Abstract
We recently showed that vaccination with a complex of cholesterol-bearing hydrophobized pullulan and NY-ESO-1 protein (CHP-NY-ESO-1) elicited antibody responses in 9 of 9 patients vaccinated in a clinical trial. In this study, we performed T cell immunomonitoring and analyzed tumor responses in these patients. To evaluate CD4 and CD8 T cell responses, an IFN-γ secretion assay was used. The assay showed low background and was sensitive for detecting antigen-specific T cells. An increase in the CD4 T cell response was observed in 2 of 2 initially sero-positive and 5 of 7 initially sero-negative patients after vaccination. An increase in the CD8 T cell response was also observed in 2 of 2 sero-positive and 5 of 7 sero-negative patients after vaccination. Analysis of peptides recognized by CD4 and CD8 T cells revealed two dominant NY-ESO-1 regions, 73-114 and 121-144. Tumor responses were observed in 3 esophageal cancer patients and a malignant melanoma patient. In 3 of 4 prostate cancer patients, prostate-specific antigen (PSA) values stabilized during the course of vaccination. The use of whole protein, containing multiple CD4 and CD8 epitopes, may be beneficial for cancer vaccines to prevent tumors from evading the immune response.
Keywords: human, cancer vaccine, CHP-NY-ESO-1, immunohistochemistry, cellular immunity
Introduction
NY-ESO-1 antigen was originally identified in esophageal cancer by serological expression cloning (SEREX) using autologous patient serum (1, 2). In normal adult tissues, NY-ESO-1 mRNA and protein expression is restricted to germ cells in the testis (1, 3). In cancer, NY-ESO-1 expression is detected in a wide range of human malignancies (3, 4). For this reason, NY-ESO-1 has emerged as a prototype of a class of antigens called cancer/testis or CT antigens (5). Among cancer/testis antigens, NY-ESO-1 has received particular attention because of its strong immunogenicity (6, 7). A spontaneous NY-ESO-1-specific antibody response is frequently observed in patients with various types of advanced stage NY-ESO-1-expressing tumors (6-12). In contrast, antibody responses are rare in patients with tumors expressing other cancer/testis antigens, e.g., MAGE or GAGE. Both CD4 and CD8 T cell responses are easily detected in patients with a serological response to NY-ESO-1 (13, 14). As a consequence of the strong natural immunogenicity of NY-ESO-1, a coordinated effort to develop therapeutic NY-ESO-1 vaccines has been organized and clinical trials with NY-ESO-1 peptide (15-18), protein (19), and viral vaccine constructs (20) have been carried out and analyzed for immunogenicity and tumor responses.
Cholesterol-bearing hydrophobized pullulan (CHP) is a newly developed antigen delivery vehicle that can be used to formulate nanoparticles, including protein antigens (21, 22). Both CD4 and CD8 T cells are efficiently activated by DCs pulsed with a complex of CHP and NY-ESO-1 protein (CHP-NY-ESO-1) in vitro (23). In a phase I clinical trial, we immunized 9 cancer patients with CHP-NY-ESO-1 and showed that the vaccine had potent capacity to induce NY-ESO-1 antibody (24). The NY-ESO-1 epitopes recognized by antibodies from vaccinated patients were similar to those recognized by antibodies in non-vaccinated cancer patients with spontaneous immunity.
In the present study, we monitored the CD4 and CD8 T cell responses in patients receiving the CHP-NY-ESO-1 vaccine and recorded tumor responses.
Results
Patient characteristics
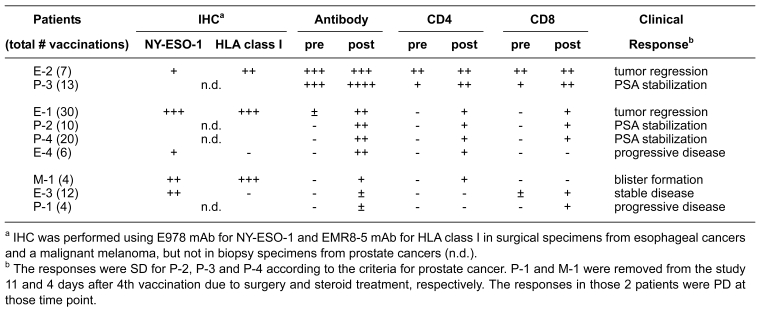
Table 1 shows the list of 9 patients who were evaluable for immune and tumor responses. There were 4 stage IV esophageal cancer patients (E-1, E-2, E-3 and E-4), 4 stage D3 prostate cancer patients (P-1, P-2, P-3 and P-4), and 1 stage IV malignant melanoma patient (M-1). Two patients (E-2 and P-3) were NY-ESO-1 antibody sero-positive before vaccination and the other 7 were sero-negative at baseline.
Table 1.
Patient characteristics.
Expression of NY-ESO-1 and HLA class I in tumors determined by immunohistochemistry (IHC)
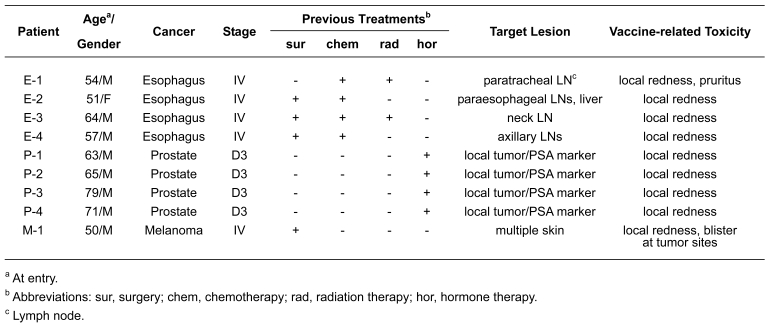
Expression of NY-ESO-1 mRNA was observed in tumor specimens from all 9 patients. Expression of NY-ESO-1 and HLA class I in tumors was analyzed by IHC. As shown in Figure 1, NY-ESO-1 expression was variable, ranging from homogeneous staining to expression in only a subset of tumor cells. No HLA class I expression was observed in tumor specimens from patients E-3 and E-4. Biopsy specimens from prostate cancer patients were not available for IHC.
Figure 1.
