Abstract
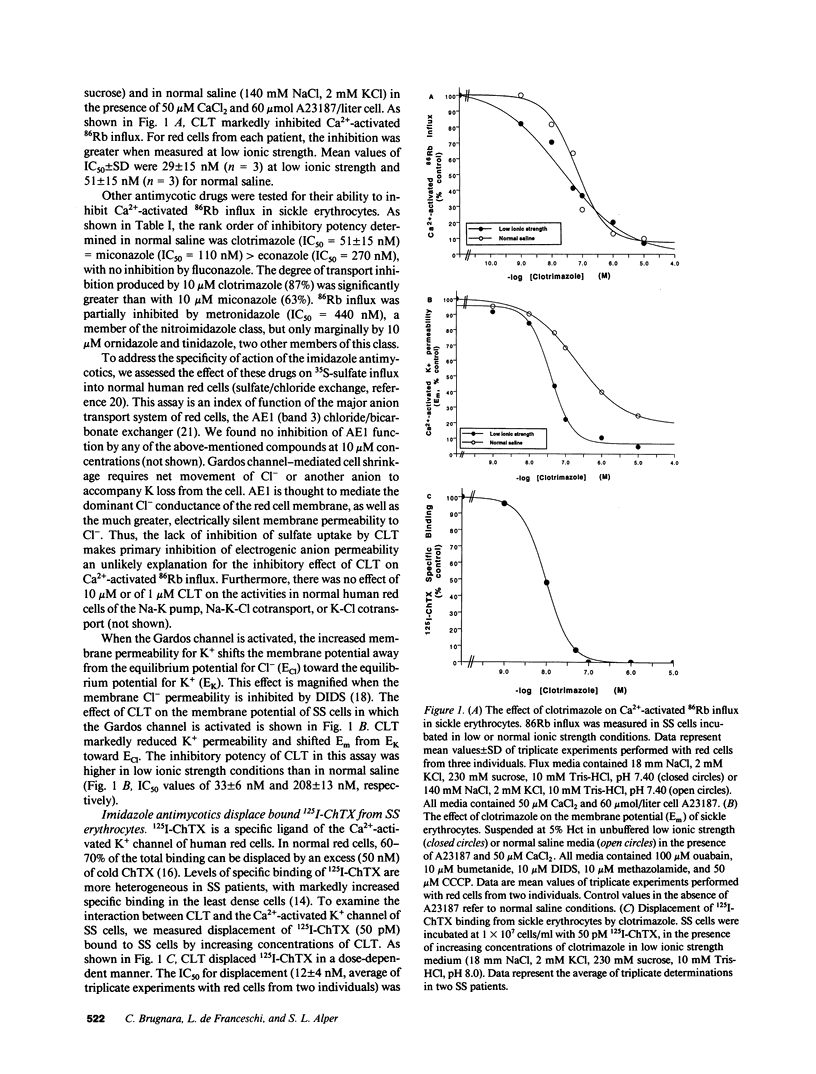
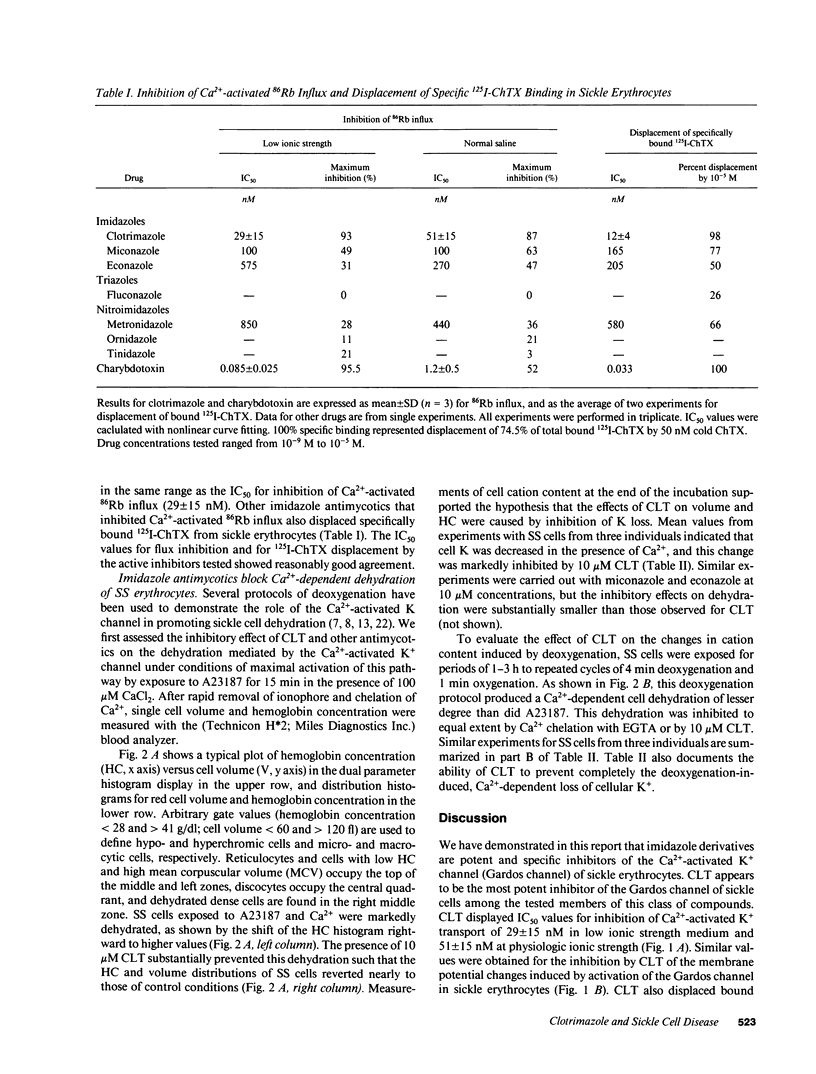
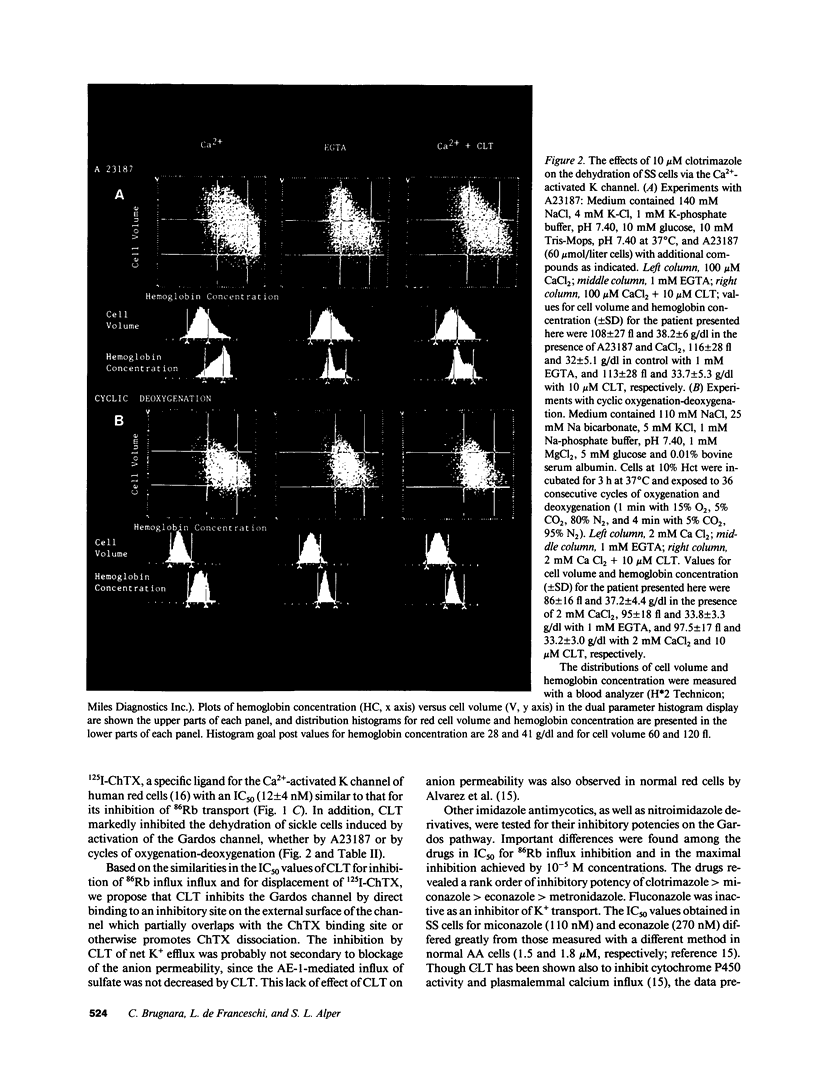
We have investigated the interaction of clotrimazole (CLT) and related compounds with the erythroid Ca(2+)-activated K+ channel, a mediator of sickle cell dehydration. We measured K+ transport, membrane potential, and cell volume upon activation of this pathway in sickle erythrocytes. CLT blocked almost completely Ca(2+)-activated K+ transport in homozygous hemoglobin S cells, with IC50 values of 29 +/- 15 nM in isotonic 20 mM salt solution and 51 +/- 15 nM in normal saline (n = 3). The inhibition of K+ transport by CLT was caused by a specific interaction with the Ca(2+)-activated K+ channel of human red cells, since it displaced bound 125I-Charybdotoxin, a specific ligand of the Gardos channel, with an IC50 (12 +/- 4 nM in isotonic 20 mM) similar to the IC50 values for flux inhibition. When homozygous hemoglobin S cells were dehydrated by incubation in the presence of 100 microM CaCl2 and the ionophore A23187, or by exposure to cycles of oxygenation and deoxygenation, CLT effectively inhibited cell dehydration and K+ loss. The IC50 of CLT for inhibition of Ca(2+)-activated K+ transport in sickle cells is significantly lower than plasma concentrations of CLT achievable after nontoxic oral doses. We therefore propose that oral administration of CLT may prevent red cell dehydration in patients with sickle cell anemia.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez J., Montero M., Garcia-Sancho J. High affinity inhibition of Ca(2+)-dependent K+ channels by cytochrome P-450 inhibitors. J Biol Chem. 1992 Jun 15;267(17):11789–11793. [PubMed] [Google Scholar]
- Beutler E., West C., Blume K. G. The removal of leukocytes and platelets from whole blood. J Lab Clin Med. 1976 Aug;88(2):328–333. [PubMed] [Google Scholar]
