Abstract
Estradiol protects against brain injury, neurodegeneration, and cognitive decline. Our previous work demonstrates that physiological levels of estradiol protect against stroke injury and that this protection may be mediated through receptor-dependent alterations of gene expression. In this report, we tested the hypothesis that estrogen receptors play a pivotal role in mediating neuroprotective actions of estradiol and dissected the potential biological roles of each estrogen receptor (ER) subtype, ERα and ERβ, in the injured brain. To investigate and delineate these mechanisms, we used ERα-knockout (ERαKO) and ERβ-knockout (ERβKO) mice in an animal model of stroke. We performed our studies by using a controlled endocrine paradigm, because endogenous levels of estradiol differ dramatically among ERαKO, ERβKO, and wild-type mice. We ovariectomized ERαKO, ERβKO, and the respective wild-type mice and implanted them with capsules filled with oil (vehicle) or a dose of 17β-estradiol that produces physiological hormone levels in serum. One week later, mice underwent ischemia. Our results demonstrate that deletion of ERα completely abolishes the protective actions of estradiol in all regions of the brain; whereas the ability of estradiol to protect against brain injury is totally preserved in the absence of ERβ. Thus, our results clearly establish that the ERα subtype is a critical mechanistic link in mediating the protective effects of physiological levels of estradiol in brain injury. Our discovery that ERα mediates protection of the brain carries far-reaching implications for the selective targeting of ERs in the treatment and prevention of neural dysfunction associated with normal aging or brain injury.
Menopause marks the end of female reproduction and is accompanied by a dramatic and permanent decrease in estrogen levels. Although the life span of women has increased significantly in the past century, the average age of menopause has remained constant. Consequently, women may now spend more than one-third of their lives in a chronic hypoestrogenic postmenopausal state. Because estradiol is an important trophic and protective factor in the adult brain (1, 2), hypoestrogenic postmenopausal women may be more vulnerable to brain injury and dysfunction caused by neurodegenerative conditions and cognitive decline. It is, therefore, crucial that we gain a complete understanding of the mechanisms underlying the neuroprotective actions of estradiol.
A growing body of evidence has begun to reveal that estrogen replacement therapy may ameliorate neural dysfunctions resulting from Alzheimer's disease (3–5) and stroke (6, 7) through multiple and complex cellular and molecular mechanisms of action. The protective role of estrogen in brain function has been examined by using a variety of in vivo and in vitro models of brain injury that mimic neurotoxic environments found in Alzheimer's disease, stroke, and other neurodegenerative conditions (8–13). These studies demonstrate that physiological and pharmacological concentrations of 17β-estradiol, the predominant and most biologically active estrogen secreted by the ovary, profoundly attenuate the extent of injury and decrease cell death in the brain.
Using in vivo and in vitro methods, our laboratory has focused on the mechanisms by which physiological levels of estradiol protect the brain against stroke injury and, specifically, whether estrogen receptors (ERs) are essential to neuroprotection. After the discovery that two ER subtypes, ERα and ERβ, exist throughout the body (14), we began to probe the potential roles of these receptors in the brain. We found that the neuroprotective effects of estradiol are not rapid and require a period of pretreatment, suggesting that ER-mediated alteration of gene expression is required to afford neuroprotection (8, 12). Indeed, our initial studies revealed that in response to injury estradiol modulates critical factors including bcl-2 (15), galanin (16), and immediate early genes (17). Furthermore, we found that ERs are differentially modulated in neurodegenerative injury. After injury, ERα mRNA is highly up-regulated in the presence and absence of estradiol. In contrast, injury down-regulates ERβ mRNA and estradiol prevents this injury-induced down-regulation (15). Finally, our in vitro studies established that 17α-estradiol, an estrogen isomer that has 100-fold less affinity for ERs, fails to protect against injury (12). In addition, these in vitro studies demonstrated that the protective actions of estradiol are blocked by ICI 182,780, an ER antagonist (12). Thus, these findings strongly implicate that estradiol acts through its receptor(s) in unique and novel ways in the injured brain.
The goal of this study was to test directly and dissect out the specific biological roles of ER subtypes, ERα and ERβ, in estradiol-mediated protection against brain injury. We used ERα-knockout (ERαKO) and ERβ-knockout (ERβKO) mice in an animal model of stroke. Our results show that ERα, but not ERβ, is the critical mechanistic link that mediates the ability of physiological levels of estradiol to protect the brain against injury.
Materials and Methods
All surgical procedures were performed in compliance with the National Institutes of Health Guide and have been approved by the University of Kentucky, Chandler Medical Center, Institutional Animal Care and Use Committee.
Animals.