Immunohistochemical analysis of tumor specimens. Expression of NY-ESO-1 and HLA class I was analyzed in four esophageal cancers (E-1, E-2, E-3 and E-4) and a malignant melanoma (M-1) using E978 and EMR-5 mAbs, respectively. Magnification was 200x, except for NY-ESO-1 expression in E-3 which was 80x.
Toxicity
Toxicity was assessed using CTC v3.0 criteria (25). All patients showed G1 grade local redness (4-7 cm diameter) at the vaccination site (Table 1). No induration and no increase in the intensity of the reaction were observed with sequential injections. G1 pruritus was observed in patient E-1. These reactions subsided within 3 days after vaccination without any medication. Blister formation was observed at tumor sites infiltrating the skin in patient M-1. No other adverse event related to the drug was observed in any patient.
CD4 and CD8 T cell responses in patients after CHP-NY-ESO-1 vaccination
CD4 and CD8 T cell responses in patients were evaluated by the IFN-γ secretion assay. The purity of CD4 T cells was generally >98%, while that of CD8 T cells was 80-90%. CD4 and CD8 T cell-enriched populations were cultured with irradiated autologous CD4- and CD8-depleted stimulatory cells in the presence of a mixture of 28 overlapping 18-mer peptides and a 30-mer C-terminal peptide spanning the entire NY-ESO-1 protein for 12 days. The cells from the stimulation culture were then assayed for IFN-γ secretion in response to paraformaldehyde (PFA)-treated CD4- and CD8-depleted PBMCs pre-pulsed with the peptides for 4 hr.
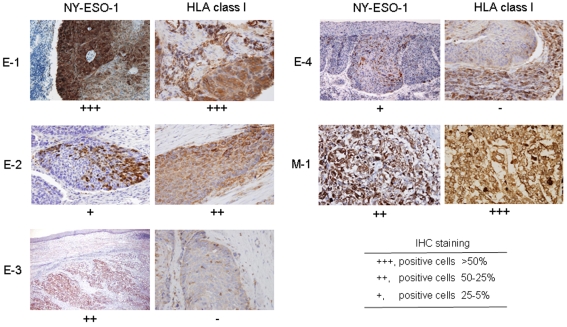
We first examined the specificity of the assay for the detection of IFN-γ-secreting T cells responding to peptides. As shown in Figure 2, even when a significant number of IFN-γ-secreting CD4 T cells were observed in cultures stimulated with the NY-ESO-1 peptides, no IFN-γ positive cells were observed in the CD4-negative fraction containing stimulatory and target cells. T cells and non-T cells in the PBMC fraction were shown to bind a bi-specific antibody that detects IFN-γ to a similar extent (data not shown). The finding indicated that detection of passively bound IFN-γ on the cells was unlikely and the low background ensured the detection of IFN-γ-secreting T cells responding to the peptides.
Figure 2.
IFN-γ secretion assay. The CD4-enriched population (2 x 106) obtained from PBMCs of a CHP-NY-ESO-1-vaccinated patient (P-2) (day 155) using MACS CD4 beads was stimulated twice with irradiated autologous CD4- and CD8-depleted cells (2 x 106) in the presence of a mixture of 28 18-mer overlapping peptides and a 30-mer C-terminal peptide spanning the entire NY-ESO-1 protein (1 µg/ml for each peptide) for 12 days. The cells (2 x 105) from the stimulation culture were assayed for IFN-γ secretion in response to PFA-treated CD4- and CD8-depleted PBMCs (2 x 105) pre-pulsed or not pulsed with the peptide mixture for 4 hr using FACS.
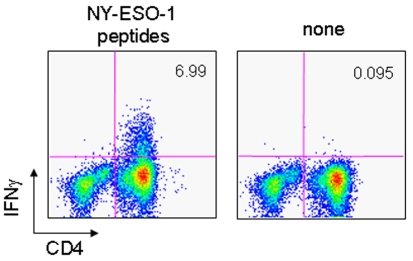
CD4 and CD8 T cell responses were analyzed in NY-ESO-1 antibody sero-positive patients. As shown in Figure 3, a significant increase in the percentage of IFN-γ-secreting CD4 and CD8 T cells was observed in patient P-3 after vaccination. In vitro stimulating twice greatly augmented the number of IFN-γ-secreting CD4 and CD8 T cells, confirming the response. On the other hand, a high number of IFN-γ secreting CD4 and CD8 T cells was observed in patient E-2 at baseline before vaccination; these remained at a similar level after vaccination.
Figure 3.
Antibody, CD4 and CD8 T cell responses in vaccinated patients. NY-ESO-1 antibody response was determined using recombinant NY-ESO-1 protein by ELISA. CD4 and CD8 T cell responses were determined by IFN-γ secretion assay. CD4- and CD8-enriched populations (2 x 106) obtained from PBMCs of patients using MACS beads were stimulated once (1˚ IVS) or twice (2˚ IVS) with irradiated autologous CD4- and CD8-depleted cells (2 x 106) in the presence of a mixture of 28 18-mer overlapping peptides and a 30-mer C-terminal peptide spanning the entire NY-ESO-1 protein (1 µg/ml for each peptide) for 12 days. The cells (2 x 105) from each stimulation culture were assayed for IFN-γ secretion in response to PFA-treated CD4- and CD8-depleted PBMCs (2 x 105) pre-pulsed or not pulsed with the peptide mixture for 4 hr using FACS. Net % of IFN-γ secreting CD4 and CD8 T cells of the total CD4 and CD8 T cells, respectively, in cultures were plotted for (A) prolonged (10-18 vaccinations) and (B) shorter (3-5 vaccinations) time periods after repeated vaccinations. Values >0.1% were considered to be significant.
CD4 and CD8 T cell responses were similarly investigated in patients who were sero-negative at baseline for NY-ESO-1 antibody. As shown in Figure 3, a CD4 T cell response was detected in 5 of 7 sero-negative patients after vaccination in cultures stimulated once and confirmed in cultures stimulated twice. The range of net % CD4 T cells was less than 1.0%, except for patient E-4 in cultures stimulated once. Two patients, E-3 and P-1, with no CD4 T cell response were evaluated up to day 169 (7 vaccinations) and day 43 (3 vaccinations), respectively. The absence of a CD4 T cell response in these patients was consistent with the low antibody response.
