentiation into insulin-producing cells. Epithelial-like cells were isolated from mouse pancreas and confirmed to be positive for a duct cell marker cytokeratin-20 (CK-20) but negative for insulin. Incubation of these cells with epidermal growth factor, exhibited a gradual increase in Akt phosphorylation and expression of pancreatic duodenal homeobox-1 (PDX-1), a regulator of β-cell differentiation. Three weeks later, these CK-20-positive cells were noted to express insulin as determined by immunofluorescent double-staining. Akt phosphorylation, PDX-1 expression, and insulin production were effectively reduced by blocking the PI3K/Akt pathway using siRNA to the p85α regulatory subunit of PI3K. Our results demonstrate that PI3K/Akt activation has a critical role for pancreatic duct cell differentiation into insulin-producing cells.
Keywords: PDX-1, PI3K, insulin, pancreatic regeneration, duct differentiation, β-cell differentiation, wortmannin, siRNA
Introduction
In the normal adult pancreas, islet β-cell mass is replenished by two major mechanisms, β-cell replication and neogenesis from progenitor cells [1;2]. Although the origin of β-cell progenitor cells is not clearly understood at present, pancreatic duct cells are one of the candidates for these progenitor cells since insulin within the pancreatic duct epithelium has been well documented in the regenerating pancreas [3]. Nevertheless, compared to islet morphogenesis during embryogenesis, differentiation of duct cells to endocrine cells in the adult pancreas is poorly understood.
Pancreatic duodenal homeobox-1 (PDX-1), which is also known as IDX-1, IPF-1 and STF-1, is a key regulator of β-cell differentiation and function [4]. PDX-1 plays critical roles in endocrine pancreatic function through its regulatory action on the expression of functional pancreatic genes including insulin [5]. PDX-1 is also essential for β-cell neogenesis as demonstrated by several in vitro models of cell differentiation to insulin-producing cells [6;7]. Among these studies, pancreatic duct cell-derived cancer cells, Panc-1, were shown to differentiate to insulin-producing cells upon forceful expression of exogenous PDX-1 [6]. Expression of PDX-1 increases in the duct during β-cell neogenesis in an animal model of pancreatic regeneration after partial pancreatectomy [8]. These findings strongly suggest that PDX-1 is an important mediator and a marker of duct cell differentiation into β-cells. However, the regulation of PDX-1 expression during β-cell neogenesis is at present not well understood.
Phosphatidylinositol 3-kinase (PI3K) pathway plays various important roles in pancreatic function, such as insulin signaling, insulin-stimulated glucose transport and glycogen synthesis [9;10]. PI3K is composed of a regulatory subunit, p85, and a catalytic subunit, p110 [11]. Activated Akt, which is phosphorylated by PI3K, causes phosphorylation of downstream target proteins that affect cell growth, cell cycle distribution, apoptosis and survival [11]. Previously, we have reported that the PI3K pathway is critical for the proliferation of pancreatic acinar cells and plays a major role in pancreatic regeneration after partial pancreatectomy [12].
We have recently demonstrated that expression of PDX-1 and activation of PI3K (as assessed by phosphorylation of Akt) occurred concomitantly in pancreatic duct cells during tissue regeneration after partial pancreatectomy. We also showed that administration of a PI3K inhibitor wortmannin suppressed the pancreatectomy-induced PDX-1 expression [13]. In this study, however, it was not clear how PI3K-mediated expression of PDX-1 is directory related to β-cell neogenesis. Here, we investigated the role of PI3K in PDX-1 expression and cell differentiation utilizing siRNA technology in a primary cultured pancreatic duct cell differentiation model. We show that both PDX-1 expression and duct cell differentiation were blocked by inhibition of PI3K, demonstrating an important role for PI3K/Akt on PDX-1-mediated differentiation of duct cells into β-cells.
