Abstract
Tumor antigens are the primary target of therapeutic cancer vaccines. We set out to define and compare the expression pattern of tumor antigen genes in esophagus carcinoma biopsies and in an allogeneic tumor lysate-based cancer vaccine, MelCancerVac®. Cells used for vaccine production were treated with the DNA methyltransferase inhibitor 5-aza-2'-deoxycytidine (5-aza-CdR) to determine whether this treatment could improve the profile of tumor antigen genes expressed in these cells. In addition, the presence of MAGE-A tumor antigen protein was evaluated in the purified tumor cell lysate used in the production of the vaccine. Quantitative PCR was used to assay 74 tumor antigen genes in patients with squamous cell carcinoma of the esophagus. 81% (13/16) of tumors expressed more than five cancer/testis (CT) antigens. A total of 96 genes were assayed in the tumor cell clone (DDM1.7) used to make tumor cell lysate for vaccine preparation. Gene expression in DDM1.7 cells was compared with three normal tissues; 16 tumor antigen genes were induced more than ten-fold relative to normal tissues. Treatment with 5-aza-CdR induced expression of an additional 15 tumor antigens to a total of 31. MAGE-A protein was detected in cell lysate by Western blot at an estimated concentration of 0.2 µg/ml or 0.01% of the total protein.
Keywords: human, esophageal cancer, MelCancerVac®, tumor antigen, 5-aza-2'-deoxycytidine, PCR
Introduction
Cancer of the esophagus is the sixth leading cause of cancer mortality worldwide. Since 1975 the incidence of esophageal adenocarcinoma has increased 6-fold, with a corresponding 7-fold increase in mortality [reviewed in (1, 2)]. Overall, cancers of the esophagus and stomach have limited therapeutic options and 5-year survival is less than 20%. Esophageal tumors are usually treated by surgical resection with poor outcomes. Furthermore, recent studies using radiation or radiation/chemotherapy combination therapy have shown limited clinical benefit. The lack of good therapeutic options for esophageal cancer indicates that immunotherapy could play a role in treating this disease by inducing anti-tumor immunity (1). Immunotherapy is a particularly attractive treatment option due to the common expression of CT antigens in esophageal tumors (3-5), and the lack of serious toxicity or adverse side effects resulting from dendritic cell-based therapies (6-8).
Cancer immunotherapy aims to activate the immune system to recognize tumor antigens, proteins that are specifically expressed by tumor cells and most importantly, displayed on the cell surface as MHC-peptide antigen complexes. Tumor antigens are defined in two main groups: (i) Tumor specific antigens (TSAs) are generally unique to tumor cells and result from mutation of normal genes or from expression of oncogenic viral proteins. (ii) Tumor associated antigens (TAAs) are normal human proteins that are abnormally overexpressed in tumor cells. An important class of TAAs is the cancer/testis (CT) class of genes; also known as cancer germline genes, these genes are only expressed in tumor cells and in testis (6, 9). Many CT class genes are regulated by DNA methylation and are therefore activated by chemicals that inhibit DNA methyltransferases, such as 5-aza-2'-deoxycytidine (5-aza-CdR) (10-13). The restricted expression of CT genes enables the immune system to recognize antigenic peptides derived from these gene products. Cytotoxic T lymphocytes have been found that recognize CT antigens in cancer patients in the absence of immunotherapy, indicating that the immune system can recognize and target such antigens (14, 15). Many clinical vaccine studies have targeted CT genes; however, only a few of these studies have shown a significant effect on disease progression in cancer patients (16, 17), with the recent exception of GlaxoSmithKline's MAGE-A3 antigen-specific cancer immunotherapeutic (ASCI) [data presented at the 2008 American Society of Clinical Oncology (ASCO) Annual Meeting in Chicago - abstracts 9065(1), 9045(2) and 7501(3) and reviewed in (18)]. Many clinical studies use only a single CT gene product or antigenic peptide to induce anti-tumor immunity. It may be that a small number of antigenic peptides are not sufficient to induce tumor rejection, while immunotherapy that targets the greatest number of possible TAAs is more likely to effectively target tumor cells.
MelCancerVac® is a therapeutic cancer vaccine that is based on loading patient-derived dendritic cells with an allogeneic tumor cell lysate. A tumor cell lysate is used as the source of tumor antigen in order to provide the most diverse and abundant source of tumor antigens. By using a cell lysate there is no selection for any particular tumor antigen or a particular recipient HLA type. Thus, this vaccine approach aims to induce immunity against the broadest possible range of tumor antigens, including both known and unknown antigens. The specific melanoma cell clone used to produce tumor cell lysate for vaccine production is DDM1.7.
In the present study we have assayed for the expression of a large number of tumor antigen genes in a total of 16 biopsies from squamous cell tumors of the esophagus. In addition, we examined the molecular expression of tumor antigen genes in DDM1.7 cells with and without treatment with 5-aza-CdR, in three normal tissues, in an esophageal tumor biopsy, and in normal human testis. The protein expression of MAGE-A was evaluated in whole cells and purified cell lysate by Western blot. Our goal is to define those tumor antigens whose expression can be monitored by reverse transcription-quantitative polymerase chain reaction (RT-QPCR) and to use this approach to identify patient tumors that have a similar expression of tumor antigens as the DDM1.7 cells used to generate tumor vaccine. This approach can be used to identify a patient group for treatment with a cancer vaccine, as in this study, or to select individual patients for treatment based on overlapping expression of tumor antigen genes in tumors and in a vaccine.
Results
Tumor antigen gene expression in esophageal tumor biopsies
Expression of 74 tumor antigen genes (64 of which are CT type tumor antigen genes) in esophageal tumor biopsies from 16 patients (Table 1) was assayed by RT-QPCR. Biopsies were taken together with matched normal tissue biopsies, in order to compare gene expression in a patient tumor to the neighboring normal tissue. However, not all normal tissue biopsies yielded usable RNA. Therefore, to enable consistent comparison of tumor samples to normal tissue, the average relative expression values for normal tissue biopsies from 8 patients was determined and compared with the relative expression level in purified esophagus RNA purchased from Ambion (Supplementary Figure 1). Gene expression was similar in all normal esophagus tissue samples; we therefore used the average relative expression in normal tissue biopsies from 8 patients as a baseline value to compare with tumor samples. In the reactions where no gene product was detected in normal tissue (such as for MAGE-A1), the value of 10-6 relative to GAPDH expression was set as the detection limit and induction of expression was calculated relative to this value. The 10-6 cutoff value was chosen for two reasons: (i) It is approximately equal to the minimal expression detected in our system (see Table 2 and Supplementary Figure 1). (ii) Dilution experiments showed this to be the detection limit for several genes that are not detected in normal tissue (data not shown). Furthermore, in order to identify only those genes that show robust gene induction we ignored changes in gene expression that were less than 10-fold in magnitude.