A CD8 T cell response was detected in 5 of 7 patients who were sero-negative at baseline for NY-ESO-1 antibody after vaccination. CD8 T cell responses were detected in patient E-1, E-3, P-2, P-4 and P-1 in cultures stimulated once. The response was confirmed in the culture stimulated twice. No CD8 T cell response was observed in patients E-4 and M-1.
Determination of NY-ESO-1 peptide regions recognized by CD4 and CD8 T cells in CHP-NY-ESO-1-vaccinated patients
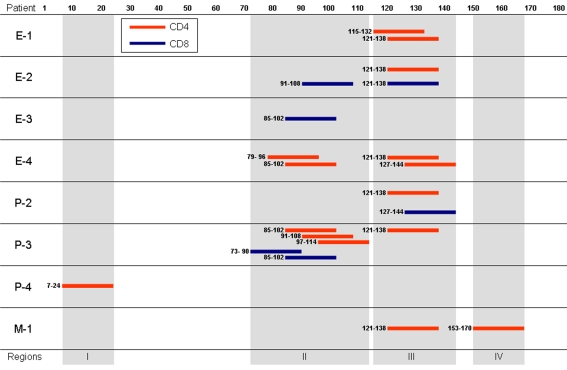
The NY-ESO-1 peptide regions recognized by CD4 and CD8 T cells were identified using an IFN-γ secretion assay and individual overlapping peptides spanning the entire NY-ESO-1 protein for stimulation. The peptides recognized by CD4 and CD8 T cells mapped to four distinct regions in the NY-ESO-1 protein (Figure 4). Regions II and III were frequently recognized by either CD4 or CD8 T cells in the patients. Regions I and IV were recognized by only CD4 T cells in patient P-4 and patient M-1, respectively. Recognition of peptide 7-24 (region I) by CD4 T cells has not been described previously.
Figure 4.
NY-ESO-1 regions recognized by CD4 and CD8 T cells. CD4- and CD8-enriched populations (2 x 106) from PBMCs of patients were stimulated two or three times with irradiated autologous CD4- and CD8-depleted PBMCs (2 x 106) in the presence of a mixture of 28 18-mer overlapping peptides and a 30-mer C-terminal peptide spanning the entire NY-ESO-1 protein. The cells (1x105) from the culture were then assayed for IFN-γ secretion in response to PFA-treated autologous CD4- and CD8-depleted PBMCs (1 x 105) pre-pulsed with individual peptides for 4 hr using FACS.
Clinical responses
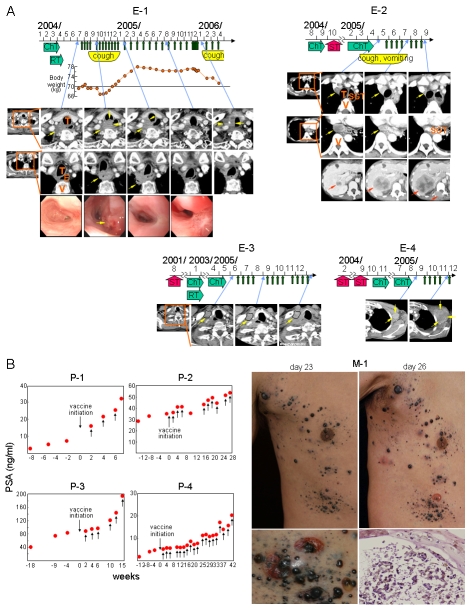
As shown in Figure 5A, vaccination was started in esophageal cancer patient E-1 two months after radiation and chemotherapy. Disease symptoms (cough, chest pain, weight loss, etc.) developed during the initial 4 months of vaccination. These then gradually improved and disappeared by 6 months (12 vaccinations). Computed tomography (CT) images showed that an enlargement of the neck and para-tracheal lymph node metastases observed at 6 months disappeared at 13 months (20 vaccinations). Endoscopy showed that a hemorrhagic tumor protrusion in the esophagus observed at 3 months (6 vaccinations) became a scar at 6 months (12 vaccinations). However, a recurrent tumor was observed in the neck lymph nodes surrounding the carotid artery at 21 months and the patient died of massive hemorrhage at 23 months. The previously involved para-esophageal lymph nodes and esophagus remained free of tumor with no recurrence of tumor at the site of the scar. In esophageal cancer patient E-2, multiple tumor metastases were found in thoracic lymph nodes and the liver 4 months after surgical treatment. The patient received chemotherapy, but showed no response. After initiating treatment with the vaccine, the thoracic tumors rapidly diminished in size and most of them disappeared by 3.5 months (7 vaccinations). Cough and vomiting also disappeared. However, the metastatic tumor in the liver grew rapidly and a new lesion was found in a vertebra. The patient was then removed from the study. Esophageal cancer patient E-3 showed recurrence after esophagectomy, radiation and chemotherapy. After initiating the vaccine, the tumor in the neck remained unexpectedly stable over a 7 month period (12 vaccinations) without additional therapy. In esophageal cancer patient E-4, no response was observed.
Figure 5.
Clinical responses in patients. (A) Esophageal cancer patient E-1: large neck (top) and para-tracheal (bottom) lymph node metastases (yellow arrow) observed in CT images at 6 months after initiating treatment with the vaccine and a hemorrhagic protrusion in the esophagus (yellow arrow) observed by endoscopy at 3 months disappeared at 13 and 6 months, respectively. Only a scar was observed in the esophagus. Tumor recurrence was noted at 21 months and the patient died of disease progression at 23 months. Abbreviations: T, trachea; E, esophagus; V, vertebra. Esophageal cancer patient E-2: para-tracheal (top) and para-esophageal (middle) lymph node metastases (yellow arrow) observed at baseline in CT images disappeared by 3.5 months after initiating vaccine treatment. However, a metastatic tumor in the liver (bottom) (red arrow) grew rapidly and a new lesion was observed in a vertebra (bottom) (red arrow). Abbreviation: SGT, substituted gastric tube emplaced by surgical treatment. Esophageal cancer patient E-3: recurrence was observed after surgical treatment, radiation and chemotherapy. However, the tumor remained unusually stable for 7 months without any other treatment during vaccination. Esophageal cancer patient E-4: liver and multiple thoracic lymph node metastases were noted 4 months after esophagectomy. He was treated by chemotherapy, but showed no response. The patient was then treated with the vaccine, but no clinical response was observed. Abbreviations: ChT, chemotherapy; RT, radiation therapy; ST, surgical treatment. (B) PSA values were plotted over time for patients P-1, P-2, P-3 and P-4 who had recurrent hormone-refractory prostate cancer. Recurrence was defined as a continuous increase of PSA values at 3 consecutive time points at least 2 weeks apart. The PSA values of patients P-3 and P-4 showed no significant increase over a period of time during vaccination. The PSA values of patient P-2 also showed limited increase during vaccination. In malignant melanoma patient M-1, tumor infiltrates in the skin became surrounded by reddish areas associated with blistering at 10 days after the 2nd vaccination. Pictures at top left and right show the skin before and after blisters appeared, respectively. The blisters ruptured (bottom left) and recovered tumor cells were apoptotic as shown by HE staining (200x magnification) and annexin V staining (not shown). The patient died of rapid growth of metastatic tumors in the lung.