Materials and methods
Cell isolation, culture, and siRNA transfection
The inferior vena cava of anesthelized mice (male 8-week-old C57BL/6 from Charles Rivers Laboratories, Wilmington, MA) was cut, and blood was removed with physiological saline perfused through the cardiac left ventricle. Whole pancreas was dissected, minced and transferred to 3ml of pre-warmed oxygenated phosphate buffered saline (PBS with Ca2+ and Mg2+) containing 0.1% BSA and 0.01% [wt/vol] soybean trypsin inhibitor (Calbiochem, La Jolla, CA). One ml of PBS containing 1mg/ml of type IV collagenase (Sigma, St. Louis, MO) was added and incubated at 37°C for 15 min. Digested tissue was washed 3 times with PBS containing BSA and soybean trypsin inhibitor and filtered through 860- and 190-μm meshes. The cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% fetal bovine serum (FBS), 1.6nM epidermal growth factor (EGF, from Molecular Probes, Eugen, OR) 0.25mg/ml soybean trypsin inhibitor, 50IU/ml penicillin and 50μg/ml streptomycin at 37°C in 5% CO2/Air. After 3-5 days, floating cells were removed by washing repeatedly with fresh DMEM. DMEM with high glucose (25mM) was used throughout the study except for the glucose challenge tests.
For PI3K blocking, the siSTABLE SMARTpool siRNA directed to PI3K p85α regulatory subunit were synthesized by Dharmacon (Lafayette, CO). To prevent recognition and cleavage of unintended mRNA targets (off-target effect) [14], the siRNA was modified by an ON-TARGET technique from the same manufacturer. For transfection, isolated pancreatic cells were seeded on 12 or 24-well plates. Seven days later, cells were washed with fresh DMEM, and p85α or non-specific control siRNA (final concentration at 50nM) was transfected using Trans TKO Transfection Reagent (Mirus, Madison, WI). For experiments using wortmannin, this reagent was added to the culture medium at a final concentration of 100nM every 6 h since wortmannin is not stable for more than 5-6 h in the medium [15]
Protein extraction and Western blot analysis
Protein was extracted as previously described [12]. Western blot analysis was also performed as previously described [12]. Briefly, equal amounts of protein were electrophoretically resolved on either 10% Novex Tris-Glycine or NuPAGE 4-12% Bis-Tris gels (Invitrogen, Carlsbad, CA), and transferred to polyvinylidene difluoride membranes. After blocked with 5% dried skimmed milk, the membranes were incubated with primary antibodies for overnight at 4°C. The primary antibodies used in the analysis were: rabbit anti-pAkt (Ser473, #9271, Cell Signaling, Beverly, MA, 1:1000 diluted), anti-Akt (#9272, Cell Signaling, 1:1000 diluted), rabbit anti-PI3K p85α (NeoMarker, Fremont, CA, 1:1000 diluted), rabbit anti-PDX-1 (Chemicon International, Temecula CA, 1:5000 diluted), and monoclonal anti-β-actin antibody (A5441; clone AC-15 from Sigma, St. Louis, MO, 1:2000 diluted). The membranes were incubated with a secondary antibody (1:5000 diluted, goat anti-rabbit or mouse IgG, Upstate, Waltham, MA) conjugated with horseradish peroxidase. The immunoreaction was visualized using enhanced chemiluminescence (ECL) system or ECL Plus (Amersham, Arlington Heights, IL).
Immunocytochemical analysis
Immunocytochemical analysis was performed with EnVision+ system (DAKO Cytomation, Carpinteria, CA) according to our previously published method [16] with a few modifications. Briefly, cells were washed with fresh PBS, fixed in 10% neutral-buffered formaldehyde for 20 min, and endogenous peroxidase activity blocked with 3% hydrogen peroxidase. The fixed cells were incubated with the primary antibody at room temperature for 3 h or overnight. Primary antibodies used for the immunocytochemical analysis were: anti-CK-20 (#sc-17112, Santa Cruz Biotechnology, Santa Cruz, CA), anti-amylase (#sc-12821 from Santa Cruz Biotechnology), anti-insulin (A0564, DAKO), anti-glucagon (G2654, DAKO), and anti-C-peptide (Lincoln Research, St. Charles MO). After rinsing, cells were incubated with secondary antibodies (Rhodamine or Alexa-488 conjugated) at room temperature for 30 min. Cells were treated with 3, 3′-diaminobenzidine chromogen solution, and the slides were counter-stained with hematoxylin. For immunofluorescent analysis, cells were washed after secondary antibody reaction and fluorescent signals in the cells were detected under the fluorescence microscope.
