Abstract
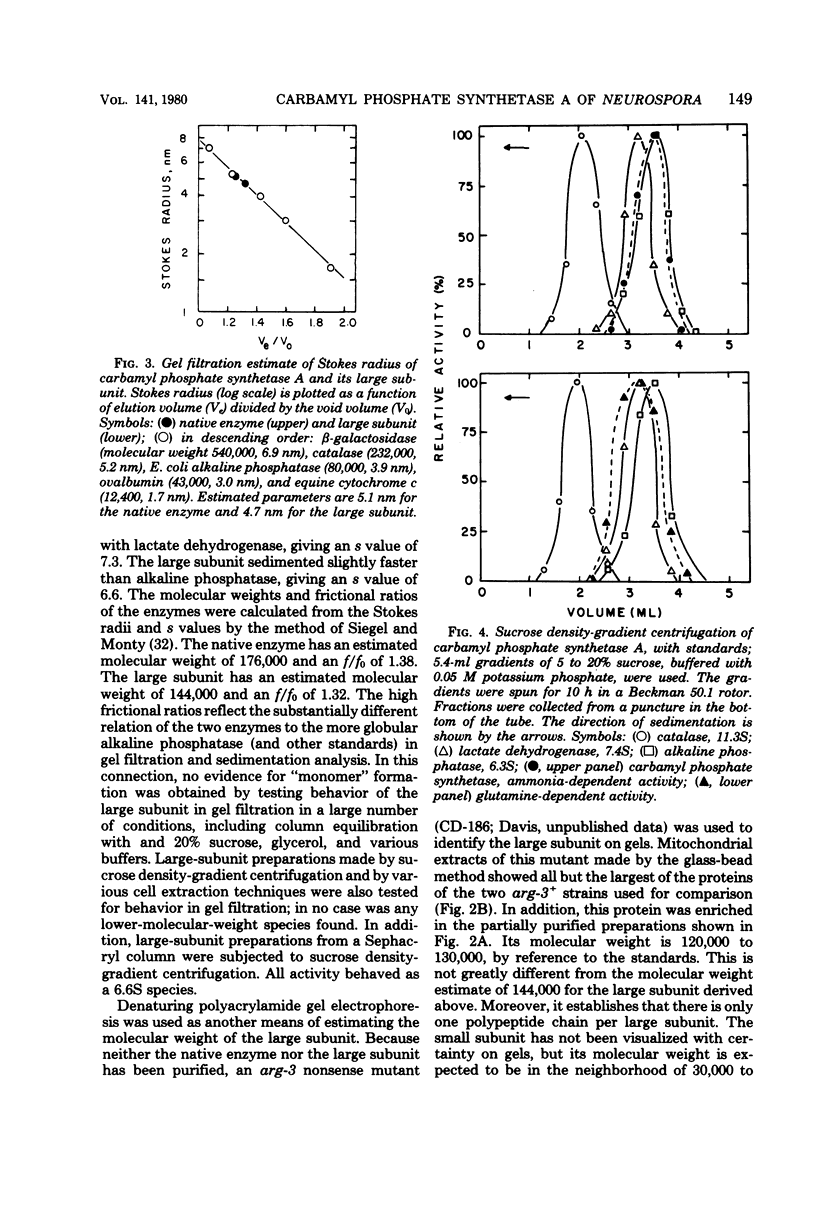
Carbamyl phosphate synthetase A of Neurospora crassa was partially purified from mitochondrial extracts. It is an extremely unstable enzyme (t 1/2 = 45 min at 25 detrees C) made up of two unequal subunits. The native enzyme has a molecular weight of approximately 175,000, and the large subunit has a molecular weight of about 125,000. Both the native enzyme and its large subunit are quite asymmetric, as revealed by slow sedimentation in sucrose gradents (7.3S and 6.6S, respectively). The small subunit has not been identified physically as a separate entity. The denaturation of the native, glutamine-dependent activity is correlated with dissociation of subunits, the larger of which retains a more stable, ammonia-dependent activity. Neither substrates nor any other agents except glycerol or polyethylene glycol appreciably stabilized the glutamine-dependent activity. Kinetic studies showed the native enzyme to have a Km for glutamine of about 0.16 mM, and a Km for NH4Cl of about 16 mM, at the optimal pH, 8.0. The enzyme, using either N donor, has a K+ requirement for activity, for which NH4+ can substitute. The glutamine leads to glutamate reaction, which requires the small subunit, also requires the large subunit and all reaction substrates for optimal activity. Other evidences of subunit interaction are the greater activity of the native enzyme, as opposed to the large subunit, with low concentrations of adenosine 5'-triphosphate-Mg2+, and in the stimulation of the ammonia-dependent activity of the native enzyme by glycine. Curiously, although the enzyme's role in biosynthesis is confined to the arginine pathway, it is completely indifferent to arginine or its precursors as feedback effectors or activators. The enzyme is compared with carbamyl phosphate synthetases of other organisms.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdelal A. T., Ingraham J. L. Carbamylphosphate synthetase from Salmonella typhimurium. Regulations, subunit composition, and function of the subunits. J Biol Chem. 1975 Jun 25;250(12):4410–4417. [PubMed] [Google Scholar]
- Anderson P. M. A glutamine- and N-acetyl-L-glutamate-dependent carbamyl phosphate synthetase activity in the teleost Micropterus salmoides. Comp Biochem Physiol B. 1976;54(2):261–263. doi: 10.1016/0305-0491(76)90154-1. [DOI] [PubMed] [Google Scholar]
- Bernhardt S. A., Davis R. H. Carbamoyl phosphate compartmentation in Neurospora: histochemical localization of aspartate and ornithine transcarbamoylases. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1868–1872. doi: 10.1073/pnas.69.7.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman B. J., Davis R. H. Cellular distribution of ornithine in Neurospora: anabolic and catabolic steady states. J Bacteriol. 1977 Apr;130(1):274–284. doi: 10.1128/jb.130.1.274-284.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassady W. E., Leiter E. H., Bergquist A., Wagner R. P. Separation of mitochondrial membranes of Neurospora crassa. II. Submitochondrial localization of the isoleucine-valine biosynthetic pathway. J Cell Biol. 1972 Apr;53(1):66–72. doi: 10.1083/jcb.53.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. J., Jones M. E. The cellular location of dihydroorotate dehydrogenase: relation to de novo biosynthesis of pyrimidines. Arch Biochem Biophys. 1976 Sep;176(1):82–90. doi: 10.1016/0003-9861(76)90143-0. [DOI] [PubMed] [Google Scholar]
- Cybis J., Davis R. H. Organization and control in the arginine biosynthetic pathway of Neurospora. J Bacteriol. 1975 Jul;123(1):196–202. doi: 10.1128/jb.123.1.196-202.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS R. H. A mutant form of ornithine transcarbamylase found in a strain of Neurospora carrying a pyrimidine-proline suppressor gene. Arch Biochem Biophys. 1962 Apr;97:185–191. doi: 10.1016/0003-9861(62)90063-2. [DOI] [PubMed] [Google Scholar]