As shown in Figure 5B, four prostate cancer patients with recurrent hormone-refractory tumors were vaccinated. Recurrence was defined by the continuous increase in PSA values at 3 consecutive time points at least 2 weeks apart. At the time of entry to this study, all 4 patients showed no subjective clinical symptoms. No metastatic lesion was found by bone scintiscan, magnetic resonance imaging (MRI)/CT and ultrasonography in patients P-1, P-2 and P-4. Bone metastasis was observed in patient P-3. No target lesion was evaluable in these patients. Patient P-1 complained of difficulty in urinating after the third vaccination. MRI images showed prostate enlargement. PSA values kept increasing and the patient died of cancer 16 months after removal from the study due to transurethral resection of prostate (TUR-P). Patients P-2, P-3 and P-4 showed no subjective symptoms during vaccination. No enlargement of the prostate was observed by MRI/CT scan in those patients. PSA levels appeared to temporarily stabilize in patients P-2, P-3 and P-4 during vaccination. However, PSA levels increased eventually. Patients P-2 and P-4 are alive after 26 and 18 months, respectively. Patient P-3 died of cancer at 11 months.
A malignant melanoma patient (M-1) showed a vigorous inflammatory response at tumor sites around 10 days after the second vaccination (Figure 5B). The tumors were surrounded by reddish areas and multiple blisters were seen at tumor sites. The patient complained of tenderness. This response lasted until the patient died. Tumor cells under blisters were apoptotic, as shown by immunohistochemistry with annexin V staining (data not shown). However, metastatic tumors in the lung grew rapidly and the patient died at day 48 after initiating treatment with the vaccine.
Discussion
The immune response to NY-ESO-1 has been extensively analyzed in cancer patients with spontaneous immunity to NY-ESO-1 (2, 6-14, 26, 27), and in patients receiving NY-ESO-1 peptide (15), protein (19), or viral vector vaccines (20). The characteristics of this immune response can be summarized in the following way: (i) The spontaneous NY-ESO-1 response is generally an integrated one - antibody, CD4 and CD8 T cells (13, 14) - with these T cells capable of recognizing naturally processed NY-ESO-1 (26, 27). (ii) Immunization with NY-ESO-1 class I and II peptides elicits CD8 and CD4 T cells, respectively, but these T cells are usually of low affinity and do not recognize naturally processed NY-ESO-1 (28). (iii) Integrated NY-ESO-1 immune responses can be generated by vaccines containing NY-ESO-1 protein (19) or viral vectors (vaccinia/fowlpox) (20). As with spontaneous NY-ESO-1 immune responses, the CD4 and CD8 T cells from patients immunized with protein or viral vector recognize a broad range of class I and II NY-ESO-1 epitopes (19, 20). (iv) Ex vivo CD4 and CD8 T cell responses to NY-ESO-1 are only rarely seen in patients with spontaneous or vaccine-induced NY-ESO-1 immunity; in vitro pre-sensitization is required to detect NY-ESO-1-specific CD4 and CD8 T cells (13, 19, 20, 29). (v) The CD4 T cell response to NY-ESO-1 appears to be under Treg suppression. Removal of CD4+/CD25+ T cells exposes a population of NY-ESO-1-specific CD4 T cells reactive to class II epitopes in normal individuals and in cancer patients with NY-ESO-1 positive or negative tumors (30).
The CHP-NY-ESO-1 vaccine used in the current study elicits humoral, CD4 and CD8 T cell responses in immunized patients. Although the number of patients was small, the response compared favorably with the frequency and characteristics of the immune response to NY-ESO-1 protein induced by the NY-ESO-1/ISCOMATRIX (19) and NY-ESO-1 vaccinia/fowlpox vaccines (20). As with these other vaccines, pre-sensitization was necessary to reveal the CD4 and CD8 T cell responses to CHP-NY-ESO-1 and these T cell responses were directed against a range of peptides, similar to those elicited by other NY-ESO-1 protein or viral vector vaccines. In past studies, intracellular cytokine staining (19) and ELISPOT (20) were the primary assays for measuring functional CD4 and CD8 T cell responses. In our study, we chose the IFN-γ secretion assay, because it showed low background and was sensitive for detecting antigen-specific T cells present at low frequency. Comparative analysis of these three assays using similar populations of T cells from CHP-NY-ESO-1 immunized patients and patients with spontaneous immunity is ongoing.
Of 9 patients enrolled in this clinical trial, we observed obvious tumor responses following vaccination in 4 patients, including 3 esophageal cancer patients (E-1, E-2, E-3) and a malignant melanoma patient (M-1) (Table 2). Patient E-1 showed the most remarkable response to vaccination with almost a complete regression of advanced disease occurring over a period of 21 months. The patient received a course of 30 vaccines in total and developed a strong NY-ESO-1 antibody response, an early CD4 T cell response and a much delayed CD8 T cell response. The tumor in this patient showed strong NY-ESO-1 and MHC expression. Despite evident NY-ESO-1 immunity, the tumor recurred in the neck lymph nodes, but the para-esophageal lymph nodes and esophagus remained free of tumor until death. Patient E-2 had a tumor with heterogeneous expression of NY-ESO-1 and strong MHC expression. The patient showed a classic pattern of spontaneous NY-ESO-1 immunity, with a strongly active CD4 and CD8 T cell response. There was no evident increase in immunological parameters during vaccination, despite the fact that local metastatic tumor regression was associated with administration of the vaccine. However, concomitant progression of a metastatic tumor in the liver was observed and may reflect the development of antigen/MHC loss variance. Patient E-3 had a large tumor mass in the neck that recurred after a total esophagectomy followed by radiation and chemotherapy. The patient remained stable for 7 months, an unusual outcome. No detectable antibody or CD4 response was seen in this patient, but a CD8 T cell response was generated after vaccination. Patient E-4 showed no tumor response. The patient developed an antibody and CD4 T cell response, but no CD8 T cell response. The tumor showed heterogeneous NY-ESO-1 expression, but no MHC expression. Patient M-1 developed multiple reddish areas and multiple blisters 1-2 cm in diameter in tumor sites at day 26 after initiating the vaccine lasting until the patient died at day 48 from rapid growth of metastatic tumors in the lung.