The two strains of transgenic mice that were used in this study were obtained from Wyeth Ayerst Laboratories. Each transgenic strain was compared with its respective genetically matched wild-type strain. Homozygous ERαKO (C57BL/6J × 129) (18) and homozygous 129Sv ERβKO mice were bred and genotyped by PCR analysis of tail samples to assess the presence of the neomycin resistance, ERα, and/or ERβ mRNAs. Briefly, ERβKO mice were generated as follows: A targeting vector was used in which a 2-kb fragment of exons 1 and 2 was replaced by a cassette including translational stop codons in all three reading frames, followed by a LoxP-flanked neomycin-resistance gene. This construct forces a translational block after the 19th amino acid. The construct was electroporated into R1 embryonic stem cells and neomycin-resistant clones were screened for homologous recombinants by Southern blot analysis. Two gene-targeted embryonic stem cell clones were identified, and each clone was expanded and used to generate germ-line chimeras by blast injection into C57BL/6 host blasts. The mice have been fully characterized to confirm the disruption of ERβ by a variety of molecular and histological techniques (P.J.S., G. R. Askew, and I.M., unpublished results).
Hormone Replacement.
Young mice (19–22 g) were ovariectomized to eliminate endogenous ovarian steroids and then implanted s.c. with a Silastic capsule [0.062 in/0.125 in, inner/outer diameter (1 in = 2.54 cm); volume, 0.035 ml] containing sesame oil (vehicle) or 17β-estradiol (180 μg/ml; n = 8–13 per group). The Silastic capsules consistently release hormone over time, producing stable levels of 17β-estradiol in serum (19).
In Vivo Cerebral Ischemia.
One week after ovariectomy and estradiol or vehicle treatment, mice were anesthetized with a mixture of chloral hydrate (350.0 mg/kg i.p.) and xylazine (4.0 mg/kg i.p.). Permanent cerebral ischemia was induced by a method modified from Huang et al. (20). Briefly, a 5/0 size blue nylon suture was fired at the tip and inserted into the external carotid artery. From the external carotid artery, it was advanced 11 mm into internal carotid artery, where it effectively occluded the anterior cerebral artery in the circle of Willis. In all mice, body temperature was maintained at normothermia until recovery from anesthesia.
Histological Preparation.
Brains were collected 24 h after the onset of ischemia, frozen, and sectioned into 16-μm sections. Coronal sections were stained with hematoxylin and eosin to clearly delineate the extent of ischemic injury. The volume of infarct was calculated by integrating the area of injury on 16-μm stained coronal sections collected from the bregma points +2.46, +1.66, +0.86, +0.06, −0.74, −1.54, −2.34, and −3.14 mm of each brain (21). Total, cortical, striatal, and hippocampal infarct volumes were quantified with a computer-assisted imaging system (nih image, Version 1.6).
Blood Flow Measurements.
A laser Doppler probe was positioned through a craniotomy at 2.5–3.0 mm posterior to bregma and 2.0 mm lateral to the sagittal suture over the right parietal cortex (21), an area that is affected by cerebral ischemia and is protected by estradiol treatment. Baseline measurements were obtained every minute for 10 min. Ischemic laser Doppler measurements began 10 min after the onset of vascular occlusion and measurements were taken every minute for 30 min.
17β-Estradiol Assay.
In a pilot study, we determined the serum concentrations produced in the mice by our physiological paradigm of estradiol replacement. Mice were ovariectomized and implanted s.c. with a Silastic capsule containing sesame oil (vehicle) or 17β-estradiol (180 μg/ml) (n = 4 per group). One week after ovariectomy and treatment, we collected arterial blood from mice and froze the sera until the time of assay. Samples (250 μl) were extracted with ether and then analyzed for levels of 17β-estradiol by radioimmunoassay.
Data Analyses.
All data are expressed as mean ± SEM. Infarct volumes and baseline laser Doppler measurements were analyzed by using a two-way analysis of variance (ANOVA). Post hoc analyses were carried out with Newman–Keuls test. Ischemic laser Doppler measurements were analyzed by using a mixed-factorial ANOVA. All differences were considered significant at P < 0.05.
Results
Effects of Estradiol in Stroke Injury.
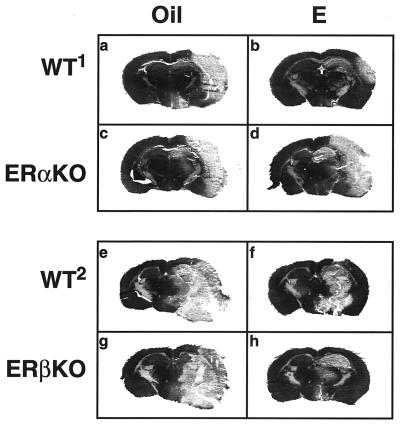
The extent of brain injury produced by permanent cerebral ischemia, an animal model of stroke, is clearly delineated by staining and is distributed throughout the right hemisphere of ERαKO, ERβKO, and the respective wild-type mice (Fig. 1 a–h). These representative brain sections clearly demonstrate that without estradiol, ischemic injury, or infarct, is extensive in all mice (Fig. 1 a, c, e, and g). Physiological levels of estradiol greatly reduce the extent of infarct in both wild-type mice (Fig. 1 b and f) and in ERβKO mice (Fig. 1h) but not in ERαKO mice (Fig. 1d). The volume of infarct, which includes significant portions of the cerebral cortex, striatum, and hippocampus, was analyzed by computer-assisted imaging.
Figure 1.
