Abstract
Trials to evaluate the efficacy of preventive HCV vaccines will need participation from high risk HCV seronegative injection drug users (IDUs). To guide trial planning, we assessed willingness of young IDU in San Francisco to participate in HCV vaccine efficacy trials and evaluate knowledge of vaccine trial concepts: placebo, randomization and blinding. During 2006 and 2007, a total of 67 participants completed the survey. A substantial proportion (88%) would definitely (44%) or probably (44%) be willing to participate in a randomized trial, but knowledge of vaccine trial concepts was low. Reported willingness to participate in an HCV vaccine trial decreased with increasing trial duration, with 67% of participants surveyed willing to participate in a trial of one year duration compared to 43% of participants willing to participate in a trial of 4 years duration. Willingness to enroll in HCV vaccine trials was higher in young IDU than reported by most at-risk populations in HIV vaccine trials. Educational strategies will be needed to ensure understanding of key concepts prior to implementing HCV vaccine trials.
Keywords: Hepatitis C, injection drug use, vaccine trial
Introduction
Hepatitis C viral infection (HCV) infects an estimated 130 million persons globally, the majority of whom develop chronic infection (HCV RNA positive in 80 to 100 percent of cases) and chronic hepatitis (elevated serum ALT in 60 to 80% of cases)[1]. HCV is responsible for one quarter of worldwide cirrhosis and hepatocellular carcinoma cases, respectively and remains the main indication for liver transplantation in the U.S.[2]
Injection drug use (IDU) is the risk behavior leading to infection in 90% of global HCV infections.[3] HCV infection is epidemic among IDUs, with annual incidence rates highest among young IDUs ranging from 9% to 38%.[4-7] The CDC estimated the number of acute HCV infections in the US has decreased from approximately 230,000 per year in the 1980s to the current level of about 19,000 per year.[8] This decrease is primarily due to screening of the blood supply followed by a reduction in IDU infection due to needle exchange and safer injection practices.[9] Despite these successes, prevalence remains high among IDU in the US. Among young (<30 years) IDUs in San Francisco, we found 50% prevalence of HCV after 5 years of injecting.[10] In a large sample of young IDUs (n=520), we recently reported a cumulative HCV incidence of 26.7/100 person-years of observation and an estimated reinfection rate of 24.6/100 person-years of observation.[11] Access to HCV treatment is limited for IDUs [12] and as with non-injecting populations, current treatment success is suboptimal. Further decreases in HCV incidence are unlikely to be achieved without large-scale and effective biomedical interventions, notably preventive vaccines.[13]
HCV, an RNA virus, has enormous genetic variability, which has challenged vaccine development. The site of greatest variability is within the E2 envelope glycoprotein, a major antibody target. [14] Several candidate vaccines designed to prevent initial HCV infection in uninfected people, reduce viral persistence in infected individuals, or sustain virological response in individuals with chronic infection, are currently in preclinical development or early stage clinical trials. Nonetheless, first generation HCV vaccines are expected to be of only low-to-moderate efficacy. Both efficacy and cross clade protection have emerged as significant factors affecting HIV vaccine trial acceptability [15] and are likely to be important determinants of HCV vaccine trial participation. Additionally, trial duration and cohort retention will be important trial design considerations. Young IDUs are a challenging population to track and retain in clinical trials for many reasons, including distrust of the medical establishment, homelessness and mobility. Despite these challenges, young IDUs are the population most at risk for HCV acquisition, the key population for immunogenicity studies and prime candidates for inclusion in future prevention vaccine trials assessing preventive efficacy. In this cross sectional study, we assess willingness to participate in preventive HCV vaccine trials and knowledge of trial concepts among young IDU.
Materials and Methods
Participants
Beginning in 2000, young (< 30 years) IDUs in San Francisco have been offered participation in multiple prospective studies under variations of the shared title of the “UFO Study”described previously.[4] In brief, young IDU were recruited by peer outreach workers familiar with neighborhoods in San Francisco where young IDU congregate, using study invitation cards and flyers, contacts with youth friendly neighborhood groups and community providers, and word of mouth to participate in HCV screening as the baseline visit for eligibility in a prospective study assessing incident HCV infection. Inclusion criteria for screening were: 1) age under 30 years 2) self reported use of injection drugs in the past 30 days 3) ability to provide informed consent and 4) understanding spoken English, and (5) after 2005, self-reported HCV negative or unknown status.