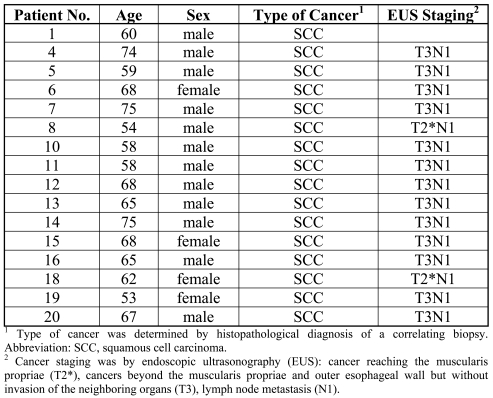
Table 1.
Patients included in this study.
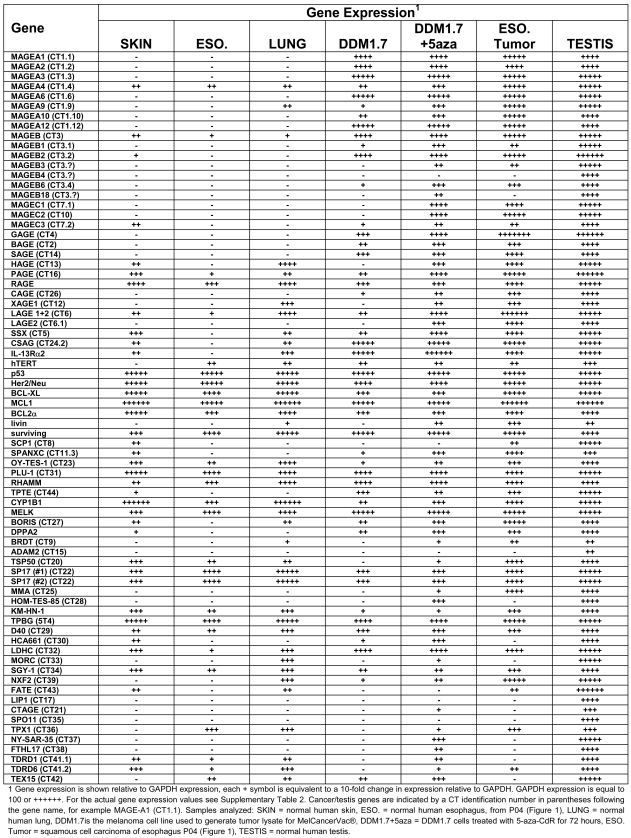
Table 2.
Analysis of tumor antigen gene expression by QPCR.
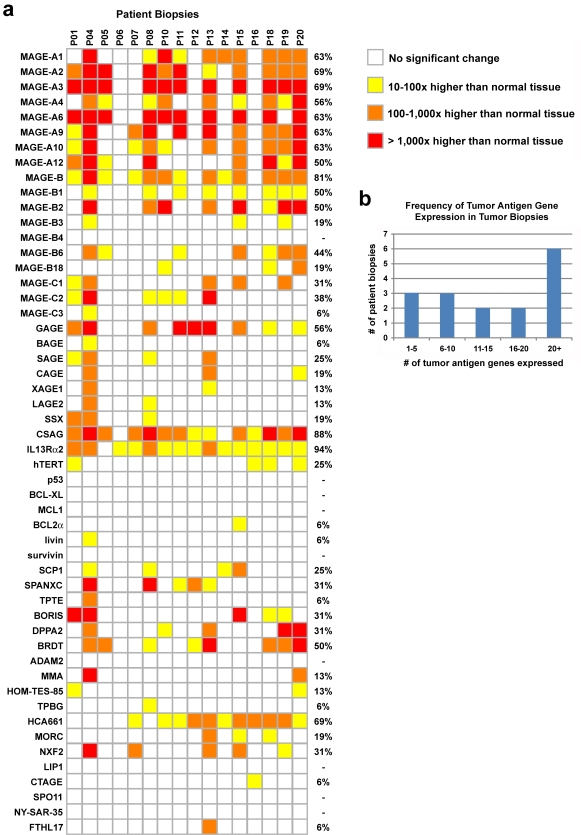
The results of gene expression analysis in esophageal tumor biopsies are shown in Figure 1. Expression of CT tumor antigens is frequent in squamous cell carcinoma of the esophagus, with 81% (13/16) of biopsies expressing more than five CT tumor antigens, while 63% (10/16) express more than 10 CT tumor antigens (Figure 1b). The most frequently expressed antigens were the MAGE-A and MAGE-B genes, as well as CSAG, IL13Rα2, BRDT, and HCA661. Tumors that express MAGE-A3 expressed high numbers of tumor antigens in general (64% expressed 20 or more tumor antigens; only one biopsy expressed less than 10 tumor antigens, P05, which expressed 9), indicating that this gene may be a good marker for CT expression overall. We used a pan-MAGE-B reaction to detect expression of multiple MAGE-B genes (MAGE-B). Detection of MAGE-B by this reaction correlated well with detection of individual MAGE-B family genes (10/13), indicating that this reaction may be used in pre-screening for MAGE-B expression. However, it should be noted that the MAGE-B primer pair is not particularly specific; it is able to amplify many MAGE-B family members by allowing amplification even in the presence of mismatched base pairs and may amplify related MAGE genes, such as MAGE-A8. A total of 9 tumor antigen genes were not detected in any of the tumor biopsies while a total of 26 tumor antigen genes were detected in fewer than 25% of the biopsies. Non-CT type tumor antigen genes were rarely induced in tumor biopsies (p53, TPBG, BCL-XL, MCL1, BCL2α, livin/BIRC7 and survivin/BIRC5), with the exception of the hTERT gene, which was modestly induced in 25% of tumor biopsies.
Figure 1.