Representative brain sections from oil- and estradiol-treated wild-type, ERαKO, and ERβKO mice that underwent permanent cerebral ischemia of the right hemisphere. In the absence of estradiol, injury is extensive in all mice (a, c, e, and g). Estradiol reduces the extent of infarct in wild-type mice of both genetic backgrounds (b and f) and in ERβKO mice (h), but not in ERαKO mice (d). The volume of infarct includes significant portions of the cerebral cortex, striatum, and hippocampus. Each brain section is a 16-μm coronal slice obtained from bregma −1.54 mm.
Estradiol-Mediated Protection Against Total Brain Injury Is Abolished in the Absence of ERα.
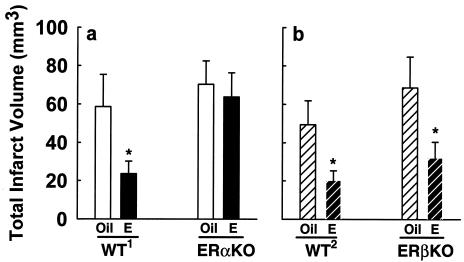
We analyzed total ischemic injury and found that the ability of physiological levels of estradiol to exert neuroprotection is completely different in wild-type and ERβKO mice than in ERαKO mice. In both wild-type backgrounds, estradiol decreases total ischemic injury by more than 50% compared with respective oil-treated controls (Fig. 2). In ERαKO mice, estradiol fails to exert any protective effect, and infarct volume is equally extensive in ovariectomized mice with oil- or estradiol-replacement (Fig. 2a). In marked contrast, in ERβKO mice, estradiol continues to exert profound protective effects against brain injury. Estradiol-induced protection in ERβKO mice is identical to its effects in wild-type mice: total ischemic injury in estradiol-treated ERβKO mice is decreased by more than 50% compared with oil-treated controls (Fig. 2b). Thus, the deletion of ERα totally abolishes the profound protection afforded by estradiol in the brain, whereas deletion of ERβ does not diminish the ability of estradiol to protect the brain against injury.
Figure 2.
Estradiol protects against total ischemic brain injury in wild-type mice of both genetic backgrounds and ERβKO mice, but not in ERαKO mice. (a) Estradiol (n = 9) significantly decreases total infarct volume in C57BL/6J × 129 wild-type mice (WT1), compared with oil-treated controls (n = 7) (*, P < 0.04). In contrast, in ERαKO mice, estradiol (n = 13) does not exert any protective effect on total infarct volume, compared with oil-treated controls (n = 13) (P = 0.48). (b) Estradiol (n = 6) significantly decreases total infarct volume in 129Sv wild-type mice (WT2), compared with oil-treated controls (n = 10) (*, P < 0.02). In ERβKO mice, estradiol (n = 8) significantly decreases total infarct, compared with oil-treated controls (n = 9) (*, P < 0.02). Values represent the mean ± SEM.
ERα Is Critical in All Brain Regions Protected by Estradiol.
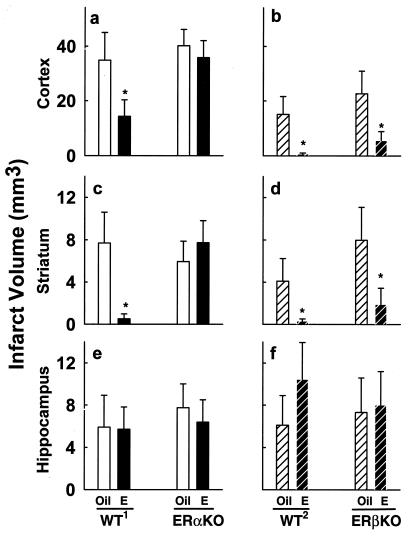
We further analyzed total infarct volume and compared treatment effects in cortex, striatum, and hippocampus to determine whether the biological role of ERα in estradiol-mediated neuroprotection is region-specific. Estradiol treatment significantly reduces cortical and striatal infarct volumes in both wild-type backgrounds and in ERβKO mice (Fig. 3a–d). Protection in these regions is truly profound because estradiol induces decreases of 59%–95% in wild-type mice and in ERβKO mice. In contrast, estradiol fails to protect cortex or striatum in ERαKO mice (Fig. 3a–d). Hippocampal injury is equivalent in all ERαKO, ERβKO, and wild-type brains, because we did not detect any protective effect of estradiol in this region (Fig. 3 e and f). Thus, in this model of cerebral ischemia, estradiol exerts profound neuroprotective effects in the cerebral cortex and striatum of wild-type and ERβKO mice, but not in ERαKO mice. ERα plays a critical role throughout the brain in all regions where we observe estradiol-mediated neuroprotection.
Figure 3.