The UFO Study had two waves of data collection, the first wave from 2000-2002 and a second wave from 2003-2008. During the second wave of data collection, a subgroup of young IDU participating in UFO-3 was asked to participate in this sub-study assessment of vaccine trial willingness, and completed a supplemental interviewer-administered survey on willingness to participate in future HCV vaccine trials and knowledge of key prevention vaccine trial concepts presented as true/false statements (n=67). These data were collected at one of the quarterly UFO-3 study visits in 2006 and 2007. Participants in the prospective cohort study completed the supplemental survey on vaccine trial knowledge and willingness if they attended a quarterly study visit during 2006 and 2007.
Study design and measures
Eligible consenting participants were interviewed, counseled, and tested for antibodies to HCV (anti-HCV) and HCV RNA. Each was remunerated $10 at screening, and $20 at results visits. Participants who were HCV negative and did not plan to travel in the next 3 months were offered enrollment into the prospective cohort (UFO-3 Study). Follow-up included monthly “check-ins” and quarterly study visits which included structured interviews to assess exposures, HCV status (including anti-HCV and HCV RNA testing), and risk reduction counseling and referrals.
The vaccine trial willingness survey instrument was adapted and constructed from previously published assessments of vaccine trial willingness.27-29 Participants were asked to rate willingness to participate in an HCV vaccine trial that began that week on a four point scale (definitely, probably, probably not and definitely not willing.) The survey evaluated baseline knowledge of prevention vaccine trial concepts including need for baseline HCV seronegativity, randomization, placebo control, blinding, adverse events, experience with vaccines, motivation for participation in, support for, or against an HCV vaccine trial, trusted sources of trial information and expectancies for positive and negative outcomes related to an HCV vaccine trial among young IDU. Questions on willingness and knowledge were asked without reference to a specific vaccine candidate or product. At survey completion, participants were provided correct answers on knowledge questions through a brief educational session on HCV and vaccine trial design concepts. The protocol and informed consent were approved by the institutional review board (IRB) of the University of California, San Francisco.
Statistical analyses
Sociodemographic information and drug use behavior at the baseline interview were compared between vaccine survey participants and HCV-negative cohort participants in the second wave of data collection who did not complete the vaccine willingness survey. Participants who did not complete the survey were those who did not have quarterly study visits during the time period of the vaccine willingness sub-study. Differences were assessed using the Chi-square test of association for categorical variables and the Kruskal-Wallis median test for continuous variables. Frequency distributions were tabulated for each questionnaire item and bivariate associations between questionnaire items and age group were analyzed using the Chi-Square test of association. In order to examine differences in vaccine trial willingness and knowledge by age, all subjects were classified into two age-stratified subgroups for data analysis: <23 and ≥23 years.
Results
Overall, demographics and IDU related behaviors for the study population of vaccine trial survey participants (n=67) and HCV antibody negative UFO participants who were not offered the supplemental survey (n=202) are summarized in Table 1. Vaccine trial survey participants reported injecting drugs for longer (median 5.1 years) compared to other HCV antibody (anti-HCV) negative UFO-3 participants (median 4.1 years) and using more frequent daily injections (median 3 compared to 2), however these differences did not reach statistical significance. Gender and age at baseline interview did not vary significantly between groups. Heroin was the drug most frequently injected by both groups, reported by two thirds of participants. Incarceration in the last 3 months was reported by one quarter of vaccine trial survey participants and one third of HCV negative cohort participants who did not complete willingness survey.
Table 1. Baseline sociodemographic characteristics and risk behaviors among young IDU in HCV vaccine trial willingness survey (n=67) and participants in HCV negative cohort study (n=202), San Francisco, 2003-2008.