Tumor antigen gene expression in esophagus tumor biopsies. (a) Gene expression in tumor samples is compared to the expression in normal esophagus tissue. Gene expression changes less than 10-fold in magnitude are not shown. Frequency of gene expression in the 16 tumor biopsies is shown on the right hand side of the figure. (b) Frequency of overall tumor antigen expression in the 16 tumor biopsies is shown.
Tumor antigen gene expression in DDM1.7 cells
A larger number of QPCR reactions was used to detect tumor antigen gene expression in the cells (DDM1.7) used to make tumor cell lysate for MelCancerVac®. The purpose of assaying tumor antigen gene expression in DDM1.7 melanoma cells is to identify highly expressed tumor antigen genes. Total RNA isolated from normal skin, lung, and esophagus tissues was used to determine the baseline expression of tumor antigen genes in normal tissue. Human testis RNA was used as a positive control for gene expression since many of the genes we assayed are CT type TAAs, which are known to have robust expression in testis. This also provides a means to compare the degree of CT gene expression in a tumor tissue to the normal gene expression level in testis. The esophagus tumor biopsy from patient 4 (P04, Figure 1) was used as an additional positive control since this sample showed robust tumor antigen gene expression.
The result of the QPCR analysis is shown in Table 2 using symbols to indicate gene expression relative to GAPDH. The actual relative expression values are given in Supplementary Table 1. For many genes the results were consistent with CT type tumor antigens, i.e. expression was very low or absent in normal tissues and elevated in tumor tissues and testis (see MAGE-A1). However, some CT genes had surprisingly high expression in normal tissue, in contrast to their classification as CT-specific. Similarly, some genes were detected at a low level in only one of the normal tissue samples, indicating partially restricted expression.
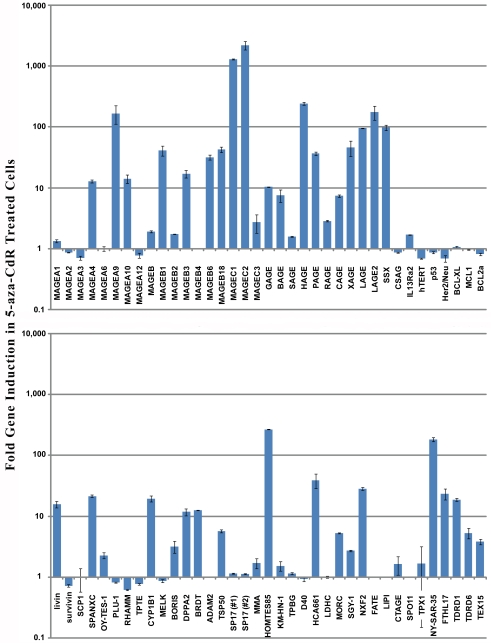
Since we were interested in determining which genes may be significantly expressed in DDM1.7 cells, we set a cutoff value for gene expression at 10-fold higher relative expression than in normal tissues. Using this cutoff we identify 16 genes as being elevated in DDM1.7 cells (MAGE-A1, MAGE-A2, MAGE-A3, MAGE-A6, MAGE-A10, MAGE-A12, MAGE-B1, MAGE-B2, MAGE-B6, GAGE, BAGE, SAGE, CAGE, CSAG, IL13-Rα2, and TPTE). Treatment of cells with the DNA methyltransferase inhibitor 5-aza-CdR nearly doubles the number of genes with elevated expression to 31 genes (MAGE-A1, MAGE-A2, MAGE-A3, MAGE-A6, MAGE-A9, MAGE-A10, MAGE-A12, MAGE-B1, MAGE-B2, MAGE-B3, MAGE-B6, MAGE-B18, MAGE-C1, MAGE-C2, GAGE, BAGE, SAGE, PAGE, CAGE, LAGE2, SSX, CSAG, IL13-Rα2, TPTE, BORIS, DPPA2, MMA, HOM-TES-85, CTAGE, NY-SAR-35, and FTHL). Furthermore, a direct comparison of gene expression in DDM1.7 cells to DDM1.7 cells treated with 5-aza-CdR shows that the expression of 27 genes is elevated by treatment with 5-aza-CdR by a factor of more than 10 (Figure 2). Significantly, only CT type tumor antigens were induced by treatment with 5-aza-CdR while other genes such as hTERT, p53, and the inhibitor of apoptosis class genes (BCL-XL, MCL1, BCL2a, and survivin) were largely unaffected. In addition, CT genes that were already significantly elevated in DDM1.7 cells (such as MAGE-A1) were not further induced by treatment with 5-aza-CdR, suggesting that these genes may be hypomethylated in DDM1.7 cells before treatment with 5-aza-CdR.
Figure 2.
Gene expression induced by 5-aza-2 -deoxycytidine in DDM1.7 cells. The graph shows the gene expression in DDM1.7 cells treated with 5-aza-CdR for 72 hours relative to DDM1.7 cells. Error bars indicate standard deviation between reaction duplicates.
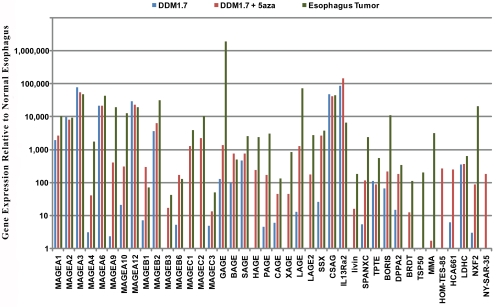
Tumor antigen gene expression in the esophageal tumor biopsy (patient 4) is more extensive than in DDM1.7 cells but is comparable to the expression seen in DDM1.7 cells treated with 5-aza-CdR (Figure 3). Only the genes that have a greater than 10-fold higher expression level than the tissue-matched normal esophagus sample (patient 4) are compared in Figure 3. Note that 37 genes have significantly higher expression in the esophageal tumor biopsy sample; of these 37 genes, 95% (35/37) are also highly expressed in DDM1.7 cells treated with 5-aza-CdR and 60% (22/37) are genes that were significantly induced by 5-aza-CdR in DDM1.7 cells.
Figure 3.
Gene expression relative to normal esophagus tissue (ESO) in an esophageal tumor (P04), DDM1.7 cells with and without 5-aza-CdR treatment. Only the genes that were expressed >10-fold higher than normal esophagus tissue in at least one of the three samples are shown. The data is taken from Table 1. Note the similarity in gene expression between 5-azaCdR treated cells and the esophageal tumor.