ERα is critical in brain regions protected by estradiol. Effects of estradiol in cerebral ischemia are region-specific in wild-type mice of both genetic backgrounds, ERαKO, and ERβKO mice. (a) Estradiol (n = 9) significantly reduces cortical infarct volume in wild-type mice (WT1), compared with oil-treated controls (n = 7) (*, P < 0.02). In contrast, estradiol (n = 13) fails to protect the cerebral cortex in ERαKO mice, compared with oil-treated controls (n = 13) (P = 0.56). (b) Estradiol (n = 6) significantly reduces cortical infarct volume in wild-type mice (WT2), compared with oil-treated controls (n = 10) (*, P < 0.02). In parallel to its effects in wild-type mice, estradiol (n = 8) significantly reduces cortical infarct volume in ERβKO mice, compared with oil-treated controls (n = 9) (*, P < 0.02). (c) Estradiol significantly reduces striatal infarct volume in WT1, compared with oil-treated controls (*, P < 0.05), but fails to protect the striatum in ERαKO mice (P = 0.81). (d) Estradiol significantly reduces striatal infarct volume in both WT2 mice and in ERβKO mice, compared with respective oil-treated controls (*, P < 0.04). (e) Estradiol does not protect against hippocampal injury in WT1 or in ERαKO mice, compared with respective oil-treated controls (P = 0.68). (f) Likewise, estradiol does not protect against hippocampal injury in WT2 or in ERβKO mice, compared with respective oil-treated controls (P = 0.441). Values represent mean ± SEM.
Estradiol-Mediated Protection via ERα Is Independent of Blood Flow.
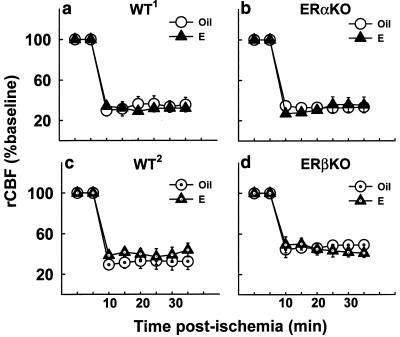
We monitored baseline and ischemic regional cerebral blood flow (rCBF) in ERαKO, ERβKO, and the respective wild-type mice by laser Doppler flowmetry over the right parietal cortex. Estradiol does not alter the baseline, or preischemic, levels of cortical perfusion in ERαKO, ERβKO, or wild-type mice, compared with the respective oil-treated controls (Table 1). Furthermore, rCBF after the onset of ischemia does not differ between oil- and estradiol-treated mice (Fig. 4) in any of the experimental groups. Thus, estradiol's protective effects in wild-type and ERβKO mice cannot be explained by differences in basal regional cerebral blood flow or by differences in the extent of circulatory compromise after permanent cerebral ischemia. ERα plays a crucial role in neuroprotection via mechanisms that are likely independent of blood flow.
Table 1.
Baseline rCBF does not differ between oil- and estradiol-treated mice
| Mice | Oil | E |
|---|---|---|
| WT1 | 12.03 ± 0.47 | 12.08 ± 0.55 |
| ERαKO | 13.18 ± 0.41* | 13.96 ± 0.62* |
| WT2 | 12.52 ± 1.03 | 12.34 ± 1.24 |
| ERβKO | 11.91 ± 0.62 | 11.89 ± 0.16 |
Baseline rCBF is slightly elevated in ERαKO compared with wild-type1 (WT1) mice (*, P < 0.02). However, estradiol (E) does not alter baseline rCBF compared with oil-treated mice in any of the experimental groups (n = 3–4 per group). Values represent the mean ± SEM.
Figure 4.
rCBF during permanent cerebral ischemia does not differ between oil- and estradiol-treated mice. Ischemia significantly reduces cortical perfusion in oil- and estradiol-treated, WT1 (a) and ERαKO mice (b) (n = 3 per group) (P < 0.001). Likewise, ischemia significantly reduces cortical perfusion in oil- and estradiol-treated, WT2 (c) and ERβKO (d) mice (n = 3 or 4 per group) (P < 0.001). Estradiol does not affect the extent of the decrease in regional cortical flow in any of the mice (a–d). Ischemic laser Doppler measurements began 10 min after the onset of vascular occlusion and were taken every minute. Each data point represents a 5-min average and is represented as a percentage of baseline. Values represent mean ± SEM.
Estradiol Replacement Therapy Achieves Physiological Levels of Estradiol in Serum.
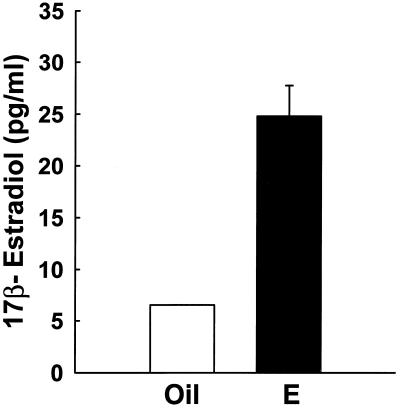
We measured concentrations of 17β-estradiol in serum to assure that our controlled endocrine paradigm of hormone replacement achieves equivalent and physiological levels of 17β-estradiol in mice. This is critical because endogenous levels of this hormone are dramatically different in ERαKO (18) versus wild-type (18, 22) and ERβ-knockout (23) mice. Our paradigm of physiological estradiol replacement in ovariectomized mice produces serum levels of approximately 25 pg/ml (Fig. 5), a level that is equivalent to basal circulating levels in the mouse estrous cycle (22).