Glucose challenge test
One day after culture medium was replaced with low glucose (5.5mM) DMEM, cells were washed 3 times with low glucose DMEM and incubated for 1 h in the the same medium. The medium was then changed to either low or high glucose (25mM) DMEM. Conditioned medium was collected 3 h later, and the insulin content was measured by a commercial ELISA kit from Crystal Chem (Downers Grove, IL).
Statistical analysis
Differences in insulin release were analyzed using analysis of variance for a two-factor factorial experiment. The two factors were defined as: i) glucose concentration (low and high), and ii) siRNA treatment (control and p85α siRNA). The results of glucose challenge test were analyzed using two-group t-test. All effects were assessed at the 0.05 levels of significance as the experiment-wise error rates. Data analysis was conducted using StatView software for Windows version 5.0 from SAS (Cary, NC).
Results
PDX-1 expression is increased in isolated pancreatic duct cells
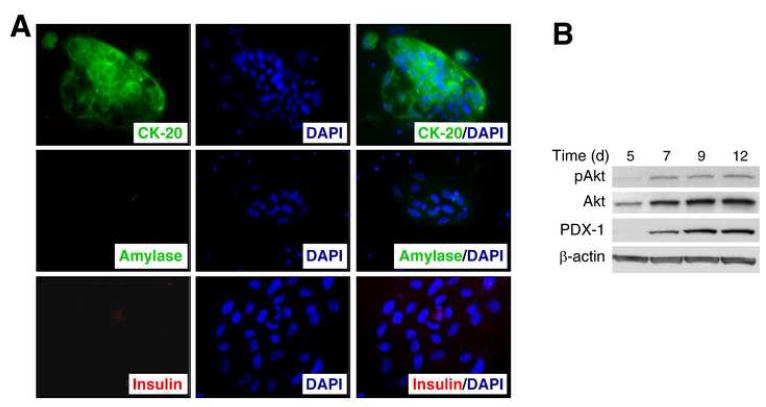
To better delineate the role of PI3K in PDX-1 expression in pancreatic duct cells, we established an in vitro primary culture model of pancreatic duct cells. Mouse pancreas was digested with collagenase and filtered, and dissociated cells were seeded in culture dishes. Within 3-4 days, monolayer colonies of epithelial-like cells became clearly visible under the microscope. Floating cells, including acinar and islet cells, were removed at this point by washing the culture repeatedly. Immunocytochemical analysis showed that these epithelial-like cells were stained positive for cytokeratin-20 (CK-20), a duct cell marker [17], but not for amylase or insulin, suggesting that these cells were derived from the pancreatic ducts (Fig 1A).
Fig. 1. Increased PDX-1 expression in cultured pancreatic duct epithelial-like cells in vitro.
(A) Immunocytochemistry of cytokeratin-20 (CK-20), amylase and insulin expression 5 d after cell isolation (magnification ×200). (B) Western blot analysis on Akt phosphorylation (pAkt) and PDX-1 expression in cultured pancreatic duct cells. The experiment was repeated 3 times with similar results.
To examine PI3K activation and PDX-1 expression, Western blot analysis was performed on whole protein samples derived from the cells (Fig. 1B). Expression of PDX-1 was increased in the cultured duct cells over the time course with associated phosphorylation of Akt. Interestingly, total Akt levels also increased 5 to 7 d after the initiation of the culture. These results confirm our recent in vivo study [13] demonstrating that PDX-1 expression, the possible initiating step of duct cell differentiation to endocrine cells [18], increases with elevated phosphorylation of Akt.