| Vaccine trial survey participants % (95% CI) or median (IQR) | HCV negative UFO cohort participants % (95% CI) or median (IQR) | p-value | |
|---|---|---|---|
| Age | 23.7 (21.1 – 27.2) | 22.8 (20.6 – 25.5) | n.s.* |
| Male | 65.7 | 68.8 | n.s. |
| Non-white race/ethnicity | 20.0 | 30.2 | n.s. |
| Less than high school education | 36.9 | 45.6 | n.s. |
| Homeless last 3 mo. | 67.2 | 67.0 | n.s. |
| Years injecting | 5.1 (2.9 – 9.2) | 4.1 (1.7 – 7.6) | n.s. |
| No. of daily injections | 3.0 (1.8 – 4.0) | 2.0 (1.0 – 3.0) | n.s. |
| Drug used most days last month - heroin | 69.4 | 59.1 | n.s. |
| Borrowed used needle last 3 mo. | 34.9 | 36.2 | n.s. |
| Incarcerated last 3 mo. | 23.4 | 30.8 | n.s. |
non-signficant, p≥0.05
Table 2 summarizes knowledge of vaccine trial concepts and willingness to enroll in HCV vaccine trial by age among the sixty-seven young IDU surveyed. Approximately, one quarter of survey participants had previously received immunizations (most commonly hepatitis B virus (HBV) and hepatitis A virus (HAV) vaccine) through the UFO Study. IDU ≥ 23 years were more than ten times as likely to have previously received a vaccine as younger IDU. (p<=0.01) Over 90% of survey participants knew that a vaccine served to prevent disease. Aside from an understanding that an HCV clinical trial would enroll only anti-HCV negative persons, vaccine trial knowledge did not vary significantly by age. Injectors ≥ 23 years of age were twice as likely as younger injectors to know that an HCV vaccine trial would be limited to anti-HCV negative participants. Less than half (45%) of all survey participants correctly reported that vaccine assignment would be random in an HCV vaccine clinical trial. Approximately half of all trial participants correctly recognized blinding of trial participants as an important design feature. The majority (67%) of survey participants correctly reported that unblinding of vaccine status would occur at trial conclusion. Over three quarters of IDU surveyed, correctly answered that adverse events related to study vaccine would be treated by study staff.
Table 2. Knowledge of clinical trial concepts and willingness to enroll in an HCV vaccine trial among young, HCV antibody negative IDUs by age in San Francisco, 2006-2007 (n=67).
| Total N (%) |
Age <23 N (%) |
Age ≥23 N (%) |
|
|---|---|---|---|
| Overall | 67 (100) | 25 (37.3) | 42 (62.7) |
| Ever received a vaccine from UFO (n=66) | 19 (28.8) | 1 (4.2) | 18 (42.9)** |
| Vaccine knowledge | |||
| What do you think a vaccine is for? | |||
| To prevent | 47 (70.2) | 21 (84.0) | 26 (61.9) |
| To treat disease | 3 (4.5) | 1 (4.0) | 2 (4.8) |
| To prevent and treat disease | 16 (23.9) | 3(12.0) | 13 (31.0) |
| The study would only enroll people not infected with hcv. [True] | 33 (57.4) | 7 (28.0) | 26 (61.9)* |
| Participants would be randomly assigned to get actual vaccine or placebo. [True] | 30 (44.8) | 13 (52.0) | 17 (40.5) |
| No one knows who received actual vaccine or placebo until trial end [True] | 39 (58.2) | 12 (48.0) | 27 (64.3) |
| Only researchers know who received actual vaccine or placebo before trial end [True] (n=66) | 30 (45.5) | 11 (45.8) | 19 (45.2) |
| Participants told whether received actual vaccine or placebo at trial end [True] | 45 (67.2) | 18 (72.0) | 27 (64.3) |
| Participants would be treated for vaccine related health problems [True] | 53 (79.0) | 19 (76.0) | 34 (81.0) |
| Willingness to enroll | |||
| Willingness to enroll by study length (n=66) | |||
| 1 year | 44 (66.7) | 10 (40.0) | 34 (82.9)** |
| 2 years | 36 (54.6) | 7 (28.0) | 29 (70.7)** |
| 3 years | 30 (45.5) | 7 (28.0) | 23 (56.1)* |
| 4 years | 28 (42.4) | 7 (28.0) | 21 (51.2) |
| Willingness to enroll if study started this week (n=66) | |||
| Definitely willing | 29 (43.9) | 7 (28.0) | 22 (53.7) |
| Probably willing | 29 (43.9) | 13 (52.0) | 16 (39.0) |
| Probably unwilling | 5 (7.6) | 3 (12.0) | 2 (4.9) |
| Definitely unwilling | 3 (4.6) | 2 (8.0) | 1 (2.4) |
| Trust to explain safety and side effects (n=66) | |||
| UFO Study | 58 (87.9) | 20 (80.0) | 38 (92.7) |
| San Francisco DPH | 37 (56.1) | 15 (60.0) | 22 (53.7) |
| UCSF Committee on Human Research | 35 (53.1) | 10 (40.0) | 25 (61.0) |