Table 2.
Study summary: Immune responses and tumor responses following vaccination with CHP-NY-ESO-1.
One of the great challenges in the field of cancer vaccines is to relate immunological responses elicited by the vaccination to tumor response. Although the underlying presumption in the field is that immune responses and tumor responses should be associated, it is unclear what element or elements of the immune response constitute an anti-tumor immune response in humans. Up to this point, our sole window on the NY-ESO-1 immune response has been peripheral blood, and future studies need to focus on CD4, CD8, Treg, DC, cytokine/chemokine, etc. responses observed at the tumor site. In addition, more attention needs to be focused on the relation of vaccine-induced tumor responses to tumor antigen and class I/II MHC expression and other features of the cancer cell, i.e. immunosuppressant factors secreted by tumor cells. Because of the strong natural immunogenicity of NY-ESO-1 and the coordinated program that has been developed to construct and test maximally immunogenic NY-ESO-1 vaccines, this complex relation between immune response and tumor response can be dissected in the necessary detail.
Abbreviations
- CHP-NY-ESO-1
cholesterol-bearing hydrophobized pullulan (CHP) and NY-ESO-1 protein complex
- PSA
prostate-specific antigen
Acknowledgements
We thank Drs. Kazuhisa Yao, Kojiro Futagami and Yutaka Gomita (Pharmacy and the Center for Clinical Research of New Drugs and Therapeutics, Okayama University Hospital), and Dr. Youichi Ueda (Department of Pharmacy, Osaka University Hospital). We also thank Ms. T. Akimoto for excellent technical assistance and Ms. J. Mizuuchi for preparation of the manuscript.
This work was supported by the Cancer Vaccine Collaborative of the Cancer Research Institute and the Ludwig Institute for Cancer Research, and by a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Chen YT, Scanlan MJ, Sahin U, Türeci O, Güre AO, Tsang S, Williamson B, Stockert E, Pfreundschuh M, Old LJ. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci USA. 1997;94:1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gnjatic S, Nishikawa H, Jungbluth AA, Güre AO, Ritter G, Jäger E, Knuth A, Chen YT, Old LJ. NY-ESO-1: Review of an immunogenic tumor antigen. Adv Cancer Res. 2006;95:1–30. doi: 10.1016/S0065-230X(06)95001-5. [DOI] [PubMed] [Google Scholar]
- 3.Jungbluth AA, Chen YT, Stockert E, Busam KJ, Kolb D, Iversen K, Coplan K, Williamson B, Altorki N, Old LJ. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int J Cancer. 2001;92:856–860. doi: 10.1002/ijc.1282. [DOI] [PubMed] [Google Scholar]
- 4.Scanlan MJ, Güre AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev. 2002;188:22–32. doi: 10.1034/j.1600-065x.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- 5.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 6.Stockert E, Jäger E, Chen YT, Scanlan MJ, Gout I, Karbach J, Arand M, Knuth A, Old LJ. A survey of the humoral immune response of cancer patients to a panel of human tumor antigens. J Exp Med. 1998;187:1349–1354. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jäger E, Stockert E, Zidianakis Z, Chen YT, Karbach J, Jäger D, Arand M, Ritter G, Old LJ, Knuth A. Humoral immune responses of cancer patients against "Cancer-Testis" antigen NY-ESO-1: Correlation with clinical events. Int J Cancer. 1999;84:506–510. doi: 10.1002/(sici)1097-0215(19991022)84:5<506::aid-ijc10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Fossa A, Berner A, Fossa SD, Hernes E, Gaudernack G, Smeland EB. NY-ESO-1 protein expression and humoral immune responses in prostate cancer. Prostate. 2004;59:440–447. doi: 10.1002/pros.20025. [DOI] [PubMed] [Google Scholar]
- 9.Fujita S, Wada H, Jungbluth AA, Sato S, Nakata T, Noguchi Y, Doki Y, Yasui M, Sugita Y, Yasuda T, Yano M, Ono T, Chen YT, Higashiyama M, Gnjatic S, Old LJ, Nakayama E, Monden M. NY-ESO-1 expression and immunogenicity in esophageal cancer. Clin Cancer Res. 2004;10:6551–6558. doi: 10.1158/1078-0432.CCR-04-0819. [DOI] [PubMed] [Google Scholar]
- 10.Sahin U, Türeci O, Chen YT, Seitz G, Villena-Heinsen C, Old LJ, Pfreundschuh M. Expression of multiple cancer/testis (CT) antigens in breast cancer and melanoma: basis for polyvalent CT vaccine strategies. Int J Cancer. 1998;78:387–389. doi: 10.1002/(SICI)1097-0215(19981029)78:3<387::AID-IJC22>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Sugita Y, Wada H, Fujita S, Nakata T, Sato S, Noguchi Y, Jungbluth AA, Yamaguchi M, Chen YT, Stockert E, Gnjatic S, Williamson B, Scanlan MJ, Ono T, Sakita I, Yasui M, Miyoshi Y, Tamaki Y, Matsuura N, Noguchi S, Old LJ, Nakayama E, Monden M. NY-ESO-1 expression and immunogenicity in malignant and benign breast tumors. Cancer Res. 2004;64:2199–2204. doi: 10.1158/0008-5472.can-03-3070. [DOI] [PubMed] [Google Scholar]
- 12.Odunsi K, Jungbluth AA, Stockert E, Qian F, Gnjatic S, Tammela J, Intengan M, Beck A, Keitz B, Santiago D, Williamson B, Scanlan MJ, Ritter G, Chen YT, Driscoll D, Sood A, Lele S, Old LJ. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer Res. 2003;63:6076–6083. [PubMed] [Google Scholar]