Pancreatic duct cells differentiate and secrete insulin in a glucose-dependent fashion in vitro
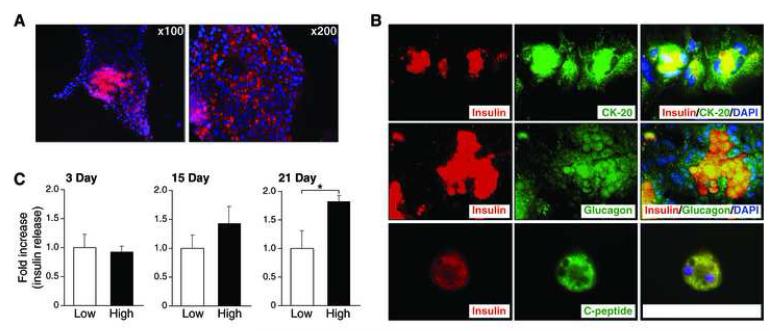
To determine whether isolated pancreatic duct cells can differentiate into insulin-producing cells, we cultured the cells in DMEM containing a high concentration of glucose (25mM) for longer periods of time; insulin immunoreactivity was assessed by immunofluorescence analysis. The isolated cells vigorously replicate for the first week, and slow down thereafter. Although insulin immunoreactivity was not detected in the cells 5 days after the initiation of the culture, a small number of insulin-positive cells appeared among duct cell colonies after 3 weeks in culture (Fig 2A). These results demonstrate that pancreatic duct cells from adult mice can differentiate into insulin-positive cells in vitro.
Fig. 2. Differentiation of cultured pancreatic duct cells into insulin-secreting cells in a glucose-dependent fashion.
(A) Immunofluorescence analysis detecting insulin expression in cultured pancreatic duct cells 3 weeks after initiation of the culture. DAPI (blue) stain was performed as counter stain (magnification ×100). (B) Double-immunofluorescence analysis confirming differentiation of isolated duct cells into insulin-producing cells. Double immunofluorescence analysis of insulin (red), CK-20 (green), glucagon (green) or C-peptide (green) were performed 3 weeks after initiation of the culture. DAPI (blue) stain was performed as counter-stain (magnification ×400). (C) Glucose challenge test to confirm a glucose-dependent insulin secretion by differentiated duct cells. (Values are mean±SEM, n=3. *: p<0.05 versus low glucose treated group).
To further confirm that the insulin-positive cells were differentiated from the pancreatic duct cells, we performed double immunofluorescence analysis of insulin, CK-20, glucagon and C-peptide. Insulin immunoreactivity was localized to the cells which also expressed CK-20, confirming that these insulin-positive cells were originated from duct cells (Fig. 2B). Interestingly, insulin-positive cells also expressed glucagon. Furthermore, double-staining analyses showed that C-peptide was co-localized with insulin in the duct cells, confirming that these insulin-positive cells indeed produced insulin. Although fibroblast-like cells were also seen in the culture, these cells were negative for CK-20, amylase, insulin, glucagon, and C-peptide (data not shown). These findings demonstrate that the cultured pancreatic duct cells can differentiate into insulin-producing cells in vitro.
Mature β-cells secrete insulin in response to high glucose concentration. To examine whether the differentiated duct cells also secrete insulin in a glucose-dependent fashion, glucose challenge tests was performed on the duct cells in vitro. For these experiments, pancreatic duct cultures were maintained in a low glucose medium (5.5mM glucose) for 24 h. Cells were then stimulated with a high glucose medium (25mM glucose) for 3 h, and insulin concentration in the culture medium was determined by ELISA. As shown in Fig 2C, insulin secretion, as measured on day 21, was noted to occur in a glucose concentration-dependent fashion; insulin secretion was not noted in cells cultured for 3 to 15 d after the initiation of the culture. These findings demonstrate that the duct-derived insulin-positive cells are functional and secrete insulin in a glucose-dependent fashion.