Assaying MAGE-A protein expression in DDM1.7 cell lysate
While the expression of tumor antigen genes is likely to indicate the presence of tumor antigen protein in cells or tissues, it remains important to verify and quantify the amount of tumor antigen protein that is present. Our vaccine product, MelCancerVac®, is based on loading patient-derived dendritic cells with a purified cell lysate made from DDM1.7 cells. In order for vaccine-induced T cells to effectively target a particular tumor antigen, the tumor antigen protein must be present in both DDM1.7 cells and in the purified cell lysate.
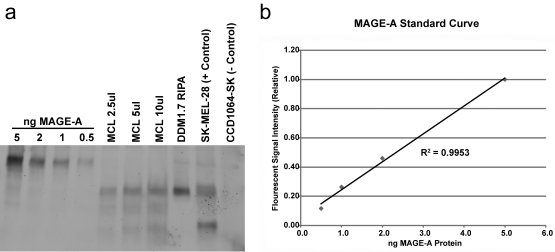
MAGE-A protein was assayed in DDM1.7 cells and purified cell lysate by Western blot with the 6C1 monoclonal antibody (Figure 4a). MAGE-A protein was detected in both purified DDM1.7 cell lysate (melanoma cell lysate, MCL) and in whole cell lysate made by lysis of DDM1.7 cells in radioimmunoprecipitation assay (RIPA) buffer. Including protease inhibitor and/or proteasome inhibitor in the RIPA lysis buffer had no effect on protein stability (data not shown). Three independently produced batches of MCL were assayed with similar results (data not shown). A standard curve of purified MAGE-A fusion protein enables us to estimate the abundance of MAGE-A protein in MCL (Figure 4b). Western blots were developed with a fluorescent scanner, allowing direct quantification of signal intensity using ImageQuant software. These data show that the MCL batch analyzed in Figure 4 contains approximately 1 ng MAGE-A/5 µl MCL or 200 ng/ml. Since the MCL batch contains a total of approximately 2.2 mg/ml of protein, MAGE-A represents approximately 0.01% of the total protein present. Attempts to detect additional CT antigens with antibodies directed against MAGE-C1, SSX, IL-13Rα2, and LAGE1/NY-ESO-1 all failed due to insufficient signal or high background (data not shown).
Figure 4.
MAGE-A protein is present in DDM1.7 cell lysate. (a) Western blot using the pan-MAGE-A monoclonal antibody 6C1 (reacts with MAGE-A1, -A2, -A3, -A4, -A6, -A10, and -A12). Samples analyzed: “ng MAGE-A” indicates the amount of MAGE-A fusion protein loaded in each lane, MCL corresponds to melanoma cell lysate, DDM1.7 RIPA refers to whole cell lysate made with RIPA buffer, SK-MEL-28 is a positive control cell lysate, CCD1064-SK is a negative control cell lysate. (b) Graph showing the MAGE-A standard curve made by measuring the fluorescent intensity of MAGE-A fusion protein in the Western blot shown above.
Discussion
In this study we show that squamous cell carcinomas of the esophagus frequently express CT type tumor antigen genes. We further demonstrate that DDM1.7 cells, used to provide tumor antigen for the production of MelCancerVac®, express many of the same tumor antigen genes found in squamous cell carcinoma of the esophagus. Treatment of DDM1.7 cells with the DNA methyltransferase inhibitor 5-aza-CdR induces further expression of CT type tumor antigen genes, thereby increasing the number antigens that may be targeted by a cancer vaccine. MAGE-A tumor antigen was detected in purified lysate prepared from DDM1.7 cells, indicating that this tumor cell lysate contains this tumor antigen protein.
In order to identify tumor antigen genes expressed in squamous cell carcinomas of the esophagus, and in DDM1.7 melanoma cells, we assayed a large number of tumor antigen genes simultaneously. Gene expression (mRNA) typically indicates whether or not the gene product (protein) is present in the cells or tissue being analyzed. However, gene expression alone cannot be used to specify the presence or absence of tumor antigen. Protein expression may be regulated at the level of protein stability rather than gene transcription, and tumor antigens may arise by abnormal processing of normally expressed proteins (19). In addition, at least one ubiquitously expressed human protein has been shown to become antigenic in cancer patients (20). Analysis of tumor antigen gene expression by QPCR is rapid, inexpensive, and easy. However, this approach can only be used to assay tumor antigens that are transcriptionally activated in tumors. Therefore, the main reason for performing this work is to identify the tumor antigen genes that can be monitored by RT-QPCR in patient tumor samples, in order to identify patients that may benefit from treatment with MelCancerVac® or other immune therapies and as a first step in designing patient-tailored immune therapy. In addition, by measuring a large number of genes simultaneously we can compare the expression levels of different tumor antigen genes directly, an insight that is missing from the many studies that examine the expression of one or a few tumor antigen genes at a time.
We found that a fairly large number of CT tumor antigen genes were expressed to a significant degree in normal tissue, apparently contradicting the initial studies that classified these genes as CT restricted. We do not have the space here to review the literature for each CT gene individually; however, two studies in particular also found expression of CT genes in normal tissues and are consistent with the results presented here. A recent study examined CT gene expression using existing gene expression data found in a multitude of gene expression databases and libraries (21). This study identified three groups of CT genes: Testis-restricted, testis/brain-restricted, and testis-selective. Genes that were identified as testis-selective had gene expression in some normal tissues that was typically less than expression in testis. Our results largely agree with the results presented in this study. However, we detected a very low level of expression in some of our normal tissue samples for genes that are classified as testis-restricted. This may result from the higher sensitivity of the RT-QPCR assay used in our study or from inadequate coverage in the gene expression libraries used in (21). Similarly, Scanlan et al. (9) surveyed CT gene expression by RT-PCR and similarly found expression in a number of normal tissues. Such results indicate that the initial classification of CT genes may be unreliable and that a comprehensive study of CT gene expression is needed to allow for better selection of antigens for immune therapy. The data presented here and in (9, 21) are a step in the right direction.