- 13.Gnjatic S, Atanackovic D, Jäger E, Matsuo M, Selvakumar A, Altorki NK, Maki RG, Dupont B, Ritter G, Chen YT, Knuth A, Old LJ. Survey of naturally occurring CD4+ T cell responses against NY-ESO-1 in cancer patients: Correlation with antibody responses. Proc Natl Acad Sci USA. 2003;100:8862–8867. doi: 10.1073/pnas.1133324100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jäger E, Nagata Y, Gnjatic S, Wada H, Stockert E, Karbach J, Dunbar PR, Lee SY, Jungbluth A, Jäger D, Arand M, Ritter G, Cerundolo V, Dupont B, Chen YT, Old LJ, Knuth A. Monitoring CD8 T cell responses to NY-ESO-1: Correlation of humoral and cellular immune responses. Proc Natl Acad Sci USA. 2000;97:4760–4765. doi: 10.1073/pnas.97.9.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jäger E, Gnjatic S, Nagata Y, Stockert E, Jäger D, Karbach J, Neumann A, Rieckenberg J, Chen YT, Ritter G, Hoffman E, Arand M, Old LJ, Knuth A. Induction of primary NY-ESO-1 immunity: CD8+ T lymphocyte and antibody responses in peptide-vaccinated patients with NY-ESO-1+ cancers. Proc Natl Acad Sci USA. 2000;97:12198–12203. doi: 10.1073/pnas.220413497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gnjatic S, Jäger E, Chen W, Altorki NK, Matsuo M, Lee SY, Chen Q, Nagata Y, Atanackovic D, Chen YT, Ritter G, Cebon J, Knuth A, Old LJ. CD8(+) T cell responses against a dominant cryptic HLA-A2 epitope after NY-ESO-1 peptide immunization of cancer patients. Proc Natl Acad Sci USA. 2002;99:11813–11818. doi: 10.1073/pnas.142417699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valmori D, Dutoit V, Ayyoub M, Rimoldi D, Guillaume P, Lienard D, Lejeune F, Cerottini JC, Romero P, Speiser DE. Simultaneous CD8+ T cell responses to multiple tumor antigen epitopes in a multipeptide melanoma vaccine. Cancer Immun. 2003;3:15. http://www.cancerimmunity.org/v3p15/030915.htm [PubMed] [Google Scholar]
- 18.Shackleton M, Davis ID, Hopkins W, Jackson H, Dimopoulos N, Tai T, Chen Q, Parente P, Jefford M, Masterman KA, Caron D, Chen W, Maraskovsky E, Cebon J. The impact of imiquimod, a Toll-like receptor-7 ligand (TLR7L), on the immunogenicity of melanoma peptide vaccination with adjuvant Flt3 ligand. Cancer Immun. 2004;4:9. http://www.cancerimmunity.org/v4p9/040710.htm [PubMed] [Google Scholar]
- 19.Davis ID, Chen W, Jackson H, Parente P, Shackleton M, Hopkins W, Chen Q, Dimopoulos N, Luke T, Murphy R, Scott AM, Maraskovsky E, McArthur G, MacGregor D, Sturrock S, Tai TY, Green S, Cuthbertson A, Maher D, Miloradovic L, Mitchell SV, Ritter G, Jungbluth AA, Chen YT, Gnjatic S, Hoffman EW, Old LJ, Cebon JS. Recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant induces broad integrated antibody and CD4(+) and CD8(+) T cell responses in humans. Proc Natl Acad Sci USA. 2004;101:10697–10702. doi: 10.1073/pnas.0403572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jäger E, Karbach J, Gnjatic S, Neumann A, Bender A, Valmori D, Ayyoub M, Ritter E, Ritter G, Jäger D, Panicali D, Hoffman E, Pan L, Oettgen H, Old LJ, Knuth A. Recombinant vaccinia/fowlpox NY-ESO-1 vaccines induce both humoral and cellular NY-ESO-1-specific immune responses in cancer patients. Proc Natl Acad Sci USA. 2006;103:14453–14458. doi: 10.1073/pnas.0606512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu XG, Schmitt M, Hiasa A, Nagata Y, Ikeda H, Sasaki Y, Akiyoshi K, Sunamoto J, Nakamura H, Kuribayashi K, Shiku H. A novel hydrophobized polysaccharide/oncoprotein complex vaccine induces in vitro and in vivo cellular and humoral immune responses against HER2-expressing murine sarcomas. Cancer Res. 1998;58:3385–3390. [PubMed] [Google Scholar]
- 22.Kitano S, Kageyama S, Nagata Y, Miyahara Y, Hiasa A, Naota H, Okumura S, Imai H, Shiraishi T, Masuya M, Nishikawa M, Sunamoto J, Akiyoshi K, Kanematsu T, Scott AM, Murphy R, Hoffman EW, Old LJ, Shiku H. Induction of HER2-specific T cell immune responses in patients with HER2-expressing cancer vaccinated with truncated HER2 protein 1-146 complexed with nanogels of cholesteryl pullulan. Clin Cancer Res. 2006;12:7397–7405. doi: 10.1158/1078-0432.CCR-06-1546. [DOI] [PubMed] [Google Scholar]
- 23.Hasegawa K, Noguchi Y, Koizumi F, Uenaka A, Tanaka M, Shimono M, Nakamura H, Shiku H, Gnjatic S, Murphy R, Hiramatsu Y, Old LJ, Nakayama E. In vitro stimulation of CD8 and CD4 T cells by dendritic cells loaded with a complex of cholesterol-bearing hydrophobized pullulan and NY-ESO-1 protein: Identification of a new HLA-DR15-binding CD4 T-cell epitope. Clin Cancer Res. 2006;12:1921–1927. doi: 10.1158/1078-0432.CCR-05-1900. [DOI] [PubMed] [Google Scholar]
- 24.Kawabata R, Wada H, Isobe M, Saika T, Sato S, Uenaka A, Miyata H, Yasuda T, Doki Y, Noguchi Y, Kumon H, Tsuji K, Iwatsuki K, Shiku H, Ritter G, Murphy R, Hoffman E, Old LJ, Monden M, Nakayama E. Antibody response against NY-ESO-1 in CHP-NY-ESO-1 vaccinated patients. Int J Cancer. 2007;120:2178–2184. doi: 10.1002/ijc.22583. [DOI] [PubMed] [Google Scholar]
- 25.Common Terminology Criteria for Adverse Events v3.0 (CTCAE). http://ctep.cancer.gov/reporting/ctc.html