PI3K inhibition blocks duct cell differentiation into insulin-secreting cells
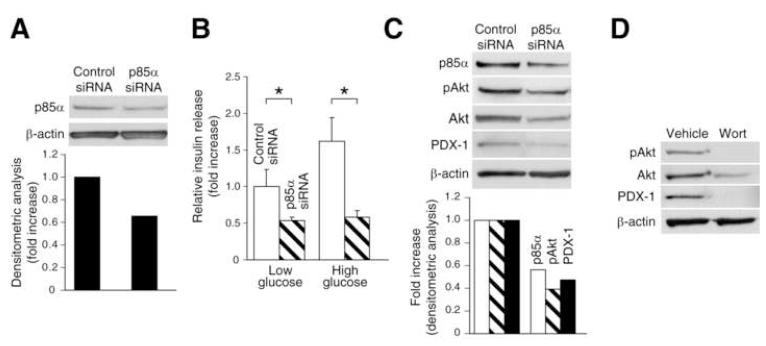
To determine the role of PI3K activation in the pancreatic duct cell differentiation to insulin-positive cells, the effect of PI3K inhibition on PDX-1 expression and insulin secretion was assessed. Isolated pancreatic duct cells were cultured for 7 d and transfected with either p85α or control siRNA. To confirm efficiency of knockdown by p85α siRNA, Western blot analysis was performed; the level of p85α was reduced to approximately 40% by p85α siRNA (Fig. 3A). Glucose challenge tests at 7 d after siRNA transfection demonstrated that glucose-dependent insulin secretion in the cultured duct cells was significantly blocked by p85α siRNA transfection (Fig. 3B). Akt phosphorylation and PDX-1 expression in the cultured duct cells were effectively reduced by p85α siRNA (Fig. 3C). These results demonstrate a critical role for PI3K activation in PDX-1 expression and duct cell differentiation into insulin-secreting cells.
Fig. 3. Effects of PI3K inhibition on duct cell differentiation in vitro.
(A) Western blot analysis confirming a reduction of p85α protein in cultured duct cells 3 days after transfection with p85α siRNA. (B) Glucose challenge test to demonstrating that PI3K inhibition by p85α siRNA blocks duct differentiation into insulin-secreting cells. Cells were transfected with p85α or control siRNA and glucose challenge test was performed 7 d later (Values are mean±SEM, n=3. *: p<0.05). (C) Western blot analysis demonstrating that transfection with p85α siRNA suppresses PDX-1 expression in cultured duct cells. (D) Western blot analysis confirming the effect of wortmannin on PDX-1 expression in cultured duct cells.
To further confirm these results, the effect of wortmannin on PDX-1 expression in cultured duct cells was also examined (Fig. 3D). Wortmannin completely blocked the expression of PDX-1 and Akt phosphorylation in the cultured duct cells (Fig. 3D). Taken together, our findings provide strong evidence that the PI3K/Akt pathway regulates PDX-1-mediated differentiation of duct cells into insulin-secreting endocrine cells.
Discussion
In the present study, we show that isolated pancreatic duct cells from normal mice differentiate into functional insulin-secreting cells. A previous report showed that pancreatic epithelioid-like cells, obtained from non-obese diabetic mice [15], formed islet-like cell clusters (ICCs) that released insulin after several weeks of culture in vitro [19]. Similar results have been reported for human pancreatic duct epithelial cells [20]. However, the sources of ICCs have not been clearly delineated in these previous reports. To determine the origin of the insulin-positive cells in our culture system, we performed double-staining immunocytochemistry and demonstrated that these cells are positive for both insulin and a duct cell marker CK-20. The insulin-positive cells were not present at 5 d, but appeared 3 weeks after the initiation of cultures, excluding a possibility that these cells are pre-existing islet-derived cells.
Interestingly, we also showed that these insulin-positive cells produce glucagon. Although mature β-cells in pancreas do not produce glucagon, endocrine cells during embryogenesis are frequently noted to express both insulin and glucagon with differentiation [21]. In addition, differentiated embryonic stem cells or intestinal epithelial cells express both insulin and glucagon [22;23]. Therefore, colocalization of insulin and glucagon in the duct cells in our study further supports the notion that these cells are newly differentiating cells. Immunochemical detection of insulin alone may overestimate genuine β-cell differentiation since cultured cells can take up exogenous insulin from the culture medium [24]. Thus, we demonstrated colocalization of insulin and C-peptide in the cells and confirmed that these cells indeed produce insulin. Taken together, we show that mouse pancreatic duct epithelial-like cells can differentiate into insulin producing cells in vitro. The mechanisms inducing differentiation of the isolated duct cells in vitro have yet to be determined. Glucose concentrations may be a possible factor for differentiation since cells in our study were usually maintained in a culture medium containing a high concentration of glucose. It would be of interest to determine how glucose concentrations affect duct cell differentiation.