Figure 5.
Estradiol replacement in ovariectomized mice achieves physiological levels of 17β-estradiol in serum. Physiological estradiol replacement in mice (n = 4 per group) produces serum levels that are equivalent to basal circulating levels in the mouse estrous cycle. The low range of sensitivity of the radioimmunoassay was determined to be approximately 5.0 pg/ml. Values represent mean ± SEM.
Discussion
The results of this study clearly establish the pivotal mechanistic role of ERs in estradiol-mediated protection against brain injury. We demonstrate that ERα, and not ERβ, is a critical link that determines the ability of physiological levels of estradiol to exert neuroprotection. These findings uncover fundamental molecular mechanisms by which low levels of estradiol protect the brain against injury, neurodegeneration, and possibly cognitive decline. Therefore, these results carry far-reaching implications for the selective targeting of ERs in the treatment and prevention of neural dysfunction associated with normal aging or brain injury.
We have uncovered a critical role for ERα in estradiol-mediated protection of the brain. Our data demonstrate that estradiol exerts profound protective actions in the brains of wild-type and ERβKO mice but fails to protect in ERαKO mice. Physiological levels of estradiol dramatically decrease stroke infarct volumes in the cerebral cortex and striatum in wild-type mice of two genetic backgrounds and in ERβKO mice. In marked contrast, the deletion of ERα totally abolishes the ability of estradiol to protect in any region of the brain. Interestingly, no hippocampal protection by estradiol was observed. We speculate that the lack of estradiol-mediated neuroprotection in this brain region may be due to severely compromised and irreversibly injured tissue in this area.
Our findings elucidate a new functional role for ERα in brain regions of the adult that were not previously thought to express the receptor (24). Importantly, this biological role for ERα extends and complements our recent finding that ERα expression is dramatically up-regulated in regions of the adult brain that are protected by estradiol (15). The induction of ERs after brain injury is specific to only the ERα subtype and not the ERβ subtype (15). Interestingly, the dramatic increase in ERα expression during injury is reminiscent of its expression during early postnatal development of the brain (25, 26), a time of extensive neurogenesis and differentiation. Therefore, perhaps, the ischemia-induced reexpression of ERα may allow recapitulation of the developmental actions of estradiol.
We performed these studies with a controlled endocrine paradigm of hormone replacement because endogenous levels of estradiol are dramatically different in ERαKO versus wild-type or ERβ-KO mice. Because of the lack of negative feedback at the level of the hypothalamus and pituitary in ERαKO mice, estradiol synthesis and secretion increases exponentially. This increase leads to serum levels that are 10- to 15-fold higher in ERαKO mice (18) than normally circulate in wild-type (18, 22) or ERβ-knockout mice (23). These chronically elevated concentrations of estradiol are considered pharmacological. Because physiological and pharmacological levels of estradiol may act through different mechanisms (1), it was critical that animals were exposed to equivalent concentrations of estradiol. To achieve equivalent levels of estradiol replacement, ERαKO, ERβKO, and their respective wild-type littermates were ovariectomized and implanted with Silastic capsules filled with oil (vehicle) or a low dose of 17β-estradiol. This dose in capsules produces serum estradiol concentrations of 25 pg/ml in mice, a level of hormone that is equivalent to basal circulating levels in the mouse estrous cycle (18, 22). We used two groups of wild-type mice because the genetic backgrounds of the ERαKO and ERβKO mice were different, and could influence the extent of injury and the effects of steroids.
Our results differ from a previous study that compared stroke injury in gonadally intact wild-type and ERα-knockout mice and concluded that protection does not depend on ERα (27). The comparison between wild-type and ERαKO mice that are gonadally intact is difficult to interpret because the estradiol concentrations in ERαKO mice are dramatically higher than in wild-type controls. This is important to consider because the mechanisms by which estradiol exerts protective actions are diverse and depend, in part, on the dose of the steroid. Thus, previous studies suggest that physiological levels of estradiol may protect through receptor-dependent mechanisms (8, 12, 13, 28), whereas higher concentrations of estradiol may act through mechanisms that do not require the presence of ERs (29–36). Hence, the former study further confirms that pharmacological levels of estradiol protect through ER-independent mechanisms, but it does not address the potential role of ERs in mediating the physiological levels of estradiol to protect. In our current study, use of a controlled endocrine paradigm of physiological hormone replacement clearly establishes that estradiol acts through ERα to exert neuroprotection.
Our results demonstrate that estradiol protects against brain injury through blood flow-independent mechanisms. Estradiol does not alter baseline, or preischemic, cortical perfusion in wild-type, ERαKO, or ERβKO mice. Likewise, the extent of decrease in regional ischemic flow is equivalent between oil- and estradiol-treated mice in all experimental groups, regardless of genetic background. It is interesting to note that preischemic perfusion is slightly increased in ERαKO mice, in both the presence and the absence of estradiol treatment. These data corroborate previous reports that show that low levels of estradiol do not modulate cerebral blood flow (8, 9, 16, 37). Thus, our current findings indicate that physiological levels of estradiol act via ERα to protect the brain through mechanisms that cannot be explained by differential levels of circulatory compromise.