| San Francisco General Hospital† | 31 (50.8) | 15 (62.5) | 16 (43.2) |
| Doctor | 39 (59.1) | 17 (68.0) | 22 (53.7) |
| Government | 17 (25.8) | 6 (24.0) | 11 (26.8) |
| Pharmaceutical company | 9 (13.6) | 5 (20.0) | 4 (9.8) |
| Media/Newspapers | 6 (9.1) | 2 (8.0) | 4 (9.8) |
p<=0.05;
p<=0.01;
n=61
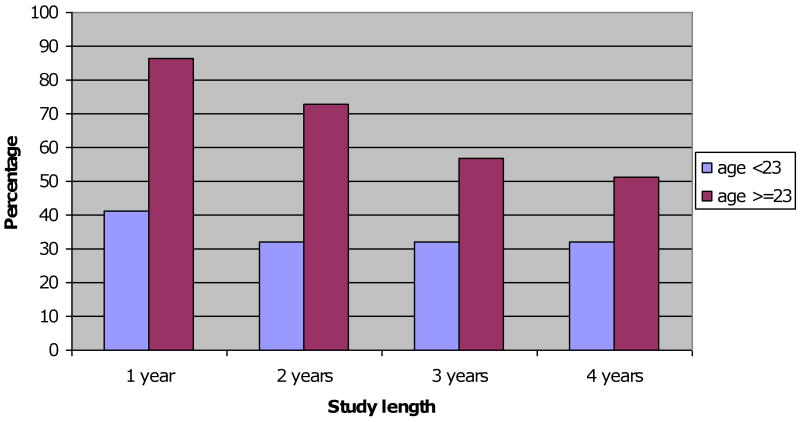
Overall, 88% (58/66) of participants who completed a supplemental survey indicated that they would definitely or probably be willing to participate in a HCV vaccine trial that began that week (Table 2). Levels of willingness to enroll in an HCV vaccine trial varied significantly by age (p=<0.01). Reported willingness to participate in an HCV vaccine trial decreased with increasing trial duration, with 67% of participants surveyed willing to participate in a trial of one year duration compared to 43% of participants willing to participate in a trial of 4 years duration. For IDU ≥ age 23, willingness to enroll in an HCV vaccine trial showed a decreasing trend with hypothetical trial duration (Figure 1).
Figure 1. Willingness to enroll in an HCV vaccine trial by trial length and age group.
The UFO Study was the most frequently reported trusted source of information on trial safety measures and adverse events, reported by over 85% of participants. Other sources of trusted information on candidate vaccine safety and adverse events reported by greater than one half of participants were: San Francisco Department of Public Health, University Human Subjects Research committee, County Hospital and their physician. Survey participants rated the following reasons as “very true” or “somewhat true” in decision making about vaccine trial participation: potential benefits for future IDU (75%), monetary benefits (55%), potential benefits to themselves (53%), and reduced chance of HCV infection due to trial participation (43%).
Discussion
In the context of the current reappraisal of the empirical approach to vaccine development [16] and the increasing recognition of the importance of two way dialogue with affected communities in vaccine trials, this study is the first to document young IDU vaccine trial knowledge and willingness to enroll in preventive hepatitis vaccine trials. We report higher levels of willingness to participate in an HCV vaccine trial (defined as definitely or probably willing to participate) than those reported by high risk adults in a hypothetical HIV vaccine trial (76-81% vs. 88%) [17, 18]and higher levels than those reported by male IDU (77%)[19] or female IDU (60%) in hypothetical HIV vaccine trials. Similar willingness to participate levels (82%) were reported in a Spanish study among female sex workers, injection and non-injection drug users and men who have sex with men which evaluated HIV vaccine trial readiness. [20] Others have reported willingness to participate in HIV vaccine efficacy studies was related to high risk sexual or injection behavior in the last six months.[18, 21] These data taken in conjunction with the risk profile of the cohort at baseline suggest that identification of willing high risk IDU participants for future HCV trials is feasible. This is consistent with prior interventions showing IDUs are amenable to participation in a variety of prospective research settings.[22-24]
Our study suggests significant resources will be needed for recruitment of IDUs for HCV vaccine trials. HCV incidence is very high among young IDU and there is a rapid saturation of the population with disease (with almost 50% becoming positive after 5 years of injecting.)[4, 25, 26] Given the challenge of finding seronegative IDU and the decreased willingness of younger IDU to participate in trials, collaboration will be needed between existing research study cohorts as well as reaching out to new IDU social networks and communities to enroll sufficient numbers of HCV seronegative IDUs. The sample size needed for an adequately powered trial of a vaccine that confers a 60% reduction in incidence of chronic infection among vaccinated participants compared to unvaccinated controls will be a total of 320 participants (160 per group). These estimates assume an 18 month follow-up period, a 10% loss to follow-up and a 14% annual incidence of chronic infection.