- 26.Jäger E, Chen YT, Drijfhout JW, Karbach J, Ringhoffer M, Jäger D, Arand M, Wada H, Noguchi Y, Stockert E, Old LJ, Knuth A. Simultaneous humoral and cellular immune response against cancer-testis antigen NY-ESO-1: Definition of human histocompatibility leukocyte antigen (HLA)-A2-binding peptide epitopes. J Exp Med. 1998;187:265–270. doi: 10.1084/jem.187.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jäger E, Jäger D, Karbach J, Chen YT, Ritter G, Nagata Y, Gnjatic S, Stockert E, Arand M, Old LJ, Knuth A. Identification of NY-ESO-1 epitopes presented by human histocompatibility antigen (HLA)-DRB4*0101-0103 and recognized by CD4+ T lymphocytes of patients with NY-ESO-1-expressing melanoma. J Exp Med. 2000;191:625–630. doi: 10.1084/jem.191.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Gal FA, Ayyoub M, Dutoit V, Widmer V, Jäger E, Cerottini JC, Dietrich PY, Valmori D. Distinct structural TCR repertoires in naturally occurring versus vaccine-induced CD8+ T-cell responses to the tumor-specific antigen NY-ESO-1. J Immunother. 2005;28:252–257. doi: 10.1097/01.cji.0000161398.34701.26. [DOI] [PubMed] [Google Scholar]
- 29.Gnjatic S, Nagata Y, Jäger E, Stockert E, Shankara S, Roberts BL, Mazzara GP, Lee SY, Dunbar PR, Dupont B, Cerundolo V, Ritter G, Chen YT, Knuth A, Old LJ. Strategy for monitoring T cell responses to NY-ESO-1 in patients with any HLA class I allele. Proc Natl Acad Sci USA. 2000;97:10917–10922. doi: 10.1073/pnas.97.20.10917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishikawa H, Jäger E, Ritter G, Old LJ, Gnjatic S. CD4+ CD25+ regulatory T cells control the induction of antigen-specific CD4+ helper T cell responses in cancer patients. Blood. 2005;106:1008–1011. doi: 10.1182/blood-2005-02-0607. [DOI] [PubMed] [Google Scholar]
- 31.Murphy R, Green S, Ritter G, Cohen L, Ryan D, Woods W, Rubira M, Cebon J, Davis ID, Sjolander A, Kypridis A, Kalnins H, McNamara M, Moloney MB, Ackland J, Cartwright G, Rood J, Dumsday G, Healey K, Maher D, Maraskovsky E, Chen YT, Hoffman EW, Old LJ, Scott AM. Recombinant NY-ESO-1 cancer antigen: Production and purification under cGMP conditions. Prep Biochem Biotechnol. 2005;35:119–134. doi: 10.1081/pb-200054732. [DOI] [PubMed] [Google Scholar]
- 32.Akiyoshi K, Kobayashi S, Shichibe S, Mix D, Baudys M, Kim SW, Sunamoto J. Self-assembled hydrogel nanoparticle of cholesterol-bearing pullulan as a carrier of protein drugs: complexation and stabilization of insulin. J Control Release. 1998;54:313–320. doi: 10.1016/s0168-3659(98)00017-0. [DOI] [PubMed] [Google Scholar]
- 33.Pittet MJ, Zippelius A, Speiser DE, Assenmacher M, Guillaume P, Valmori D, Lienard D, Lejeune F, Cerottini JC, Romero P. Ex vivo IFN-γ secretion by circulating CD8 T lymphocytes: implications of a novel approach for T cell monitoring in infectious and malignant diseases. J Immunol. 2001;166:7634–7640. doi: 10.4049/jimmunol.166.12.7634. [DOI] [PubMed] [Google Scholar]
- 34.Desombere I, Meuleman P, Rigole H, Willems A, Irsch J, Leroux-Roels G. The interferon gamma secretion assay: a reliable tool to study interferon gamma production at the single cell level. J Immunol Methods. 2004;286:167–185. doi: 10.1016/j.jim.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Lecoeur H, Ledru E, Prevost MC, Gougeon ML. Strategies for phenotyping apoptotic peripheral human lymphocytes comparing ISNT, annexin-V and 7-AAD cytofluorometric staining methods. J Immunol Methods. 1997;209:111–123. doi: 10.1016/s0022-1759(97)00138-5. [DOI] [PubMed] [Google Scholar]
- 36.Tsukahara T, Kawaguchi S, Torigoe T, Asanuma H, Nakazawa E, Shimozawa K, Nabeta Y, Kimura S, Kaya M, Nagoya S, Wada T, Yamashita T, Sato N. Prognostic significance of HLA class I expression in osteosarcoma defined by anti-pan HLA class I monoclonal antibody, EMR8-5. Cancer Sci. 2006;97:1374–1380. doi: 10.1111/j.1349-7006.2006.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uenaka A, Ono T, Akisawa T, Wada H, Yasuda T, Nakayama E. Identification of a unique antigen peptide pRL1 on BALB/c RL male 1 leukemia recognized by cytotoxic T lymphocytes and its relation to the Akt oncogene. J Exp Med. 1994;180:1599–1607. doi: 10.1084/jem.180.5.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Materials and methods
Cholesterol-bearing hydrophobized pullulan and NY-ESO-1 protein (CHP-NY-ESO-1) complex preparation
Preparation of recombinant NY-ESO-1 protein for use in the vaccine has been described elsewhere (31). The complex consisting of cholesterol-bearing hydrophobized pullulan (CHP) and NY-ESO-1 protein was formulated as described previously (32).
Study design
A phase I clinical trial of CHP-NY-ESO-1 vaccine was designed to evaluate the safety, immune response and tumor response. Patients had advanced cancers expressing NY-ESO-1 as assessed by RT-PCR using specific primers (9) and immunohistochemistry, and were injected subcutaneously at biweekly intervals with 100 µg of NY-ESO-1 recombinant protein formulated with 2 mg of CHP. The protocol was approved by the Ethics Committee of the Ludwig Institute for Cancer Research, New York (LUD 2002-005), and Osaka and Okayama Universities.