It is interesting to speculate that the ER knockout mice may differ in target tissue sensitivities to estradiol; however, our data do not hint that this might be the case in our paradigm. We examined our data to determine whether the absence of ERα or ERβ might alter sensitivity to the neuroprotective effects of estradiol. Our data do not reveal an exaggerated protection in brains of ERβKO mice compared with the respective wild-type mice. This result indicates that, in the context of cerebral ischemia, the absence of ERβ does not induce hypersensitivity to estradiol's neuroprotective effects through ERα. Furthermore, in the absence of ERα, no hormone-mediated protection is afforded, indicating that estradiol requires ERα, not ERβ, to protect. Whether ERβ contributes to injury in ERαKO mice is unknown and our data do not allow us to ask this question. Whether altered sensitivity to estradiol exists in other paradigms or brain regions of ER knockout mice remains to be determined.
Our discovery that ERα is a crucial mechanistic component of estradiol-mediated protection highlights emerging roles for this receptor in traditional and novel actions that reach far beyond the reproductive axis and its classical targets. The long-held view that estradiol simply binds to its receptor, causing receptor transactivation, dimerization, DNA binding, and transcription of genes (38) may be too simple. Accumulating evidence shows that ERs may also activate second messenger signaling pathways, such as adenylyl cyclase, phosphoinositol 3-kinase, and/or mitogen-activated protein kinase, or involve cross-talk with growth factor receptors, such as trkA and the IGF-I receptor (13, 39–42). These novel ER-mediated mechanisms may lead to altered gene expression downstream and/or increased phosphorylation of proteins that promote estradiol's actions.
The predominant mechanism of estradiol action may depend on the brain region, the type of neural injury or stimulus, and/or the dose of hormone administered. It should be noted that physiological levels of estradiol generally require receptor-mediated genomic or nongenomic function for neuroprotection. Neuroprotection induced by physiological levels of estradiol often, but not always (11), requires pretreatment, is specific to the 17β-estradiol stereoisomer, and/or can be blocked by ER antagonists (8, 12, 13, 28). In contrast, neuroprotection by pharmacological levels of estradiol appears to protect predominantly through non-ER-mediated antioxidant and/or membrane/channel effects (29–36).
The diverse and multifactorial ER-mediated interactions may induce a variety of cellular responses that promote trophic and protective effects in the brain. Physiological levels of estradiol can enhance the plasticity of synaptic connections (43–47), regulate the expression of neurotrophins and cognate receptors (48–51), and elevate the expression of cell survival factors (15, 52) in the brain. Any or all ER-mediated actions of estradiol that enhance the integrity and plasticity of the brain may ultimately promote neuroprotection through enhanced cellular function, resistance to injury, and/or facilitated regeneration from injury.
In summary, our discovery that the ER subtype ERα is a critical link in the protective actions of physiological concentrations of estradiol in neurodegenerative injury carries far-reaching implications for the selective targeting of ERs in the treatment of disease states, particularly for aging postmenopausal women. Since the recent discovery that two ERs exist in the body (14), biomedical researchers have aggressively investigated the specific biological roles of ERα and ERβ in normal and pathological processes throughout the body (23, 53–57). We have now identified a critical biological role for ERα in the brain. As we continue to gain greater insights into the actions of ER subtypes in different organ systems, we will be better able to design estrogens that selectively elicit protective effects. Our findings suggest that estrogens that specifically target ERα in the brain may promote protective actions in neurodegenerative conditions such as Alzheimer's disease and stroke.
Acknowledgments
This research was supported by a Glenn/American Federation for Aging Research (AFAR) scholarship (D.B.D.), a Merck/AFAR research scholarship (D.B.D.), and the National Institutes of Health: AG12891 (M.S.K.), NS31220 (M.S.K.), and AG02224 and AG17164 (P.M.W.). D.B.D. and S.W.R. are predoctoral trainees on National Institutes of Health Training Grant AG00242 (P.M.W.).