Young IDUs in our cohort were less willing to enroll in an HCV vaccine trial than older IDUs. A recent study among high risk adults including IDUs reported a similar association between HIV vaccine trial willingness and older age, [20]while others have not found such an association [21]. Multivariate analysis, limited by our modest sample size, evaluated willingness to participate in an HCV vaccine trial for trials of 1-3 years in duration. For HCV vaccine trials of 1-2 years in duration, age greater than 23 years is an independent predictor of willingness to participate, along with planning to stay in the San Francisco area and a higher knowledge score. For an HCV vaccine trial of 3 years duration, the age effect was not significant, with trust in physicians, media or government, no plans to leave San Francisco and a higher knowledge score predicting trial willingness.
Our findings also show that work needs to be done to educate young IDU vaccine candidates on key trial concepts, especially randomization and blinding as well as the need for baseline seronegative status. While a basic knowledge of a vaccine's role in preventing disease acquisition was higher in our study than an earlier study in Philadelphia IDU[21], knowledge of placebo in a theoretical HIV prevention vaccine trial appeared higher among 4,892 adults at high risk for HIV infection than suggested by participants in our study[27]. These authors reported prototype consent procedures which included intensive educational efforts at baseline, followed by targeted semiannual ‘booster’ educational sessions were associated with statistically significant and sustained increases in participant knowledge of key concepts. The impact of these prototype consent procedures was similar for IDUs compared to other risk groups. [27]
Our study provides encouraging results on the use of existing public health research infrastructure to recruit IDU for hepatitis vaccine trials. One third of survey willingness participants had previously received a vaccine through the UFO Study. We report high levels of knowledge on trial coverage of vaccine related adverse events. Trusted sources of vaccine trial information reported by participants were UFO Study staff, physicians and local public health authorities. Advertising vaccine trial opportunities for IDU should occur in collaboration with public health partners in settings where valued services such as syringe and needle exchange, drop-in counseling, treatment and legal services are offered.
Particular strengths of this study were the assessment of willingness with common instruments for IDU in hepatitis vaccine trials, a population for which there are no data with regard to preparedness for future hepatitis vaccine trials. Limitations of this study include its modest sample size and lack of longitudinal data on willingness to participate and vaccine trial concept knowledge. With the cross sectional data we present here, we cannot determine if willingness to participate changes over time or if changes if vaccine trial knowledge affect changes in willingness. Others have reported willingness to participate in HIV vaccine trials was significantly lower at six, 12 and 18 month follow-up visits compared to baseline in study populations including IDUs.[19] In this study, we report a large proportion of young IDU are willing to participate in future HCV vaccine efficacy trials. Participant counseling and community education need to address seronegativity at baseline in participants, and other key vaccine trial concepts.
Acknowledgments
Funding: This study and the authors were supported by U.S. National Institutes for Health (NIH) 2 R01 DA016017-03A1. Additional support was received from: NIH grants - 5 U19 AI40034-13 (Dr. Page, and Ms. Evans), and K01 DA023365 (Dr. Hahn).