- Bookchin R. M., Ortiz O. E., Lew V. L. Evidence for a direct reticulocyte origin of dense red cells in sickle cell anemia. J Clin Invest. 1991 Jan;87(1):113–124. doi: 10.1172/JCI114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugnara C., Bunn H. F., Tosteson D. C. Regulation of erythrocyte cation and water content in sickle cell anemia. Science. 1986 Apr 18;232(4748):388–390. doi: 10.1126/science.3961486. [DOI] [PubMed] [Google Scholar]
- Brugnara C., De Franceschi L., Alper S. L. Ca(2+)-activated K+ transport in erythrocytes. Comparison of binding and transport inhibition by scorpion toxins. J Biol Chem. 1993 Apr 25;268(12):8760–8768. [PubMed] [Google Scholar]
- Canessa M., Spalvins A., Nagel R. L. Volume-dependent and NEM-stimulated K+,Cl- transport is elevated in oxygenated SS, SC and CC human red cells. FEBS Lett. 1986 May 5;200(1):197–202. doi: 10.1016/0014-5793(86)80538-5. [DOI] [PubMed] [Google Scholar]
- Clark M. R., Unger R. C., Shohet S. B. Monovalent cation composition and ATP and lipid content of irreversibly sickled cells. Blood. 1978 Jun;51(6):1169–1178. [PubMed] [Google Scholar]
- Eaton W. A., Hofrichter J. Hemoglobin S gelation and sickle cell disease. Blood. 1987 Nov;70(5):1245–1266. [PubMed] [Google Scholar]
- Ellory J. C., Kirk K., Culliford S. J., Nash G. B., Stuart J. Nitrendipine is a potent inhibitor of the Ca(2+)-activated K+ channel of human erythrocytes. FEBS Lett. 1992 Jan 20;296(2):219–221. doi: 10.1016/0014-5793(92)80383-r. [DOI] [PubMed] [Google Scholar]
- GARDOS G. The function of calcium in the potassium permeability of human erythrocytes. Biochim Biophys Acta. 1958 Dec;30(3):653–654. doi: 10.1016/0006-3002(58)90124-0. [DOI] [PubMed] [Google Scholar]
- Glader B. E., Nathan D. G. Cation permeability alterations during sickling: relationship to cation composition and cellular hydration of irreversibly sickled cells. Blood. 1978 May;51(5):983–989. [PubMed] [Google Scholar]
