Abstract
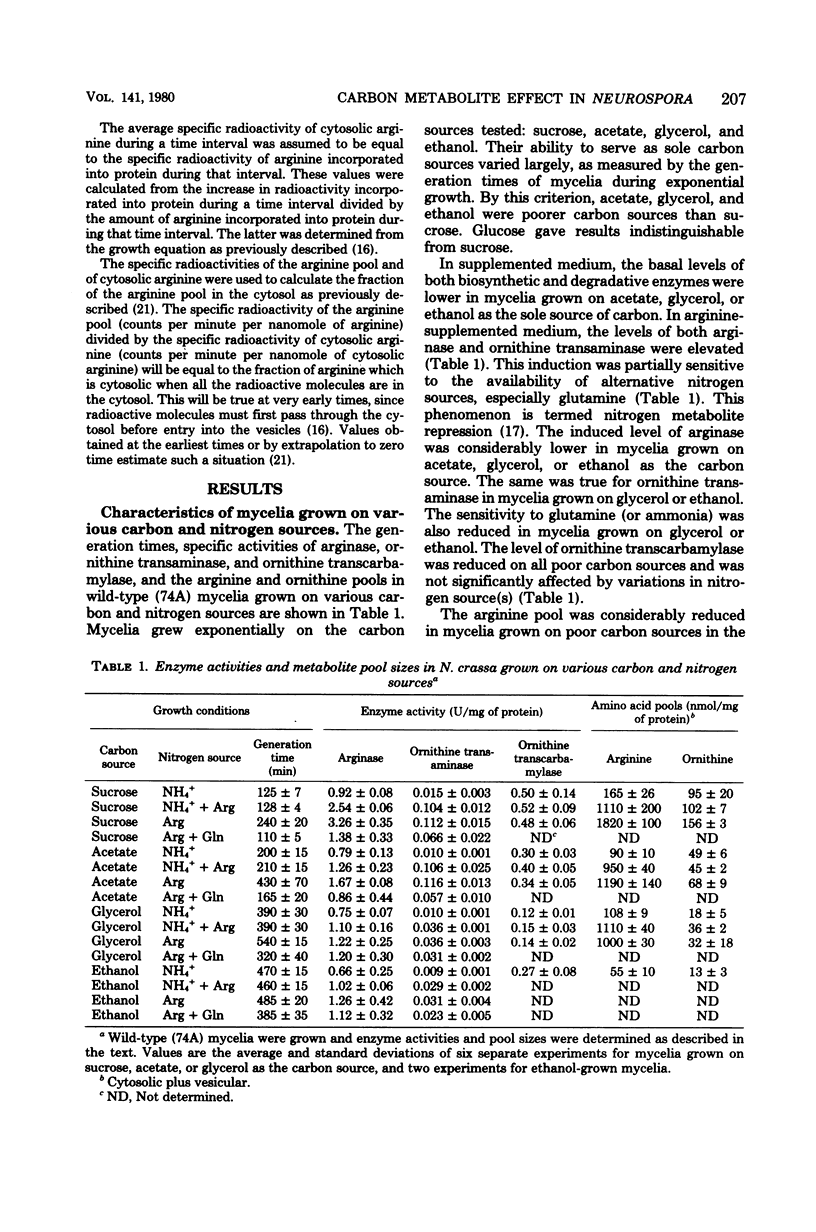
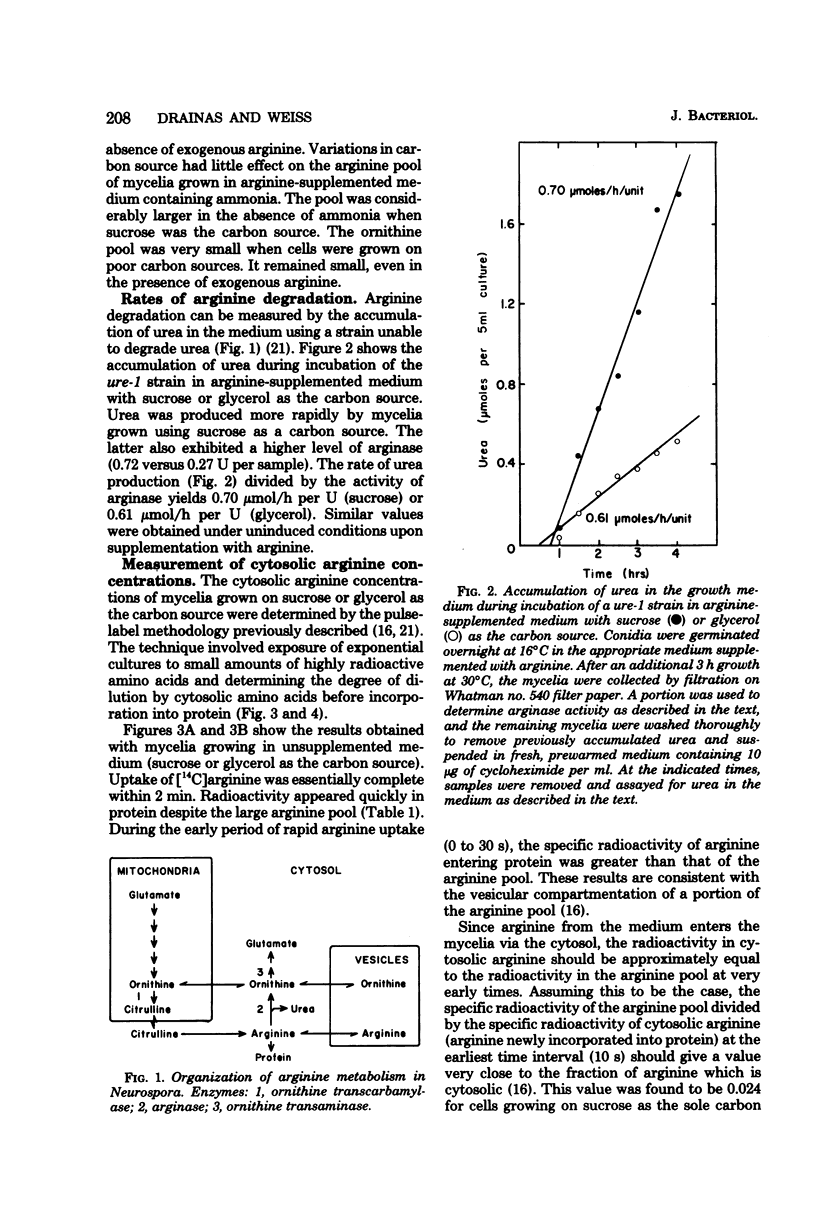
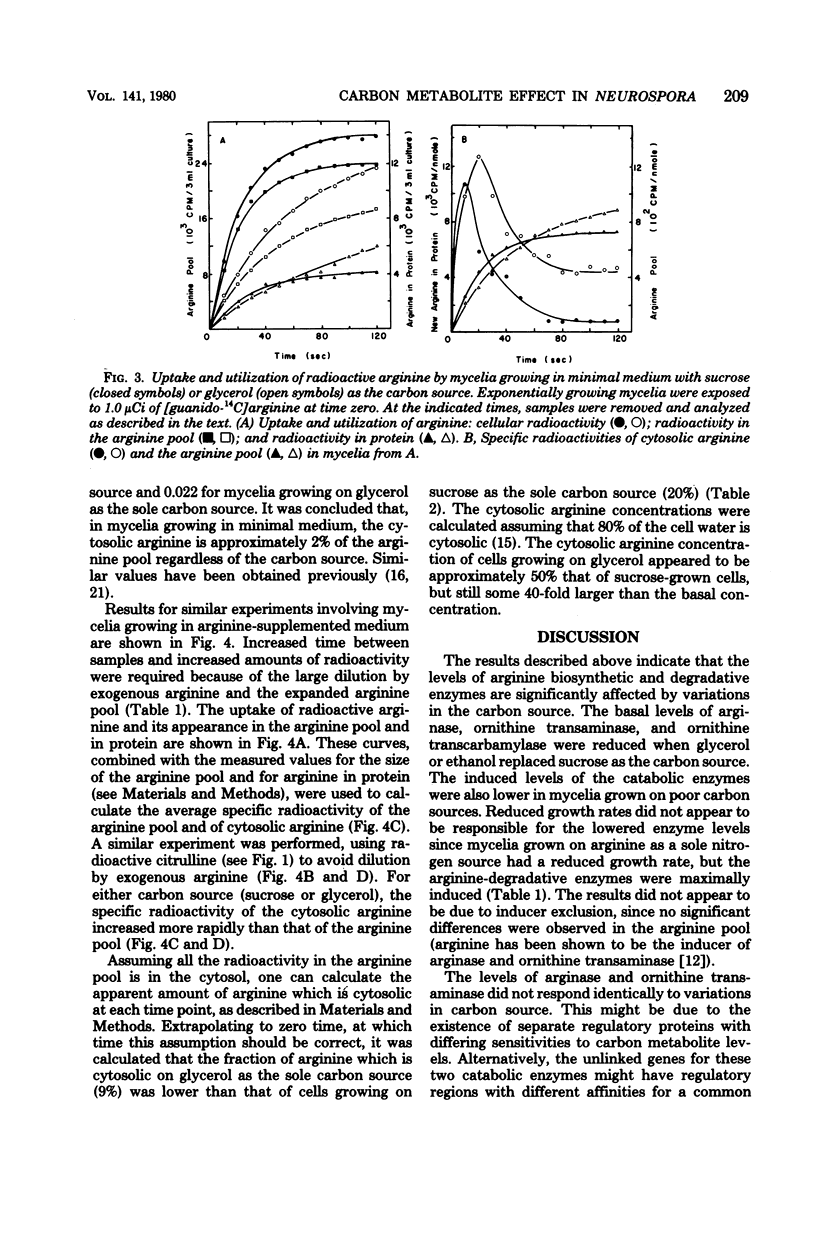
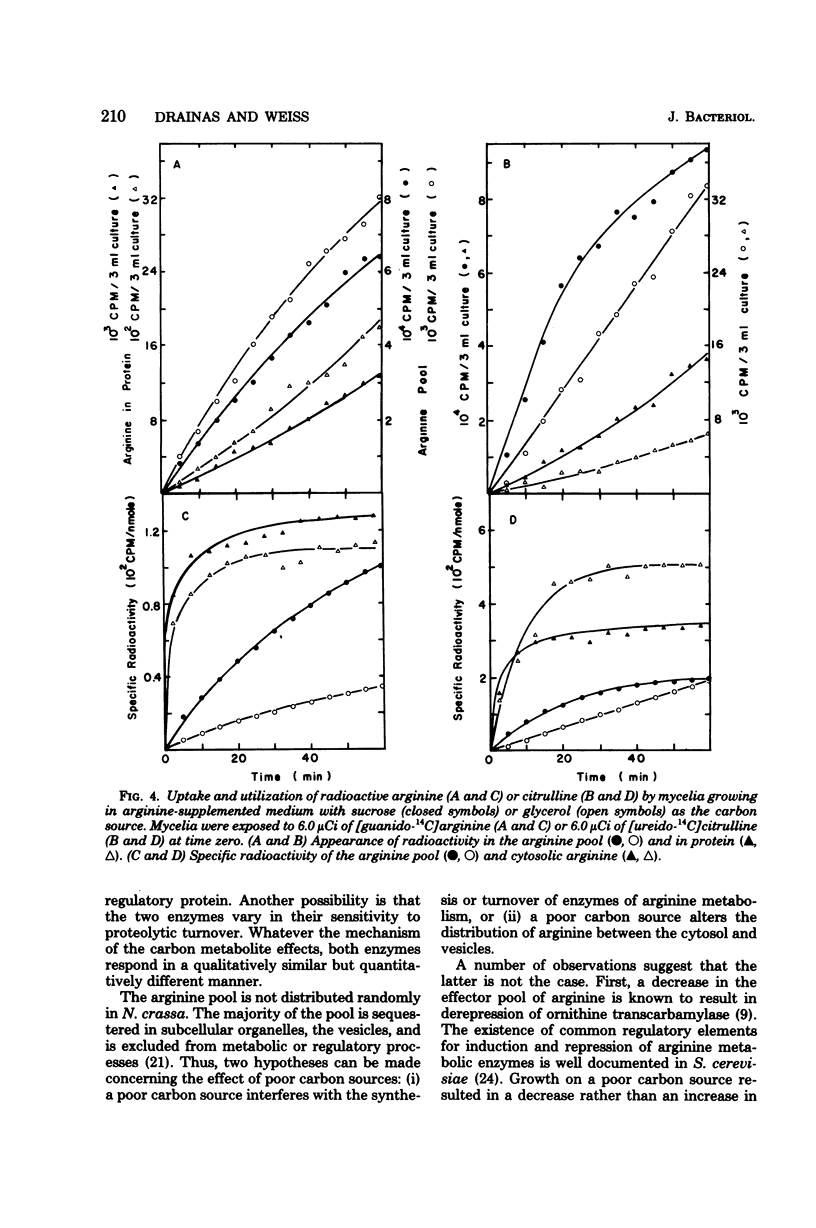
The levels of enzymes and metabolites of arginine metabolism were determined in exponential cultures of Neurospora crassa grown on various carbon sources. The carbon sources decreased in effectiveness (as determined by generation times) in the following order: sucrose, acetate, glycerol, and ethanol. The basal and induced levels of the catabolic enzymes, arginase (EC 3.5.3.1) and ornithine transaminase (EC 2.6.1.13), were lower in mycelia grown on poor carbon sources. Arginase was more sensitive to variations in carbon source than was ornithine transaminase. Induction of both enzymes was sensitive to nitrogen metabolite control, but this sensitivity was reduced in mycelia grown on glycerol or ethanol. The pools of arginine and ornithine were reduced in mycelia grown in unsupplemented medium containing poor carbon sources, but the biosynthetic enzyme ornithine transcarbamylase (EC 2.1.3.3) was not derepressed. The arginine pools were similar, regardless of carbon source, in mycelia grown in arginine-supplemented medium. The ornithine pool was reduced by growth on poor carbon sources. The rate of arginine