Abstract
Ornithine transcarbamylase was stabilized in cell-free extracts by the presence of either carbamyl phosphate or glycerol. The enzyme was rapidly purified by a procedure consisting of ion-exchange chromatography and electrofocusing. The native molecular weight of the enzyme was determined by gel filtration to be 110,000. A subunit molecular weight of 36,000 was determined by polyacrylamide electrophoresis under dissociating conditions. These findings indicated a trimeric quaternary structure for the enzyme. The isoelectric point of the purified enzyme was 4.75, and no evidence of multiple forms of active enzyme was found in either crude or purified preparations. An inactive form of the enzyme appeared upon storage in the absence of stabilization buffer.
Full text
PDF

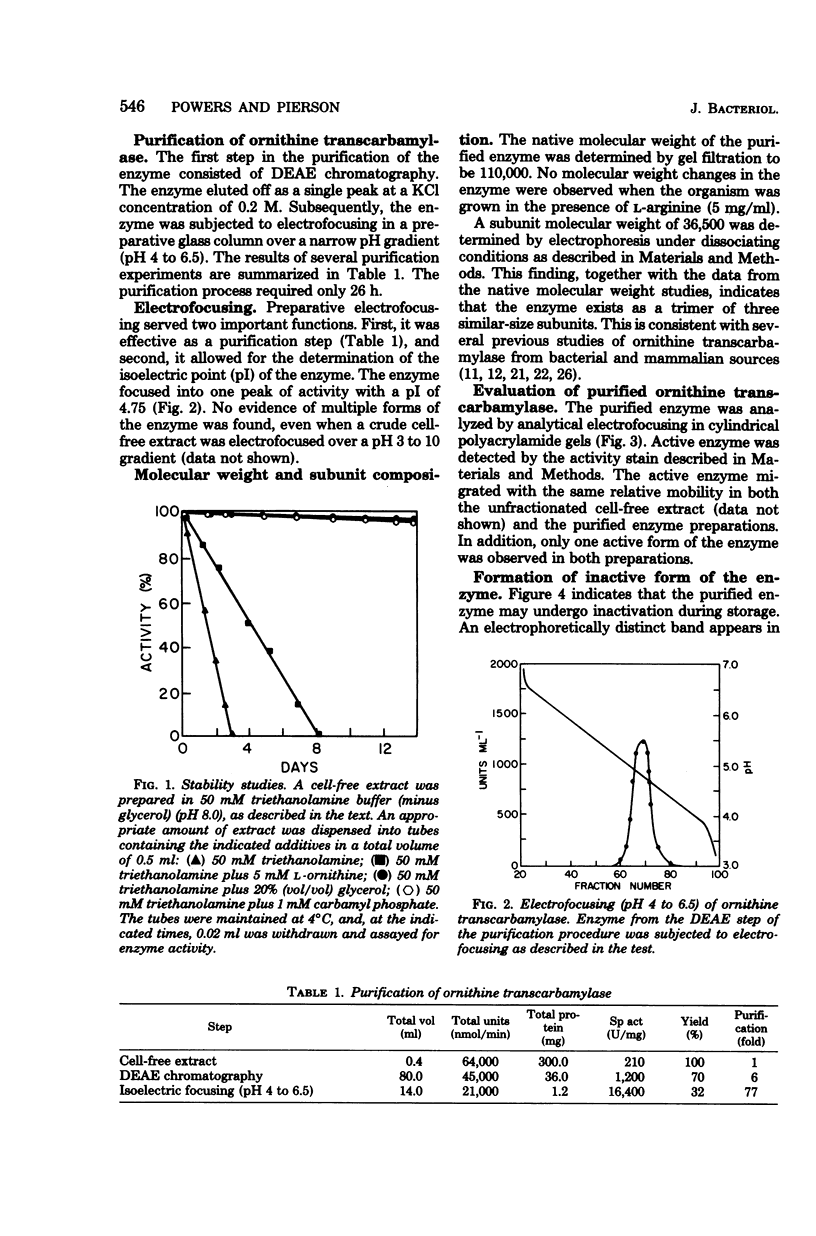
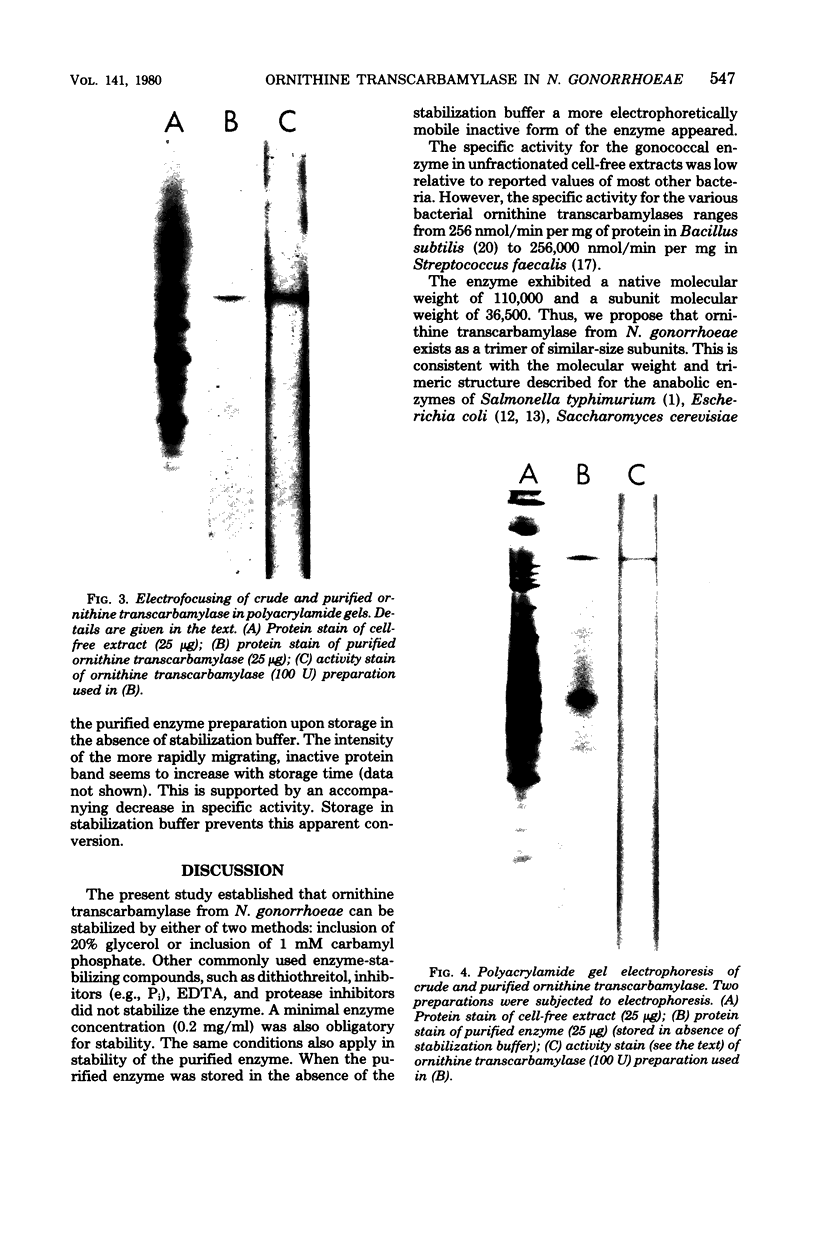


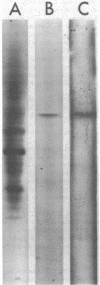
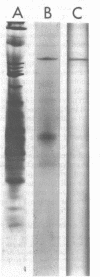
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdelal A. T., Kennedy E. H., Nainan O. Ornithine transcarbamylase from Salmonella typhimurium: purification, subunit composition, kinetic analysis, and immunological cross-reactivity. J Bacteriol. 1977 Mar;129(3):1387–1396. doi: 10.1128/jb.129.3.1387-1396.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron D. N., Buttery J. E. Electrophoretic separation and differentiation of enzymes from human and from porcine liver. J Clin Pathol. 1972 May;25(5):415–421. doi: 10.1136/jcp.25.5.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carifo K., Catlin B. W. Neisseria gonorrhoeae auxotyping: differentiation of clinical isolates based on growth responses on chemically defined media. Appl Microbiol. 1973 Sep;26(3):223–230. doi: 10.1128/am.26.3.223-230.