The authors would like to acknowledge the ongoing participation of all the UFO Study participants whose involvement continues to help provide important information to public health practice and research for prevention of HCV and other blood borne infections. We also acknowledge the following individuals for their dedication to the study: Erin Antunez, Alice Asher, Pam Axelson, Clara Brandt, Alya Briceño, Caycee Cullen, Rosary Giuliano-Deaderick, Noah Gaiser, Gina Limon, Martha Montgomery, Peter Morse, Bob Thawley and Michele Thorsen. Finally, we acknowledge recognize the important contributions the UFO Study receives from the San Francisco Department of Public Health, including the Communicable Disease Branch who provide free immunizations, the Housing and Urban Health Clinic, the Tenderloin Health (TH), the San Francisco Community Clinic Consortium/Street Outreach Services (SFCCC–SOS), and the Homeless Youth Alliance (HYA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barrera JM, Bruguera M, Ercilla MG, Gil C, Celis R, Gil MP, et al. Persistent hepatitis C viremia after acute self-limiting posttransfusion hepatitis C. Hepatology. 1995;21(3):639–44. [PubMed] [Google Scholar]
- 2.Perz JF, Alter MJ. The coming wave of HCV related liver disease: dilemmas and challenges. Journal of Hepatology. 2006;44:441–3. doi: 10.1016/j.jhep.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Hellard M, Sacks-Davis R, Gold J. Hepatitis C treatment for injection drug users: a review of the available evidence. Clinical Infectious Diseases. 2009;49(4):561–73. doi: 10.1086/600304. [DOI] [PubMed] [Google Scholar]
- 4.Hahn JA, Page-Shafer K, Lum PJ, Bourgois P, Stein E, Evans JL, et al. Hepatitis C virus seroconversion among young injection drug users: relationships and risks. Journal of Infectious Diseases. 2002;186(11):1558–64. doi: 10.1086/345554. [DOI] [PubMed] [Google Scholar]
- 5.Thorpe LE, Ouellet LJ, Hershow R, Bailey SL, Williams IT, Williamson J, et al. Risk of hepatitis C virus infection among young adult injection drug users who share injection equipment. American Journal of Epidemiology. 2002;155(7):645–53. doi: 10.1093/aje/155.7.645. [DOI] [PubMed] [Google Scholar]
- 6.Des Jarlais DC, Diaz T, Perlis T, Vlahov D, Maslow C, Latka M, et al. Variability in the incidence of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus infection among young injecting drug users in New York City. American Journal of Epidemiology. 2003;157(5):467–71. doi: 10.1093/aje/kwf222. [DOI] [PubMed] [Google Scholar]
- 7.Garten RJ, Lai S, Zhang J, Liu W, Chen J, Vlahov D, et al. Rapid transmission of hepatitis C virus among young injecting heroin users in Southern China. International Journal of Epidemiology. 2004;33(1):182–8. doi: 10.1093/ije/dyh019. [DOI] [PubMed] [Google Scholar]
- 8.Wasley A, Grytdal S, Gallagher K. Surveillance for acute viral hepatitis-United States, 2006. Morbidity Mortality Weekly Reports Surveillance Summaries. 2008;57 [PubMed] [Google Scholar]
- 9.Hurley SF, Joeely DJ, Kaldor JM. Effectiveness of needle exchange programmes for prevention of HIV infection. Lancet. 1997;349:1797–1800. doi: 10.1016/S0140-6736(96)11380-5. [DOI] [PubMed] [Google Scholar]
- 10.Hahn J, Page-Shafer K, Lum P, Ochoa K, Moss A. Hepatitis C virus infection and needle exchange use among young injection drug users in San Francisco. Hepatology. 2001;34:180–7. doi: 10.1053/jhep.2001.25759. [DOI] [PubMed] [Google Scholar]
- 11.Page K, Hahn JA, Evans J, Shiboski S, Lum P, Delwart E, et al. Acute hepatitis C virus infection in young adult injection drug users: A prospective study of incident infection, resolution and reinfection. Journal of Infectious Diseases. 2009;200:1216–1226. doi: 10.1086/605947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagan H, Latka MH, Campbell JV, Golub ET, Garfein RS, Thomas DA, et al. Eligibility for treatment of hepatitis C virus infection among young injection drug users in 3 US cities. Clinical Infectious Diseases. 2006;42(5):669–72. doi: 10.1086/499951. [DOI] [PubMed] [Google Scholar]