Analysis of tumor antigen gene expression in squamous cell carcinoma biopsies shows that CT antigen expression occurs in a large fraction of this patient group (81%). The frequency of MAGE-A gene expression was similar to that observed in previous studies using immunohistochemistry (3, 5). Activation of the MAGE-A3 gene is always accompanied by activation of additional CT genes, making MAGE-A3 a good marker for CT gene activation overall. As seen in other studies, CT gene activation is clustered; if a single CT gene is activated, it is likely that additional genes are also activated (22). This observation supports the idea that CT gene activation results from a loss of DNA methylation. The gene expression profile of IL13Rα2 and CSAG differs from the "MAGE" genes in that increased expression is not always linked with MAGE-A3 expression. IL13Rα2 and CSAG are ubiquitously expressed in normal tissues [(9) and our study] and do not appear to be significantly induced by 5-aza-CdR, perhaps indicating that activation of these genes occurs by a different mechanism than activation of the "MAGE" genes. Overall these results indicate that it may be possible to treat squamous cell carcinoma of the esophagus with immune therapy, such as vaccination or adoptive T cell transfer targeting CT antigens. In support of this idea, dendritic cells loaded with total RNA from esophagus tumor cells are able to activate T cell-dependent killing of primary esophagus tumor cell cultures as compared to normal cells (23). Tumor antigen expression in DDM1.7 cells treated with 5-aza-CdR overlaps with 95% of the tumor antigen genes expressed in the tumor biopsy from patient 4. This indicates that MelCancerVac® contains many tumor antigens that are shared with squamous cell tumors of the esophagus. In addition, these results underline the necessity to screen patients for CT antigen expression before attempting immune therapy that targets CT antigens, as some patients do not express CT antigens at all.
We find that treatment with 5-aza-CdR induces the activation of many CT tumor antigen genes. This simple treatment doubles the number of known tumor antigens expressed by DDM1.7 and broadens the range of antigens targeted by MelCancerVac®. The comparison presented here does not identify all of the genes that may be induced by 5-aza-CdR, as some genes are already expressed at a high level in DDM1.7 and may not be further induced by treatment with 5-aza-CdR. For example, a number of studies have shown that MAGE-A1 is induced by 5-aza-CdR (24-26). However, in DDM1.7 MAGE-A1 expression is elevated before treatment with 5-aza-CdR and is not further induced by treatment with 5-aza-CdR. In fact, a large number of CT genes are induced by treatment with 5-aza-CdR (MAGE-A, LAGE-1, LAGE-2, SSX-2, CAGE, GAGE, HAGE, and PAGE-5 [(10-12) and reviewed in (13)]). In contrast, some CT genes were not induced by treatment with 5-aza-CdR in our study (MAGE-B4, SCP1, OY-TES-1, PLU-1, TPTE, ADAM2, SP17, MMA, D40, LDHC, SGY-1, FATE, LIP1, and SPO11). Of these genes, SP17 and LDHC have previously been shown to be regulated by DNA methylation and induced by treatment with 5-aza-CdR (27, 28). Therefore, treatment with 5-aza-CdR may not induce gene expression in an equivalent manner in different tissues or cell lines.
DDM1.7 cells treated with 5-aza-CdR have overlapping expression with 95% of the genes found to be overexpressed in the esophageal tumor biopsy sample with the highest degree of tumor antigen expression (patient 4). Therefore, a high degree of CT tumor antigen expression is mirrored by cells treated with 5-aza-CdR. This observation suggests that DNA demethylation is responsible for much of the CT tumor antigen expression observed in some squamous cell tumors of the esophagus. Several studies have shown that promoter methylation regulates the expression of CT genes (29-32). Since some tumor biopsy samples have little or no tumor antigen gene expression whatsoever (Figure 1), these data further suggest that loss of DNA methylation only occurs in some tumors, resulting in the expression of CT type tumor antigens, while other tumors are unaffected. It is therefore important to treat only those patients that express CT tumor antigens with therapies designed to target CT antigens. DNA methyltransferase inhibitors, such as 5-aza-CdR and related compounds, have been approved for use as therapeutic agents targeting myelodysplastic syndrome and myelogenous leukemia (33, 34). These compounds inhibit tumor cell growth by reactivating genes that have been silenced by DNA methylation in cancer cells. However, treatment of patients with 5-aza-CdR may also facilitate immunotherapy by inducing CT antigen expression in patient tumors, an observation that has also been made by others (35).
As mentioned above, the expression of a tumor antigen gene is not sufficient in itself to indicate the presence of tumor antigen protein in cells or tissues. The tumor cell lysate component of MelCancerVac® is prepared by lysing DDM1.7 cells and removing insoluble cellular debris by several rounds of centrifugation and sterile ultrafiltration. Therefore, it is possible that protein may be lost during the lysis and purification steps. Unfortunately, only a few quality antibodies are commercially available for assaying tumor antigen expression by Western blot. In this study we detected MAGE-A protein using the pan-MAGE-A 6C1 monoclonal antibody (the antibody reacts with MAGE-A1, -A2, -A3, -A4, -A6, -A10, and -A12). We detect MAGE-A in the purified tumor cell lysate, indicating that MAGE-A proteins in the lysate can be loaded onto patient dendritic cells. Quantification of proteins by Western blots is often imperfect; however, we estimated the amount of MAGE-A protein present by comparing Western blot signal intensity to a standard curve made with purified MAGE-A fusion protein. Although the relative quantity of MAGE-A is a small fraction of the total protein (0.01%), this amount may be sufficient to induce MAGE-A specific CTL responses in patients vaccinated with lysate-loaded dendritic cells.
In conclusion, in this study we identify tumor antigen genes that may be monitored for expression by RT-QPCR. While not suitable for all tumor antigens, this approach provides a rapid and inexpensive method to screen patients for tumor antigen gene expression to identify patient groups suitable for immunotherapy or to select individual patients for immunotherapy, based on overlapping expression of tumor antigen genes in a patient tumor and in a cancer vaccine. Treatment with the DNA methyltransferase inhibitor 5-aza-CdR induces CT type tumor antigen gene expression and may be used to improve tumor antigen gene expression in tumor cell lysate-based cancer vaccines. Tumor antigen protein abundance should be analyzed in lysate-based therapies in order to determine if the amount of tumor antigen protein present is sufficient to induce an immune response.