- Halperin J. A., Brugnara C., Tosteson M. T., Van Ha T., Tosteson D. C. Voltage-activated cation transport in human erythrocytes. Am J Physiol. 1989 Nov;257(5 Pt 1):C986–C996. doi: 10.1152/ajpcell.1989.257.5.C986. [DOI] [PubMed] [Google Scholar]
- Heel R. C., Brogden R. N., Pakes G. E., Speight T. M., Avery G. S. Miconazole: a preliminary review of its therapeutic efficacy in systemic fungal infections. Drugs. 1980 Jan;19(1):7–30. doi: 10.2165/00003495-198019010-00002. [DOI] [PubMed] [Google Scholar]
- Horiuchi K., Ballas S. K., Asakura T. The effect of deoxygenation rate on the formation of irreversibly sickled cells. Blood. 1988 Jan;71(1):46–51. [PubMed] [Google Scholar]
- Kaji D. M. Nifedipine inhibits calcium-activated K transport in human erythrocytes. Am J Physiol. 1990 Aug;259(2 Pt 1):C332–C339. doi: 10.1152/ajpcell.1990.259.2.C332. [DOI] [PubMed] [Google Scholar]
- Latorre R., Oberhauser A., Labarca P., Alvarez O. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- Lew V. L., Freeman C. J., Ortiz O. E., Bookchin R. M. A mathematical model of the volume, pH, and ion content regulation in reticulocytes. Application to the pathophysiology of sickle cell dehydration. J Clin Invest. 1991 Jan;87(1):100–112. doi: 10.1172/JCI114958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanick M. A., Gunn R. B. Proton-sulfate co-transport: mechanism of H+ and sulfate addition to the chloride transporter of human red blood cells. J Gen Physiol. 1982 Jan;79(1):87–113. doi: 10.1085/jgp.79.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas N., Johnson A., Wyatt J., Croisille L., Reeves J., Tycko D., Groner W. Automated quantitation of cell density distribution and hyperdense cell fraction in RBC disorders. Blood. 1989 Jul;74(1):442–447. [PubMed] [Google Scholar]