Abbreviations
- ER
estrogen receptor
- ERα
ER subtype α
- ERβ
ER subtype β
- ERαKO
ERα knockout
- ERβKO
ERβ knockout
- rCBF
regional cerebral blood flow
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.041483198.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.041483198
References
- 1.Wise P M, Dubal D B, Wilson M E, Rau S W, Liu Y. Front Neuroendocrinol. 2001;22:33–66. doi: 10.1006/frne.2000.0207. [DOI] [PubMed] [Google Scholar]
- 2.Hurn P D, Macrae I M. J Cereb Blood Flow Metab. 2000;20:631–652. doi: 10.1097/00004647-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Paganini-Hill A, Henderson V W. Am J Epidemiol. 1994;140:256–261. doi: 10.1093/oxfordjournals.aje.a117244. [DOI] [PubMed] [Google Scholar]
- 4.Kawas C, Resnick S, Morrison A, Brookmeyer R, Corrada M, Zonderman A, Bacal C, Lingle D, Metter E. Neurology. 1997;48:1517–1521. doi: 10.1212/wnl.48.6.1517. [DOI] [PubMed] [Google Scholar]
- 5.Paganini-Hill A, Henderson V W. Arch Intern Med. 1996;156:2213–2217. [PubMed] [Google Scholar]
- 6.Paganini-Hill A. Prog Cardiovasc Dis. 1995;38:223–242. doi: 10.1016/s0033-0620(95)80014-x. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt R, Fazekas F, Reinhart B, Kapeller P, Fazekas G, Offenbacher H, Eber B, Schumacher M, Freidl W. J Amer Geriat Soc. 1996;44:1307–1313. doi: 10.1111/j.1532-5415.1996.tb01400.x. [DOI] [PubMed] [Google Scholar]
- 8.Dubal D B, Kashon M L, Pettigrew L C, Ren J M, Finklestein S P, Rau S W, Wise P M. J Cereb Blood Flow Metab. 1998;18:1253–1258. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Rusa R, Alkayed N J, Crain B J, Traystman R J, Kimes A S, London E D, Klaus J A, Hurn P D. Stroke. 1999;30:1665–1670. doi: 10.1161/01.str.30.8.1665. [DOI] [PubMed] [Google Scholar]
- 10.Simpkins J W, Rajakumar G, Zhang Y-Q, Simpkins C E, Greenwald D, Yu C J, Bodor N, Day A L. J Neurosurg. 1997;87:724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- 11.Green P S, Gridley K E, Simpkins J W. Neuroscience. 1998;84:7–10. doi: 10.1016/s0306-4522(97)00595-2. [DOI] [PubMed] [Google Scholar]
- 12.Wilson M E, Dubal D B, Wise P M. Brain Res. 2000;873:235–242. doi: 10.1016/s0006-8993(00)02479-3. [DOI] [PubMed] [Google Scholar]
- 13.Singer C A, Figueroa-Masot C D, Batchelor R H, Dorsa D M. J Neurosci. 1999;19:2455–2463. doi: 10.1523/JNEUROSCI.19-07-02455.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuiper G G J M, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J-A. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubal D B, Shughrue P J, Wilson M E, Merchenthaler I, Wise P M. J Neurosci. 1999;19:6385–6393. doi: 10.1523/JNEUROSCI.19-15-06385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubal D B, Wise P M. Endocrinology. 2001;142:43–48. doi: 10.1210/endo.142.1.7911. [DOI] [PubMed] [Google Scholar]
- 17.Rau S W, Dubal D B, Wise P M. Soc Neurosci Abstr. 2000;26:778. [Google Scholar]
- 18.Couse J F, Curtis S W, Washburn T F, Lindzey J, Golding T S, Lubahn D B, Smithies O, Korach K S. Mol Endocrinol. 1995;9:1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- 19.Wise P M, Camp-Grossman P, Barraclough C A. Biol Reprod. 1981;24:820–830. doi: 10.1095/biolreprod24.4.820. [DOI] [PubMed] [Google Scholar]
- 20.Huang Z, Huang P L, Panahian N, Dalkara T, Fishman M C, Moskowitz M A. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- 21.Franklin K B J, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. New York: Academic; 1997. [Google Scholar]
- 22.Nelson J F, Felicio L S, Osterburg H H, Finch C E. Endocrinology. 1992;130:805–810. doi: 10.1210/endo.130.2.1733727. [DOI] [PubMed] [Google Scholar]
- 23.Couse J F, Korach K S. Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 24.Shughrue P J, Lane M V, Merchenthaler I. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Toran-Allerand C D, Miranda R C, Hochberg R B, MacLusky N J. Brain Res. 1992;576:25–41. doi: 10.1016/0006-8993(92)90606-a. [DOI] [PubMed] [Google Scholar]
- 26.Shughrue P J, Stumpf W E, MacLusky N J, Zielinski J E, Hochberg R B. Endocrinology. 1990;126:1112–1124. doi: 10.1210/endo-126-2-1112. [DOI] [PubMed] [Google Scholar]
- 27.Sampei K, Goto G, Alkayed N J, Crain B J, Korach K S, Traystman R J, Demas G E, Nelson R J, Hurn P D. Stroke (Dallas) 2000;31:738–744. doi: 10.1161/01.str.31.3.738. [DOI] [PubMed] [Google Scholar]
- 28.Singer C A, Rogers K L, Strickland T M, Dorsa D M. Neurosci Lett. 1996;212:13–16. doi: 10.1016/0304-3940(96)12760-9. [DOI] [PubMed] [Google Scholar]