Abbreviations
- 5-aza-CdR
5-aza-2'-deoxycytidine
- CT
cancer/testis
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- MCL
melanoma cell lysate
- QPCR
quantitative polymerase chain reaction
- RT-QPCR
reverse transcription-QPCR
References
- 1.Milano F, Krishnadath KK. Novel therapeutic strategies for treating esophageal adenocarcinoma: the potential of dendritic cell immunotherapy and combinatorial regimens. Hum Immunol. 2008;69:614–624. doi: 10.1016/j.humimm.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Khushalani N. Cancer of the esophagus and stomach. Mayo Clin Proc. 2008;83:712–722. [PubMed] [Google Scholar]
- 3.Haier J, Owzcareck M, Guller U, Spagnoli GC, Bürger H, Senninger N, Kocher T. Expression of MAGE-A cancer/testis antigens in esophageal squamous cell carcinomas. Anticancer Res. 2006;26:2281–2287. [PubMed] [Google Scholar]
- 4.Kan T, Yamasaki S, Kondo K, Teratani N, Kawabe A, Kaganoi J, Meltzer SJ, Imamura M, Shimada Y. A new specific gene expression in squamous cell carcinoma of the esophagus detected using representational difference analysis and cDNA microarray. Oncology. 2006;70:25–33. doi: 10.1159/000091183. [DOI] [PubMed] [Google Scholar]
- 5.Akcakanat A, Kanda T, Tanabe T, Komukai S, Yajima K, Nakagawa S, Ohashi M, Hatakeyama K. Heterogeneous expression of GAGE, NY-ESO-1, MAGE-A and SSX proteins in esophageal cancer: Implications for immunotherapy. Int J Cancer. 2006;118:123–128. doi: 10.1002/ijc.21219. [DOI] [PubMed] [Google Scholar]
- 6.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 7.Burgdorf SK, Fischer A, Claesson MH, Kirkin AF, Dzhandzhugazyan KN, Rosenberg J. Vaccination with melanoma lysate-pulsed dendritic cells, of patients with advanced colorectal carcinoma: report from a phase I study. J Exp Clin Cancer Res. 2006;25:201–206. [PubMed] [Google Scholar]
- 8.Burgdorf SK, Fischer A, Myschetzky PS, Munksgaard SB, Zocca MB, Claesson MH, Rosenberg J. Clinical responses in patients with advanced colorectal cancer to a dendritic cell based vaccine. Oncol Rep. 2008;20:1305–1311. [PubMed] [Google Scholar]
- 9.Scanlan MJ, Simpson AJ, Old LJ. The cancer/testis genes: review, standardization, and commentary. Cancer Immun. 2004;4:1. http://www.cancerimmunity.org/v4p1/031220.htm [PubMed] [Google Scholar]
- 10.Roman-Gomez J, Jimenez-Velasco A, Agirre X, Castillejo JA, Navarro G, San Jose-Eneriz E, Garate L, Cordeu L, Cervantes F, Prosper F, Heiniger A, Torres A. Epigenetic regulation of human cancer/testis antigen gene, HAGE, in chronic myeloid leukemia. Haematologica. 2007;92:153–162. doi: 10.3324/haematol.10782. [DOI] [PubMed] [Google Scholar]
- 11.Fukutomi S, Seki N, Koda K, Miyazaki M. Identification of methylation-silenced genes in colorectal cancer cell lines: genomic screening using oligonucleotide arrays. Scand J Gastroenterol. 2007;42:1486–1494. doi: 10.1080/00365520701491173. [DOI] [PubMed] [Google Scholar]
- 12.Calabrò L, Fonsatti E, Altomonte M, Pezzani L, Colizzi F, Nanni P, Gattei V, Sigalotti L, Maio M. Methylation-regulated expression of cancer testis antigens in primary effusion lymphoma: immunotherapeutic implications. J Cell Physiol. 2005;202:474–477. doi: 10.1002/jcp.20133. [DOI] [PubMed] [Google Scholar]
- 13.Zendman AJ, Ruiter DJ, van Muijen GN. Cancer/testis-associated genes: identification, expression profile, and putative function. J Cell Physiol. 2003;194:272–288. doi: 10.1002/jcp.10215. [DOI] [PubMed] [Google Scholar]
- 14.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 15.Valmori D, Dutoit V, Liénard D, Rimoldi D, Pittet MJ, Champagne P, Ellefsen K, Sahin U, Speiser D, Lejeune F, Cerottini JC, Romero P. Naturally occurring human lymphocyte antigen-A2 restricted CD8+ T-cell response to the cancer testis antigen NY-ESO-1 in melanoma patients. Cancer Res. 2000;60:4499–4506. [PubMed] [Google Scholar]
- 16.Rescigno M, Avogadri F, Curigliano G. Challenges and prospects of immunotherapy as cancer treatment. Biochim Biophys Acta. 2007;1776:108–123. doi: 10.1016/j.bbcan.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Copier J, Dalgleish AG, Britten CM, Finke LH, Gaudernack G, Gnjatic S, Kallen K, Kiessling R, Schuessler-Lenz M, Singh H, Talmadge J, Zwierzina H, Håkansson L. Improving the efficacy of cancer immunotherapy. Eur J Cancer. 2009;45:1424–1431. doi: 10.1016/j.ejca.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Romero P. Current state of vaccine therapies in non-small-cell lung cancer. Clin Lung Cancer. 2008;9(Suppl 1):S28–S36. doi: 10.3816/clc.2008.s.005. [DOI] [PubMed] [Google Scholar]
- 19.Singh R, Bandyopadhyay D. MUC1: a target molecule for cancer therapy. Cancer Biol Ther. 2007;6:481–486. doi: 10.4161/cbt.6.4.4201. [DOI] [PubMed] [Google Scholar]
- 20.Savage PA, Vosseller K, Kang C, Larimore K, Riedel E, Wojnoonski K, Jungbluth AA, Allison JP. Recognition of a ubiquitous self antigen by prostate cancer-infiltrating CD8+ T lymphocytes. Science. 2008;319:215–220. doi: 10.1126/science.1148886. [DOI] [PubMed] [Google Scholar]