- Ohnishi S. T., Katagi H., Katagi C. Inhibition of the in vitro formation of dense cells and of irreversibly sickled cells by charybdotoxin, a specific inhibitor of calcium-activated potassium efflux. Biochim Biophys Acta. 1989 Feb 9;1010(2):199–203. doi: 10.1016/0167-4889(89)90161-4. [DOI] [PubMed] [Google Scholar]
- Rosa R. M., Bierer B. E., Thomas R., Stoff J. S., Kruskall M., Robinson S., Bunn H. F., Epstein F. H. A study of induced hyponatremia in the prevention and treatment of sickle-cell crisis. N Engl J Med. 1980 Nov 13;303(20):1138–1143. doi: 10.1056/NEJM198011133032002. [DOI] [PubMed] [Google Scholar]
- Sawyer P. R., Brogden R. N., Pinder R. M., Speight T. M., Avery Clotrimazole: a review of its antifungal activity and therapeutic efficacy. Drugs. 1975;9(6):424–447. doi: 10.2165/00003495-197509060-00003. [DOI] [PubMed] [Google Scholar]
- Schofield A. E., Reardon D. M., Tanner M. J. Defective anion transport activity of the abnormal band 3 in hereditary ovalocytic red blood cells. Nature. 1992 Feb 27;355(6363):836–838. doi: 10.1038/355836a0. [DOI] [PubMed] [Google Scholar]
- Seo M., iida H., Miura Y. Basic experiments with clotrimazole administered orally. Curr Med Res Opin. 1977;5(2):169–178. doi: 10.1185/03007997709110160. [DOI] [PubMed] [Google Scholar]
- Wang W., Cassola A., Giebisch G. Arachidonic acid inhibits the secretory K+ channel of cortical collecting duct of rat kidney. Am J Physiol. 1992 Apr;262(4 Pt 2):F554–F559. doi: 10.1152/ajprenal.1992.262.4.F554. [DOI] [PubMed] [Google Scholar]
- Weuta H. Clinical studies with oral clotrimazole. Postgrad Med J. 1974 Jul;50 (Suppl 1):45–48. [PubMed] [Google Scholar]
- Wolff D., Cecchi X., Spalvins A., Canessa M. Charybdotoxin blocks with high affinity the Ca-activated K+ channel of Hb A and Hb S red cells: individual differences in the number of channels. J Membr Biol. 1988 Dec;106(3):243–252. doi: 10.1007/BF01872162. [DOI] [PubMed] [Google Scholar]