- Davis R. H. Carbamyl phosphate synthesis in Neurospora crassa. I. Preliminary characterization of arginine-specific carbamyl phosphokinase. Biochim Biophys Acta. 1965 Aug 24;107(1):44–53. doi: 10.1016/0304-4165(65)90387-9. [DOI] [PubMed] [Google Scholar]
- Davis R. H. Metabolite distribution in cells. Science. 1972 Nov 24;178(4063):835–840. doi: 10.1126/science.178.4063.835. [DOI] [PubMed] [Google Scholar]
- Greenawalt J. W. The isolation of outer and inner mitochondrial membranes. Methods Enzymol. 1974;31:310–323. doi: 10.1016/0076-6879(74)31033-6. [DOI] [PubMed] [Google Scholar]
- Ito K., Nakanishi S., Terada M., Tatibana M. Control of pyrimidine biosynthesis in mammalian tissues. II. Glutamine-utilizing carbamoyl phosphate synthetase of various experimental tumors: distribution, purification and characterization. Biochim Biophys Acta. 1970 Dec 16;220(3):477–490. doi: 10.1016/0005-2744(70)90279-2. [DOI] [PubMed] [Google Scholar]
- Jones M. E. Regulation of pyrimidine and arginine biosynthesis in mammals. Adv Enzyme Regul. 1970;9:19–49. doi: 10.1016/s0065-2571(71)80036-5. [DOI] [PubMed] [Google Scholar]
- Lacroute F., Piérard A., Grenson M., Wiame J. M. The biosynthesis of carbamoyl phosphate in Saccharomyces cerevisiae. J Gen Microbiol. 1965 Jul;40(1):127–142. doi: 10.1099/00221287-40-1-127. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lusty C. J. Carbamoylphosphate synthetase I of rat-liver mitochondria. Purification, properties, and polypeptide molecular weight. Eur J Biochem. 1978 Apr 17;85(2):373–383. doi: 10.1111/j.1432-1033.1978.tb12249.x. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Makoff A. J., Radford A. Genetics and biochemistry of carbamoyl phosphate biosynthesis and its utilization in the pyrimidine biosynthetic pathway. Microbiol Rev. 1978 Jun;42(2):307–328. doi: 10.1128/mr.42.2.307-328.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. W., Jr Radioassay for enzymic production of glutamate from glutamine. Anal Biochem. 1972 Mar;46(1):239–243. doi: 10.1016/0003-2697(72)90417-4. [DOI] [PubMed] [Google Scholar]
- Mori M., Tatibana M. Glutaminase activity of glutamine-dependent carbamoyl-phosphate synthase from rat ascites hepatoma. Regulation by adenosine triphosphate-magensium and magnesium ion. Biochim Biophys Acta. 1977 Jul 8;483(1):90–99. doi: 10.1016/0005-2744(77)90011-0. [DOI] [PubMed] [Google Scholar]
- O'Donovan G. A., Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970 Sep;34(3):278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'neal T. D., Naylor A. W. Some regulatory properties of pea leaf carbamoyl phosphate synthetase. Plant Physiol. 1976 Jan;57(1):23–28. doi: 10.1104/pp.57.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus T. J., Switzer R. L. Characterization of pyrimidine-repressible and arginine-repressible carbamyl phosphate synthetases from Bacillus subtilis. J Bacteriol. 1979 Jan;137(1):82–91. doi: 10.1128/jb.137.1.82-91.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piérard A. Control of the activity of Escherichia coli carbamoyl phosphate synthetase by antagonistic allosteric effectors. Science. 1966 Dec 23;154(3756):1572–1573. doi: 10.1126/science.154.3756.1572. [DOI] [PubMed] [Google Scholar]