- 21.Hofmann O, Caballero OL, Stevenson BJ, Chen YT, Cohen T, Chua R, Maher CA, Panji S, Schaefer U, Kruger A, Lehvaslaiho M, Carninci P, Hayashizaki Y, Jongeneel CV, Simpson AJ, Old LJ, Hide W. Genome-wide analysis of cancer/testis gene expression. Proc Natl Acad Sci U S A. 2008;105:20422–20427. doi: 10.1073/pnas.0810777105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bredenbeck A, Hollstein VM, Trefzer U, Sterry W, Walden P, Losch FO. Coordinated expression of clustered cancer/testis genes encoded in a large inverted repeat DNA structure. Gene. 2008;415:68–73. doi: 10.1016/j.gene.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Milano F, Rygiel AM, Buttar N, Bergman JJ, Sondermeijer C, van Baal JW, ten Brinke A, Kapsenberg M, van Ham SM, Peppelenbosch MP, Krishnadath KK. An ex vivo readout for evaluation of dendritic cell-induced autologous cytotoxic T lymphocyte responses against esophageal cancer. Cancer Immunol Immunother. 2007;56:1967–1977. doi: 10.1007/s00262-007-0341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber J, Salgaller M, Samid D, Johnson B, Herlyn M, Lassam N, Treisman J, Rosenberg SA. Expression of the MAGE-1 tumor antigen is up-regulated by the demethylating agent 5-aza-2'-deoxycytidine. Cancer Res. 1994;54:1766–1771. [PubMed] [Google Scholar]
- 25.Adair SJ, Hogan KT. Treatment of ovarian cancer cell lines with 5-aza-2'-deoxycytidine upregulates the expression of cancer-testis antigens and class I major histocompatibility complex-encoded molecules. Cancer Immunol Immunother. 2009;58:589–601. doi: 10.1007/s00262-008-0582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serrano A, Garcia A, Abril E, Garrido F, Ruiz-Cabello F. Methylated CpG points identified within MAGE-1 promoter are involved in gene repression. Int J Cancer. 1996;68:464–470. doi: 10.1002/(SICI)1097-0215(19961115)68:4<464::AID-IJC11>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Zhang Y, Ramsahoye B, Bowen D, Lim SH. Sp17 gene expression in myeloma cells is regulated by promoter methylation. Br J Cancer. 2004;91:1597–1603. doi: 10.1038/sj.bjc.6602160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang H, Goldberg E. Homo sapiens lactate dehydrogenase c (Ldhc) gene expression in cancer cells is regulated by transcription factor Sp1, CREB, and CpG island methylation. J Androl. 2009;30:157–167. doi: 10.2164/jandrol.108.005785. [DOI] [PubMed] [Google Scholar]
- 29.Woloszynska-Read A, James SR, Link PA, Yu J, Odunsi K, Karpf AR. DNA methylation-dependent regulation of BORIS/CTCFL expression in ovarian cancer. Cancer Immun. 2007;7:21. http://www.cancerimmunity.org/v7p21/071122.htm [PMC free article] [PubMed] [Google Scholar]
- 30.Wischnewski F, Pantel K, Schwarzenbach H. Promoter demethylation and histone acetylation mediate gene expression of MAGE-A1, -A2, -A3, and -A12 in human cancer cells. Mol Cancer Res. 2006;4:339–349. doi: 10.1158/1541-7786.MCR-05-0229. [DOI] [PubMed] [Google Scholar]
- 31.Yao X, Hu JF, Li T, Yang Y, Sun Z, Ulaner GA, Vu TH, Hoffman AR. Epigenetic regulation of the taxol resistance-associated gene TRAG-3 in human tumors. Cancer Genet Cytogenet. 2004;151:1–13. doi: 10.1016/j.cancergencyto.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 32.Cho B, Lee H, Jeong S, Bang YJ, Lee HJ, Hwang KS, Kim HY, Lee YS, Kang GH, Jeoung DI. Promoter hypomethylation of a novel cancer/testis antigen gene CAGE is correlated with its aberrant expression and is seen in premalignant stage of gastric carcinoma. Biochem Biophys Res Commun. 2003;307:52–63. doi: 10.1016/s0006-291x(03)01121-5. [DOI] [PubMed] [Google Scholar]
- 33.Ghoshal K, Bai S. DNA methyltransferases as targets for cancer therapy. Drugs Today (Barc) 2007;43:395–422. doi: 10.1358/dot.2007.43.6.1062666. [DOI] [PubMed] [Google Scholar]
- 34.Oki Y, Issa JP. Review: recent clinical trials in epigenetic therapy. Rev Recent Clin Trials. 2006;1:169–182. doi: 10.2174/157488706776876490. [DOI] [PubMed] [Google Scholar]
- 35.Coral S, Sigalotti L, Covre A, Nicolay HJ, Natali PG, Maio M. 5-AZA-2'-deoxycytidine in cancer immunotherapy: a mouse to man story. Cancer Res. 2007;67:2900–2901. doi: 10.1158/0008-5472.CAN-06-2986. [DOI] [PubMed] [Google Scholar]
Materials and methods
Patients
Between July 2007 and September 2008, twenty patients with a suspicion of esophageal squamous cell carcinoma were referred to the Department of Gastroenterology and Hepatology of the Academic Medical Center (AMC) for investigation by upper gastrointestinal endoscopy. The study was approved by the Medical Ethics Committee of the AMC. All patients gave informed consent with written permission for the study. Patients underwent endoscopic ultrasonography for tumor and local node stage classification and biopsies were taken for confirmation of the diagnosis. All the procedures were performed by one endoscopist/investigator. During the procedure, 4 biopsies were taken for the study: 2 from normal tissue and 2 from the tumor. At least one matching biopsy, taken from the same spot of the study biopsies, was collected for histopathological diagnosis. This so-called 'correlating biopsy' served as a control for the diagnosis and presence of cancerous tissue in the study biopsies. Out of the twenty patients biopsied, sixteen were included. Two patients did not show cancerous tissue in the correlating biopsy, one had adenocarcinoma of the esophagus, and one biopsy had an insufficient yield of RNA to perform the analysis. Of the sixteen patients four were females, mean age was 61 (range 53 to 75). At endoscopy, two patients had cancer reaching the muscularis propriae (T2), the other fourteen had cancers beyond the muscularis propriae and outer esophageal wall but without invasion of the neighboring organs (T3). All patients had lymph node metastasis (N1). Details are given in Table 1.