degradation was proportional to the level of arginase in both sucrose- and glycerol-grown mycelia. The distribution of arginine between cytosol and vesicles was only slightly altered by growth on glycerol instead of sucrose. The slightly smaller cytosolic arginine concentration did not appear to be sufficient to account for the alterations in basal and induced enzyme levels. The results suggest a possible carbon metabolite effect on the expression or turnover of a variety of genes for enzymes of arginine metabolism in Neurospora.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartnik E., Weglenski P., Piotrowska M. Ammonium and glucose repression of the arginine catabolic enzymes in Aspergillus nidulans. Mol Gen Genet. 1973 Oct 16;126(1):75–84. doi: 10.1007/BF00333484. [DOI] [PubMed] [Google Scholar]
- Basabe J. R., Lee C. A., Weiss R. L. Enzyme assays using permeabilized cells of Neurospora. Anal Biochem. 1979 Jan 15;92(2):356–360. doi: 10.1016/0003-2697(79)90670-5. [DOI] [PubMed] [Google Scholar]
- CHINARD F. P. Photometric estimation of proline and ornithine. J Biol Chem. 1952 Nov;199(1):91–95. [PubMed] [Google Scholar]
- CROKAERT R., SCHRAM E. Dosage des N-carbamoyldérivés d'acides aminés par la diacétylmonoxime. Bull Soc Chim Biol (Paris) 1958;40(7-8):1093–1106. [PubMed] [Google Scholar]