PI3K activation promotes differentiation of various cell types, such as splenic B cells [25], osteoclasts [26], chondrocytes [27], and adipose cells [28]. Our present study identified yet another role for PI3K to promote differentiation of pancreatic duct cells to endocrine cells. Our findings demonstrate that inhibition of PI3K using p85α siRNA effectively suppressed PDX-1 expression and cell differentiation of pancreatic duct cells. In contrast to our findings, Ptasznik et al [29] showed that addition of PI3K inhibitors, wortmannin or LY294002, promoted differentiation of human fetal ICCs to islets. In this report, the authors concluded that PI3K is negative regulator for differentiation of human fetal islet cells from ICCs. The discrepancy between these two studies possibly suggests that mechanisms for ICC differentiation and duct cell differentiation are different. Another possible explanation may relate to the experimental design and timing of the inhibitor treatment. Since wortmannin is not stable in culture medium and PI3K can be strongly re-activated several hours after treatment [15], we have treated cells with wortmannin every 6 h to ensure a continuous suppression of PI3K. In addition, we used p85α siRNA to suppress PI3K as gene knockdown by siRNA is more specific and stable for a longer time to inhibit target genes.
PI3K is divided into three classes, i.e., Class I, II and III [11]. Class IA PI3K, which is made up of p85 regulatory and p110 catalytic subunit in mammalian cells, regulates cell proliferation, cell survival and apoptosis in various cell types [11]. In the current study, we clearly demonstrated that Class IA PI3K plays a central role in PDX-1 expression in pancreatic duct cell during pancreatic regeneration. At present, it is not known whether other classes of PI3K also mediate PDX-1 expression in duct cells. Blocking each class of PI3K by specific siRNA should help to address this question. PI3K inhibition by both wortmannin or LY294002 has previously been shown to enhance insulin release from isolated islets [10]. Moreover, hyperinsulinemia is noted in p110α or β knockout mice [30]; PI3K inhibition induces insulin secretion. In this in vitro experiment using siRNA, our results showed that insulin secretion from p85α siRNA treated cells was reduced in both low and high glucose treatment, suggesting that p85α siRNA blocks duct cell differentiation into insulin-secreting cells with β-cell-like function.
In conclusion, our findings strongly suggest that activation of PI3K, especially Class IA PI3K, plays a critical role in PDX-1-mediated duct differentiation into insulin-secreting cells. Although islet transplantation is theoretically an ideal solution for patients with type I insulin-dependent diabetes mellitus [31], a problem of donor insufficiency still exists. Consequently, an establishment of an islet neogenesis technique to alleviate this problem would be highly significant. Our findings regarding duct cell differentiation into insulin-secreting cells could be one step in elucidating the molecular mechanisms of pancreatic duct cell differentiation in adult animals.