Preparation of RNA and cDNA
Total human RNA was purchased from Stratagene (MVP Total RNA, Human Skin #540031; the distributor in Denmark is A.H. Diagnostics, Aarhus, Denmark) and Ambion (Human Lung Total RNA #AM7968, Human Esophagus Total RNA #6842; Austin TX, USA). RNA was isolated from cell lines and esophagus tissue biopsies using the Qiagen RNeasy mini kit (Qiagen AB, Solna, Sweden) with DNAse treatment in solution. Total RNAs purchased from Stratagene and Ambion were also treated with DNase and re-purified using the Qiagen RNeasy mini kit. Cell culture samples (approximately 2 x 106 cells) were lysed in Qiagen lysis buffer (RLT) and stored at -80˚C until purification was performed. Esophagus tissue biopsies (20 mg - 30 mg) were placed in RNAlater (Ambion) reagent overnight at 4˚C then stored at -80˚C until RNA was purified. Tissue biopsies were frozen in liquid nitrogen and homogenized with a mini-pestle in a microfuge tube while frozen and, when thawed, in the presence of Qiagen RLT lysis buffer. Homogenized tissue lysate was then treated by centrifuging with a QIAshredder column to remove tissue debris and the sample further homogenized before proceeding with the Qiagen RNeasy purification. Total RNA was quantified using a UV spectrophotometer or by using Ribogreen reagent (Invitrogen, Taastrup, Denmark) with a RNA standard curve. Complementary DNA (cDNA) was synthesized from total RNA using Superscript III reverse transcriptase (Invitrogen) as per the manufacturer's protocol. Each 25 µl reaction contained 1 µg total RNA, 400 nM oligo dT24, and 400 nM random hexamer. Reverse transcription reactions were diluted with 100 µl H2O and stored at -20˚C.
Quantitative PCR
Quantitative PCR was performed using a Stratagene Mx3000P instrument and the data analyzed with MxPro software. Individual reactions contained 10 µl Brilliant SYBR Green Master Mix (Stratagene), 2 µl cDNA (equivalent to 16 ng total RNA), oligonucleotides at a final concentration of 500 nM and H2O for a total volume of 20 µl. The thermo cycles were as follows: 1 cycle of 10 min at 95˚C; 40 cycles of 30 s at 95˚C, 60 s at 56˚C, 30 s at 72˚C, and 1 dissociation cycle of 30 s at 95˚C, 30 s at 55˚C, slow ramp to 95˚C. All QPCR runs included no reverse transcriptase control reactions and a dissociation analysis of the final products in order to confirm the formation of specific PCR products from mRNA. PCR products amplified from testis RNA were analyzed by agarose gel electrophoresis to confirm that the PCR products were the correct length (data not shown). QPCR reactions were performed in duplicate for each sample. RT-QPCR was used to detect the presence of 96 tumor antigen genes using 74 reactions that amplify sequences from 153 transcripts. Transcripts amplified and PCR primers used can be found in Supplementary Table 2. Relative gene expression was determined by comparing gene expression to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression. In each QPCR reaction run a GAPDH standard curve was made by 1,000-fold serial dilution (10x at each point) and the PCR efficiency was determined. The PCR efficiency was then used to calculate the abundance of cDNA for each gene relative to GAPDH. We included QPCR analysis of an additional two housekeeping genes, TATA box-binding protein (TBP) and hydroxymethylbilane synthase (HMBS), in order to ensure that GAPDH expression is constant in all samples. The relative expression value for the three control genes is shown in Supplementary Figure 2 and Supplementary Figure 3. These results indicate that the relative expression of GAPDH to the other control genes is fairly constant between different samples. Furthermore, analysis of the same sample on different days (compare TBP1, TBP2, and TBP3 in Supplementary Figure 2) indicates that the QPCR method is reproducible.
Western blot
Western blotting was performed using PAGEgel precast 10% gels and PAGEgel low molecular weight running buffer and transfer buffer (PAGEgel, San Diego, CA, USA). Gels were transferred to a nitrocellulose membrane and blocked with 2% ECL advance blocking agent (GE/Amersham, Hillerød, Denmark) for 1 hour at room temperature. Blots were incubated with MAGE-A 6C1 monoclonal antibody (Santa Cruz Biotech, distributed by Tebu-Bio, Roskilde, Denmark) at 1/100 dilution overnight at 4˚C, followed by goat anti-mouse HRP secondary antibody (GE/Amersham) at 1/5000 for 1 hour at room temperature. The blots were then washed extensively and visualized by using ECLplus reagent (GE/Amersham) and a Typhoon scanner to detect a chemifluorescence signal. The MAGE-A standard curve was generated using purified recombinant MAGE-A fusion protein (Santa Cruz Biotech).
Cell culture
DDM1.7 melanoma cells were grown in RPMI media supplemented with glutamine and 2% human serum at 37˚C and 5% CO2. Cells were treated with 1 µM 5-aza-CdR (prepared at 10 mM in sterile H2O, Sigma, Brøndby, Denmark) for 72 h.
Supplemental data
Download from http://www.cancerimmunity.org/v9p9/090909_suppl_fig1.pdf (311 KB PDF file).
Download from http://www.cancerimmunity.org/v9p9/090909_suppl_fig2.pdf (319 KB PDF file).
Download from http://www.cancerimmunity.org/v9p9/090909_suppl_fig3.pdf (470 KB PDF file).
Download from http://www.cancerimmunity.org/v9p9/090909_suppl_tab1.pdf (254 KB PDF file).
Download from http://www.cancerimmunity.org/v9p9/090909_suppl_tab2.pdf (221 KB PDF file).
Download from http://www.cancerimmunity.org/v9p9/090909_suppl_data.pdf (1.4 MB PDF file).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Download from http://www.cancerimmunity.org/v9p9/090909_suppl_fig1.pdf (311 KB PDF file).
Download from http://www.cancerimmunity.org/v9p9/090909_suppl_fig2.pdf (319 KB PDF file).
Download from http://www.cancerimmunity.org/v9p9/090909_suppl_fig3.pdf (470 KB PDF file).
Download from http://www.cancerimmunity.org/v9p9/090909_suppl_tab1.pdf (254 KB PDF file).
Download from http://www.cancerimmunity.org/v9p9/090909_suppl_tab2.pdf (221 KB PDF file).
Download from http://www.cancerimmunity.org/v9p9/090909_suppl_data.pdf (1.4 MB PDF file).