- 29.Culmsee C, Vedder H, Ravati A, Junker V, Otto D, Ahlemeyer B, Krieg J-C, Krieglstein J. J Cereb Blood Flow Metab. 1999;19:1263–1269. doi: 10.1097/00004647-199911000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Regan R F, Guo Y. Brain Res. 1997;764:133–140. doi: 10.1016/s0006-8993(97)00437-x. [DOI] [PubMed] [Google Scholar]
- 31.Mooradian A D. J Steroid Biochem Mol Biol. 1993;45:509–511. doi: 10.1016/0960-0760(93)90166-t. [DOI] [PubMed] [Google Scholar]
- 32.Behl C, Skutella T, Lezoualch F, Post A, Widmann M, Newton C J, Holsboer F. Mol Pharmacol. 1997;51:535–541. [PubMed] [Google Scholar]
- 33.Green P S, Gordon K, Simpkins J W. J Steroid Biochem Moc Biol. 1997;63:229–235. doi: 10.1016/s0960-0760(97)00124-6. [DOI] [PubMed] [Google Scholar]
- 34.Goodman Y, Bruce A J, Cheng B, Mattson M P. J Neurochem. 1996;66:1836–1844. doi: 10.1046/j.1471-4159.1996.66051836.x. [DOI] [PubMed] [Google Scholar]
- 35.Hall E D, Braughler J M. In: in Molecular and Cellular Approaches to the Treatment of Neurological Disease. Waxman S G, editor. New York: Raven; 1993. pp. 81–105. [Google Scholar]
- 36.Green P S, Bishop J, Simpkins J W. J Neurosci. 1997;17:511–515. doi: 10.1523/JNEUROSCI.17-02-00511.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carswell H V, Anderson N H, Morton J J, McCulloch J, Dominiczak A F, Macrae I M. J Cereb Blood Flow Metab. 2000;20:931–936. doi: 10.1097/00004647-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Tsai M-J, O'Malley B. Ann Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 39.Singh M, Setalo G, Jr, Guan X, Warren M, Toran-Allerand C D. J Neurosci. 1999;19:1179–1188. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toran-Allerand C D, Singh M, Setalo G. Front Neuroendocrinol. 1999;20:97–121. doi: 10.1006/frne.1999.0177. [DOI] [PubMed] [Google Scholar]
- 41.Murphy D D, Segal M. Proc Natl Acad Sci USA. 1997;94:1482–1487. doi: 10.1073/pnas.94.4.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou Y, Watters J J, Dorsa D M. Endocrinology. 1996;137:2163–2166. doi: 10.1210/endo.137.5.8612562. [DOI] [PubMed] [Google Scholar]
- 43.McEwen B S, Alves S E, Bulloch K, Weiland N G. Neurology. 1997;48:S8–S15. doi: 10.1212/wnl.48.5_suppl_7.8s. [DOI] [PubMed] [Google Scholar]
- 44.Woolley C S, McEwen B S. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murphy D D, Cole N B, Segal M. Proc Natl Acad Sci, USA. 1998;95:11412–11417. doi: 10.1073/pnas.95.19.11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McEwen B S, Tanapat P, Weiland N G. Endocrinology. 1999;140:1044–1047. doi: 10.1210/endo.140.3.6570. [DOI] [PubMed] [Google Scholar]
- 47.Gould E, Woolley C S, Frankfurt M, McEwen B S. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sohrabji F, Miranda R C, Toran-Allerand C D. J Neurosci. 1994;14:459–471. doi: 10.1523/JNEUROSCI.14-02-00459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh M, Meyer E M, Simpkins J W. Endocrinology. 1995;136:2320–2324. doi: 10.1210/endo.136.5.7720680. [DOI] [PubMed] [Google Scholar]
- 50.McMillan P J, Singer C A, Dorsa D M. J Neurosci. 1996;16:1860–1865. doi: 10.1523/JNEUROSCI.16-05-01860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gibbs R B, Wu D, Hersh L B, Pfaff D W. Exp Neurol. 1994;129:70–80. doi: 10.1006/exnr.1994.1148. [DOI] [PubMed] [Google Scholar]
- 52.Pike C J. J Neurochem. 1999;72:1552–1563. doi: 10.1046/j.1471-4159.1999.721552.x. [DOI] [PubMed] [Google Scholar]
- 53.Weihua Z, Saji S, Makinen S, Cheng G, Jensen E V, Warner M, Gustafsson J A. Proc Natl Acad Sci USA. 2000;97:5936–5941. doi: 10.1073/pnas.97.11.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vidal O, Lindberg M K, Hollberg K, Baylink D J, Andersson G, Lubahn D B, Mohan S, Gustafsson J A, Ohlsson C. Proc Natl Acad Sci USA. 2000;97:5474–5479. doi: 10.1073/pnas.97.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhai P, Eurell T E, Cooke P S, Lubahn D B, Gross D R. Am J Physiol Heart Circ Physiol. 2000;278:H1640–H1647. doi: 10.1152/ajpheart.2000.278.5.H1640. [DOI] [PubMed] [Google Scholar]
- 56.Iafrati M D, Karas R H, Aronovitz M, Kim S, Sullivan T R, Lubahn D B, O'Donnell T F, Jr, Korach K S, Mendelsohn M E. Nat Med. 1997;3:545–548. doi: 10.1038/nm0597-545. [DOI] [PubMed] [Google Scholar]
- 57.Karas R H, Hodgin J B, Kwoun M, Krege J H, Aronovitz M, Mackey W, Gustafsson J A, Korach K S, Smithies O, Mendelsohn M E. Proc Natl Acad Sci USA. 1999;96:15133–15136. doi: 10.1073/pnas.96.26.15133. [DOI] [PMC free article] [PubMed] [Google Scholar]