- Carsiotis M., Jones R. F. Cross-pathway regulation: tryptophan-mediated control of histidine and arginine biosynthetic enzymes in Neurospora crassa. J Bacteriol. 1974 Sep;119(3):889–892. doi: 10.1128/jb.119.3.889-892.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsiotis M., Jones R. F., Wesseling A. C. Cross-pathway regulation: histidine-mediated control of histidine, tryptophan, and arginine biosynthetic enzymes in Neurospora crassa. J Bacteriol. 1974 Sep;119(3):893–898. doi: 10.1128/jb.119.3.893-898.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybis J. J., Davis R. H. Acetylglutamate kinase: a feedback-sensitive enzyme of arginine biosynthesis in Neurospora. Biochem Biophys Res Commun. 1974 Sep 23;60(2):629–634. doi: 10.1016/0006-291x(74)90287-3. [DOI] [PubMed] [Google Scholar]
- Davis R. H. Carbamyl phosphate synthesis in Neurospora crassa. II. Genetics, metabolic position, and regulation of arginine-specific carbamyl phosphokinase. Biochim Biophys Acta. 1965 Aug 24;107(1):54–68. doi: 10.1016/0304-4165(65)90388-0. [DOI] [PubMed] [Google Scholar]
- Davis R. H. Compartmentation and regulation of fungal metabolism: genetic approaches. Annu Rev Genet. 1975;9:39–65. doi: 10.1146/annurev.ge.09.120175.000351. [DOI] [PubMed] [Google Scholar]