Advanced cancer patients, including 4 patients with esophageal cancer (E-1, E-2, E-3 and E-4), 4 patients with prostate cancer (P-1, P-2, P-3 and P-4), and 1 patient with malignant melanoma (M-1), were enrolled after a withdrawal period after surgery, chemotherapy, radiation or antiandrogen therapy of at least 4 weeks. In prostate cancer patients, hormone refractory was defined as a continuous increase in PSA values at 3 time points at least 2 weeks apart with values >4.0 ng/ml.
Blood samples
Blood was drawn from patients and healthy donors with informed consent. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using Histopaque 1077 (Sigma-Aldrich, St. Louis, MO). A CD4 T cell-enriched population was obtained from PBMCs using CD4 microbeads with a large-scale column and a magnetic device (Miltenyi Biotec, Auburn, CA). A CD8 T cell-enriched population was then obtained from the residual cells using CD8 microbeads. The final residual cells were used as a CD4- and CD8-depleted population. The 3 populations were stored in liquid N2 until use. HLA typing of PBMCs was determined by sequence-specific oligonucleotide probing and sequence-specific priming of genomic DNA using standard procedures.
Overlapping peptides
The following series of 28 overlapping NY-ESO-1 18-mer peptides spanning the protein were synthesized: 1-18, 7-24, 13-30, 19-36, 25-42, 31-48, 37-54, 43-60, 49-66, 55-72, 61-78, 67-84, 73-90, 79-96, 85-102, 91-108, 97-114, 103-120, 109-126, 115-132, 121-138, 127-144, 133-150, 139-156, 145-162, 149-166, 153-170, and 156-173. A 30-mer peptide, 151-180, was also synthesized. These peptides were synthesized using standard solid-phase methods based on N-(9-fluorenyl)-methoxycarbonyl (Fmoc) chemistry on a Multiple Peptide Synthesizer (AMS422; ABIMED, Langenfeld, Germany) at Okayama University.
In vitro stimulation of CD4 and CD8 T cells
Frozen cells were thawed and resuspended in AIM-V (Invitrogen, Carlsbad, CA) medium supplemented with 5% heat-inactivated pooled human serum (CM), and kept at room temperature for 2 hr. CD4- and CD8-enriched populations (2 x 106) were cultured with irradiated (30 Gy), autologous CD4- and CD8-depleted PBMCs (2 x 106) in the presence of the 28 18-mer overlapping peptides and a 30-mer C-terminal peptide spanning the entire NY-ESO-1 protein (1 µg/ml for each peptide) in 2 ml of CM supplemented with 10 units/ml rIL-2 (Takeda Chemical Industries, Osaka, Japan) and 10 ng/ml rIL-7 (Peprotech, London, UK) in a 24-well culture plate at 37˚C in a 5% CO2 atmosphere for 12 days. For the second stimulation, 1 x 106 instead of 2 x 106 responder cells were used in the culture described above.
IFN-γ secretion assay
The IFN-γ secretion assay (33, 34) was carried out according to the manufacturer's protocol (Miltenyi Biotec). Briefly, 2 x 105 responder CD4 and CD8 T cells were stimulated with PFA (0.2%)-treated autologous CD4- and CD8-depleted PBMCs (2 x 105) pre-pulsed with the peptides for 4 hr at 37˚C in a 5% CO2 atmosphere. The cells were then washed and suspended in 100 µl of cold RPMI medium, and treated with a bi-specific CD45 and IFN-γ mouse antibody (IFN-γ catch reagent) (2 µl) for 5 min on ice. The cells were then diluted in AIM-V medium (1 ml) and placed on a slow rotating device (Miltenyi Biotec) to allow IFN-γ secretion at 37˚C in a 5% CO2 atmosphere. After incubation for 45 min, the cells were washed with cold buffer and treated with 7AAD (7-amino-Actinomycin D, Becton Dickinson, Mountain View, CA) (35), PE-conjugated anti-IFN-γ (detection reagent), and FITC-conjugated anti-CD4 or CD8 mAbs for staining. After incubation for 10 min at 4˚C, the cells were washed and analyzed with a FACScan (Becton Dickinson). Dead cells were sorted out by 7AAD staining. The data were analyzed with FlowJo software (Tree Star, Ashland, OR). A net population of IFN-γ secreting CD4 and CD8 T cells of more than 0.1% was considered to be significant.
Immunohistochemistry (IHC)
IHC was performed as described previously (3). E978 (6) and EMR8-5 (Funakoshi, Tokyo, Japan) (36) mAbs were used to analyze NY-ESO-1 and HLA class I expression, respectively. The reaction was evaluated as +++ (>50% stained cells), ++ (25-50% stained cells), + (5-25% stained cells) and - (<5% stained cells).
ELISA
Recombinant NY-ESO-1 protein (1 µg/ml) in coating buffer (15 mM Na2CO3, 30 mM NaHCO3, pH 9.6) was adsorbed onto 96-well Polysorp immunoplates (Nunc, Roskilde, Denmark) and incubated overnight at 4˚C. Plates were washed with PBS and blocked with 200 µl/well of 5% FCS/PBS for 1 h at room temperature. After washing, 100 µl of serially diluted serum was added to each well and incubated for 2 hrs at room temperature. After extensive washing, horseradish peroxidase (HRP)-conjugated goat anti-human IgG (Medical & Biological Laboratories, Nagoya, Japan) was added to the wells, and the plates were incubated for 1 h at room temperature. After washing and development, absorbance at 490 nm was read. Recombinant murine Akt protein (37) and ovalbumin (OVA, albumin from chicken egg white; Sigma, St. Louis, MO) were used as control proteins.
Disease assessment
The response of prostate cancer patients was assessed according to the following criteria: Complete response (CR), post-treatment PSA values <4 ng/ml at 2 consecutive time points at least 2 weeks apart. Partial response (PR), decline of post-treatment PSA values by >50% of the pre-treatment value at 2 consecutive time points at least 2 weeks apart. Minimal response (MR), decline of post-treatment PSA values by >25% of the pre-treatment value at 2 consecutive time points at least 2 weeks apart. Progressive disease (PD), two consecutive increases in PSA values at least 2 weeks apart, each >50% above pre-treatment value. Stable disease (SD), patients not meeting the above criteria.