ACKNOWLEDGEMENTS
We thank Ms. Karen Martin for manuscript preparation and Mr. Tatsuo Uchida statistical analysis. This work was supported from grants from P01DK35608, R37AG10885, R01DK48498 and R01AG025273 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Weir GC, Laybutt DR, Kaneto H, Bonner-Weir S, Sharma A. Beta-cell adaptation and decompensation during the progression of diabetes. Diabetes. 2001;50(Suppl 1):S154–9. doi: 10.2337/diabetes.50.2007.s154. [DOI] [PubMed] [Google Scholar]
- [2].Hayashi KY, Tamaki H, Handa K, Takahashi T, Kakita A, Yamashina S. Differentiation and proliferation of endocrine cells in the regenerating rat pancreas after 90% pancreatectomy. Arch Histol Cytol. 2003;66:163–74. doi: 10.1679/aohc.66.163. [DOI] [PubMed] [Google Scholar]
- [3].Zenilman ME, Perfetti R, Swinson K, Magnuson T, Shuldiner AR. Pancreatic regeneration (reg) gene expression in a rat model of islet hyperplasia. Surgery. 1996;119:576–84. doi: 10.1016/s0039-6060(96)80270-4. [DOI] [PubMed] [Google Scholar]
- [4].Melloul D, Marshak S, Cerasi E. Regulation of pdx-1 gene expression. Diabetes. 2002;51(Suppl 3):S320–5. doi: 10.2337/diabetes.51.2007.s320. [DOI] [PubMed] [Google Scholar]
- [5].Hui H, Perfetti R. Pancreas duodenum homeobox-1 regulates pancreas development during embryogenesis and islet cell function in adulthood. Eur J Endocrinol. 2002;146:129–41. doi: 10.1530/eje.0.1460129. [DOI] [PubMed] [Google Scholar]
- [6].Hui H, Wright C, Perfetti R. Glucagon-like peptide 1 induces differentiation of islet duodenal homeobox-1-positive pancreatic ductal cells into insulin-secreting cells. Diabetes. 2001;50:785–96. doi: 10.2337/diabetes.50.4.785. [DOI] [PubMed] [Google Scholar]
- [7].Petersen HV, Serup P, Leonard J, Michelsen BK, Madsen OD. Transcriptional regulation of the human insulin gene is dependent on the homeodomain protein STF1/IPF1 acting through the CT boxes. Proc Natl Acad Sci U S A. 1994;91:10465–9. doi: 10.1073/pnas.91.22.10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sharma A, Zangen DH, Reitz P, Taneja M, Lissauer ME, Miller CP, Weir GC, Habener JF, Bonner-Weir S. The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes. 1999;48:507–13. doi: 10.2337/diabetes.48.3.507. [DOI] [PubMed] [Google Scholar]
- [9].White MF. The insulin signalling system and the IRS proteins. Diabetologia. 1997;40(Suppl 2):S2–17. doi: 10.1007/s001250051387. [DOI] [PubMed] [Google Scholar]
- [10].Zawalich WS, Tesz GJ, Zawalich KC. Inhibitors of phosphatidylinositol 3-kinase amplify insulin release from islets of lean but not obese mice. J Endocrinol. 2002;174:247–58. doi: 10.1677/joe.0.1740247. [DOI] [PubMed] [Google Scholar]
- [11].Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- [12].Watanabe H, Saito H, Rychahou P, Uchida T, Evers B. Aging is associated with decreased pancreatic acinar cell regeneration and phosphatidylinositol 3-kinase/Akt activation. Gastroenterology. 2005 doi: 10.1053/j.gastro.2005.03.016. [DOI] [PubMed] [Google Scholar]
- [13].Watanabe H, Saito H, Nishimura H, Ueda J, Evers BM. Activation of phosphatidylinositol-3 kinase regulates pancreatic duodenal homeobox-1 in duct cells during pancreatic regeneration. Pancreas. 2008 doi: 10.1097/MPA.0b013e318157753e. in press in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Scacheri PC, Rozenblatt-Rosen O, Caplen NJ, Wolfsberg TG, Umayam L, Lee JC, Hughes CM, Shanmugam KS, Bhattacharjee A, Meyerson M, Collins FS. Short interfering RNAs can induce unexpected and divergent changes in the levels of untargeted proteins in mammalian cells. Proc Natl Acad Sci U S A. 2004;101:1892–7. doi: 10.1073/pnas.0308698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kimura K, Hattori S, Kabuyama Y, Shizawa Y, Takayanagi J, Nakamura S, Toki S, Matsuda Y, Onodera K, Fukui Y. Neurite outgrowth of PC12 cells is suppressed by wortmannin, a specific inhibitor of phosphatidylinositol 3-kinase. J Biol Chem. 1994;269:18961–7. [PubMed] [Google Scholar]