- Davis R. H., Lawless M. B., Port L. A. Arginaseless Neurospora: genetics, physiology, and polyamine synthesis. J Bacteriol. 1970 May;102(2):299–305. doi: 10.1128/jb.102.2.299-305.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H., Mora J. Mutants of Neurospora crassa deficient in ornithine-delta-transmainase. J Bacteriol. 1968 Aug;96(2):383–388. doi: 10.1128/jb.96.2.383-388.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HESS J., KITO E., MARTIN R. P., VAN PILSUM J. F. Determination of creatine, creatinine, arginine, guanidinoacetic acid, guanidine, and methylguanidine in biological fluids. J Biol Chem. 1956 Sep;222(1):225–235. [PubMed] [Google Scholar]
- KORITZ S. B., COHEN P. P. Colorimetric determination of carbamylamino acids and related compounds. J Biol Chem. 1954 Jul;209(1):145–150. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- SLAYMAN C. W., TATUM E. L. POTASSIUM TRANSPORT IN NEUROSPORA. I. INTRACELLULAR SODIUM AND POTASSIUM CONCENTRATIONS, AND CATION REQUIREMENTS FOR GROWTH. Biochim Biophys Acta. 1964 Nov 29;88:578–592. [PubMed] [Google Scholar]
- Subramanian K. N., Weiss R. L., Davis R. H. Use of external, biosynthetic, and organellar arginine by Neurospora. J Bacteriol. 1973 Jul;115(1):284–290. doi: 10.1128/jb.115.1.284-290.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaca G., Mora J. Nitrogen regulation of arginase in Neurospora crassa. J Bacteriol. 1977 Sep;131(3):719–725. doi: 10.1128/jb.131.3.719-725.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L., Anterasian G. P. Control of arginine metabolism in Neurospora. Induction of ornithine aminotransferase. J Biol Chem. 1977 Oct 25;252(20):6974–6980. [PubMed] [Google Scholar]
- Weiss R. L. Compartmentation and control of arginine metabolism in Neurospora. J Bacteriol. 1976 Jun;126(3):1173–1179. doi: 10.1128/jb.126.3.1173-1179.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L., Davis R. H. Control of arginine utilization in Neurospora. J Bacteriol. 1977 Feb;129(2):866–873. doi: 10.1128/jb.129.2.866-873.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L., Kukora J. R., Adams J. The relationship between enzyme activity, cell geometry, and fitness in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1975 Mar;72(3):794–798. doi: 10.1073/pnas.72.3.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfner M., Yep D., Messenguy F., Fink G. R. Integration of amino acid biosynthesis into the cell cycle of Saccharomyces cerevisiae. J Mol Biol. 1975 Aug 5;96(2):273–290. doi: 10.1016/0022-2836(75)90348-4. [DOI] [PubMed] [Google Scholar]