- Piérard A., Schröter B. Structure-function relationships in the arginine pathway carbamoylphosphate synthase of Saccharomyces cerevisiae. J Bacteriol. 1978 Apr;134(1):167–176. doi: 10.1128/jb.134.1.167-176.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C. W., Holwell J. H., Abdelal A. T. Purification and properties of the arginine-specific carbamoyl-phosphate synthase from Saccharomyces cerevisiae. J Gen Microbiol. 1978 May;106(1):145–151. doi: 10.1099/00221287-106-1-145. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott H. H., Ullrich V., Staudinger H. Enzymatic properties of L-kynurenine 3-hydroxylase (EC 1.14.1.2) in Neurospora crassa. Hoppe Seylers Z Physiol Chem. 1970 Jan;351(1):99–101. [PubMed] [Google Scholar]
- Shoaf W. T., Jones M. E. Initial steps in pyrimidine synthesis in Ehrlich ascites carcinoma. Biochem Biophys Res Commun. 1971 Nov 5;45(3):796–802. doi: 10.1016/0006-291x(71)90487-6. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Tatibana M., Ito K. Control of pyrimidine biosynthesis in mammalian tissues. I. Partial purification and characterization of glutamine-utilizing carbamyl phosphate synthetase of mouse spleen and its tissue distribution. J Biol Chem. 1969 Oct 10;244(19):5403–5413. [PubMed] [Google Scholar]
- Tatibana M., Shigesada K. Control of pyrimidine biosynthesis in mammalian tissues. IV. Requirements of a quantitative assay of glutamine-dependent carbamyl phosphate synthetase and effect of magnesium ion as an essential activator. J Biochem. 1972 Sep;72(3):537–547. doi: 10.1093/oxfordjournals.jbchem.a129933. [DOI] [PubMed] [Google Scholar]
- Thuriaux P., Ramos F., Piérard A., Grenson M., Wiame J. M. Regulation of the carbamoylphosphate synthetase belonging to the arginine biosynthetic pathway of Saccharomyces cerevisiae. J Mol Biol. 1972 Jun 20;67(2):277–287. doi: 10.1016/0022-2836(72)90241-0. [DOI] [PubMed] [Google Scholar]
- Thwaites W. M. A mutation reducing feedback regulation by arginine in suppressed pyr-3 mutants in Neurospora. Genetics. 1967 Apr;55(4):769–781. doi: 10.1093/genetics/55.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramell P. R., Campbell J. W. Carbamyl phosphate synthesis in a land snail, Strophocheilus oblongus. J Biol Chem. 1970 Dec 25;245(24):6634–6641. [PubMed] [Google Scholar]
- Trotta P. P., Burt M. E., Haschemeyer R. H., Meister A. Reversible dissociation of carbamyl phosphate synthetase into a regulated synthesis subunit and a subunit required for glutamine utilization. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2599–2603. doi: 10.1073/pnas.68.10.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrestarazu L. A., Vissers S., Wiame J. M. Change in location of ornithine carbamoyltransferase and carbamoylphosphate synthetase among yeasts in relation to the arginase/ornithine carbamoyltransferase regulatory complex and the energy status of the cells. Eur J Biochem. 1977 Oct 3;79(2):473–481. doi: 10.1111/j.1432-1033.1977.tb11830.x. [DOI] [PubMed] [Google Scholar]
- Weiss R. L., Davis R. H. Intracellular localization of enzymes of arginine metabolism in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5403–5408. [PubMed] [Google Scholar]
- Wellner V. P., Meister A. Enhancement of the glutaminase activity of carbamyl phosphate synthetase by alterations in the interaction between the heavy and light subunits. J Biol Chem. 1975 May 10;250(9):3261–3266. [PubMed] [Google Scholar]
- Williams L. G., Bernhardt S. A., Davis R. H. Evidence for two discrete carbamyl phosphate pools in Neurospora. J Biol Chem. 1971 Feb 25;246(4):973–978. [PubMed] [Google Scholar]
- Williams L. G., Bernhardt S., Davis R. H. Copurification of pyrimidine-specific carbamyl phosphate synthetase and aspartate transcarbamylase of Neurospora crassa. Biochemistry. 1970 Oct 27;9(22):4329–4335. doi: 10.1021/bi00824a013. [DOI] [PubMed] [Google Scholar]
- Williams L. G., Davis R. H. Pyrimidine-specific carbamyl phosphate synthetase in Neurospora crassa. J Bacteriol. 1970 Aug;103(2):335–341. doi: 10.1128/jb.103.2.335-341.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