- [16].Li J, Hellmich MR, Greeley GH, Jr., Townsend CM, Jr., Evers BM. Phorbol ester-mediated neurotensin secretion is dependent on the PKC-alpha and -delta isoforms. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1197–206. doi: 10.1152/ajpgi.00177.2002. [DOI] [PubMed] [Google Scholar]
- [17].Bouwens L, De Blay E. Islet morphogenesis and stem cell markers in rat pancreas. J Histochem Cytochem. 1996;44:947–51. doi: 10.1177/44.9.8773559. [DOI] [PubMed] [Google Scholar]
- [18].Sumi S, Gu Y, Hiura A, Inoue K. Stem cells and regenerative medicine for diabetes mellitus. Pancreas. 2004;29:e85–9. doi: 10.1097/00006676-200410000-00017. [DOI] [PubMed] [Google Scholar]
- [19].Ramiya VK, Maraist M, Arfors KE, Schatz DA, Peck AB, Cornelius JG. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med. 2000;6:278–82. doi: 10.1038/73128. [DOI] [PubMed] [Google Scholar]
- [20].Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A, O’Neil JJ. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci U S A. 2000;97:7999–8004. doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Slack JM. Developmental biology of the pancreas. Development. 1995;121:1569–80. doi: 10.1242/dev.121.6.1569. [DOI] [PubMed] [Google Scholar]
- [22].Blyszczuk P, Czyz J, Kania G, Wagner M, Roll U, St-Onge L, Wobus AM. Expression of Pax4 in embryonic stem cells promotes differentiation of nestin-positive progenitor and insulin-producing cells. Proc Natl Acad Sci U S A. 2003;100:998–1003. doi: 10.1073/pnas.0237371100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Suzuki A, Nakauchi H, Taniguchi H. Glucagon-like peptide 1 (1-37) converts intestinal epithelial cells into insulin-producing cells. Proc Natl Acad Sci U S A. 2003;100:5034–9. doi: 10.1073/pnas.0936260100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rajagopal J, Anderson WJ, Kume S, Martinez OI, Melton DA. Insulin staining of ES cell progeny from insulin uptake. Science. 2003;299:363. doi: 10.1126/science.1077838. [DOI] [PubMed] [Google Scholar]
- [25].Donahue AC, Hess KL, Ng KL, Fruman DA. Altered splenic B cell subset development in mice lacking phosphoinositide 3-kinase p85α. Int Immunol. 2004;16:1789–1798. doi: 10.1093/intimm/dxh180. [DOI] [PubMed] [Google Scholar]
- [26].Sugatani T, Hruska KA. Akt1/Akt2 and mTOR/Bim play critical roles in osteoclast differentiation and survival, respectively, while Akt is dispensable for cell survival in isolated osteoclast precursors. J Biol Chem. 2004 doi: 10.1074/jbc.M410480200. [DOI] [PubMed] [Google Scholar]
- [27].Ihara-Watanabe M, Uchihashi T, Miyauchi Y, Sakai N, Yamagata M, Ozono K, Michigami T. Involvement of phosphoinositide 3-kinase signaling pathway in chondrocytic differentiation of ATDC5 cells: application of a gene-trap mutagenesis. J Cell Biochem. 2004;93:418–26. doi: 10.1002/jcb.20185. [DOI] [PubMed] [Google Scholar]
- [28].Magun R, Burgering BM, Coffer PJ, Pardasani D, Lin Y, Chabot J, Sorisky A. Expression of a constitutively activated form of protein kinase B (c-Akt) in 3T3-L1 preadipose cells causes spontaneous differentiation. Endocrinology. 1996;137:3590–3. doi: 10.1210/endo.137.8.8754791. [DOI] [PubMed] [Google Scholar]
- [29].Ptasznik A, Beattie GM, Mally MI, Cirulli V, Lopez A, Hayek A. Phosphatidylinositol 3-kinase is a negative regulator of cellular differentiation. J Cell Biol. 1997;137:1127–36. doi: 10.1083/jcb.137.5.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Brachmann SM, Ueki K, Engelman JA, Kahn RC, Cantley LC. Phosphoinositide 3-kinase catalytic subunit deletion and regulatory subunit deletion have opposite effects on insulin sensitivity in mice. Mol Cell Biol. 2005;25:1596–607. doi: 10.1128/MCB.25.5.1596-1607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hakim N, Papalois V. Pancreas and islet transplantation. Ann Ital Chir. 2004;75:1–7. [PubMed] [Google Scholar]