- 13.Page-Shafer K, Hahn JA, Lum PJ. Preventing hepatitis C virus infection in injection drug users: risk reduction is not enough. AIDS. 2007;21(14):1967–9. doi: 10.1097/QAD.0b013e3282ef7701. [DOI] [PubMed] [Google Scholar]
- 14.Simmonds P. Genetic diversity and evolution of hepatitis C virus. Journal of General Virology. 2004;85:3173–88. doi: 10.1099/vir.0.80401-0. [DOI] [PubMed] [Google Scholar]
- 15.Hu DJ, Vitek CR, Bartholow B, Mastro TD. Key issues for a potential human immunodeficiency virus vaccine. Clinical Infectious Diseases. 2003;36(5):638–44. doi: 10.1086/367891. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan M. Moving candidate vaccines ito development from research: lessons from HIV. Immunology and Cell Biology. 2009;87:366–370. doi: 10.1038/icb.2009.30. [DOI] [PubMed] [Google Scholar]
- 17.Buchbinder SP, Metch B, Holte SE, Scheer S, Coletti A, Vittinghoff E. Determinants of enrollment in a preventive HIV vaccine trial: hypothetical versus actual willingness and barriers to participation. Journal of Acquired Immune Deficiency Syndrome. 2004;36(1):604–12. doi: 10.1097/00126334-200405010-00009. [DOI] [PubMed] [Google Scholar]
- 18.Koblin BA, Heagerty P, Sheon A, Buchbinder S, Celum C, Douglas JM, et al. Readiness of high risk populations in the HIV network for prevention trials to participate in HIV vaccine efficacy trials in the United States. AIDS. 1998;12(7):785–793. doi: 10.1097/00002030-199807000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Koblin BA, Holte S, Lenderking B, Heagerty P. Readiness for HIV vaccine trials: changes in willingness and knowledge among high-risk populations in the HIV network for prevention trials. The HIVNET Vaccine Preparedness Study Protocol Team. Journal of Acquired Immune Deficiency Syndrome. 2000;24(5):451–7. doi: 10.1097/00126334-200008150-00010. [DOI] [PubMed] [Google Scholar]
- 20.Etcheverry MF, de Lazzari E, Fuchs JD, Merono M, Sierra E, Del Romero J, et al. Pilot study assessing HIV vaccine trial readiness among female sex workers, injection and non-injection drug users, and mwn who have sex with men in Spain. AIDS Behavior. 2010;14:607–617. doi: 10.1007/s10461-008-9486-x. [DOI] [PubMed] [Google Scholar]
- 21.Meyers K, Metzger DS, Navaline H, Woody GE, McLellan AT. HIV vaccine trials: Will intravenous drug users enroll? American Journal of Public Health. 1994;84(5):761–766. doi: 10.2105/ajph.84.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dolan KA, Shearer J, White B, Zhou J, Kaldor J, Wodak AD. Four-year follow-up of imprisoned male heroin users and methadone treatment: mortality, re-incarceration and hepatitis C infection. Addiction. 2005;100(6):820–8. doi: 10.1111/j.1360-0443.2005.01050.x. [DOI] [PubMed] [Google Scholar]
- 23.Gibson DR, Flynn NM, Perales D. Effectiveness of syringe exchange programs in reducing HIV risk behavior and HIV seroconversion among injecting drug users. AIDS. 2001;15:1329–41. doi: 10.1097/00002030-200107270-00002. [DOI] [PubMed] [Google Scholar]
- 24.Wood E, Tyndall MW, Zhang R, Stoltz JA, Lai C, Montaner JS, et al. Attendance at supervised injecting facilities and use of detoxification services. N Engl J Med. 2006;354:2512–4. doi: 10.1056/NEJMc052939. [DOI] [PubMed] [Google Scholar]
- 25.Hagan H, Thiede H, Des Jarlais D. Hepatitis C infection among injection drug users: Survival analysis of time to seroconversion. Epidemiology. 2004;15:543–549. doi: 10.1097/01.ede.0000135170.54913.9d. [DOI] [PubMed] [Google Scholar]
- 26.Judd A, Hickman M, Jones S, McDonald T, Parry JV, Stimson GV, et al. Incidence of hepatitis C virus and HIV among new injecting drug users in London: prospective cohort study. British Medical Journal. 2005;330(7481):24–25. doi: 10.1136/bmj.38286.841227.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coletti AS, Heagerty P, Sheon AR, Gross M, Koblin BA, Metzger DS, et al. Randomized, controlled evaluation of a prototype informed consent process for HIV vaccine efficacy trials. Journal of Acquired Immune Deficiency Syndrome. 2003;32(2):161–9. doi: 10.1097/00126334-200302010-00008. [DOI] [PubMed] [Google Scholar]