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W. Nutritional profiles of Neisseria gonorrhoeae, Neisseria meningitidis, and Neisseria lactamica in chemically defined media and the use of growth requirements for gonococcal typing. J Infect Dis. 1973 Aug;128(2):178–194. doi: 10.1093/infdis/128.2.178. [DOI] [PubMed] [Google Scholar]
- Catlin B. W., Pace P. J. Auxotypes and penicillin susceptibilities of Neisseria gonorrhoeae isolated from patients with gonorrhea involving two or more sites. Antimicrob Agents Chemother. 1977 Aug;12(2):147–156. doi: 10.1128/aac.12.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halleux P., Legrain C., Stalon V., Piérard A., Wiame J. M. Regulation of the catabolic ornithine carbamoyltransferase of Pseudomonas fluorescens. A study of the quaternary structure. Eur J Biochem. 1972 Dec 4;31(2):386–393. doi: 10.1111/j.1432-1033.1972.tb02545.x. [DOI] [PubMed] [Google Scholar]
- Knapp J. S., Holmes K. K. Disseminated gonococcal infections caused by Neisseria gonorrhoeae with unique nutritional requirements. J Infect Dis. 1975 Aug;132(2):204–208. doi: 10.1093/infdis/132.2.204. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Legrain C., Halleux P., Stalon V., Glansdorff N. The dual genetic control of ornithine carbamolytransferase in Escherichia coli. A case of bacterial hybrid enzymes. Eur J Biochem. 1972 May;27(1):93–102. doi: 10.1111/j.1432-1033.1972.tb01814.x. [DOI] [PubMed] [Google Scholar]
- Legrain C., Stalon V., Glansdorff N. Escherichia coli ornithine carbamolytransferase isoenzymes: evolutionary significance and the isolation of lambdaargF and lambdaargI transducing bacteriophages. J Bacteriol. 1976 Oct;128(1):35–38. doi: 10.1128/jb.128.1.35-38.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain C., Stalon V., Noullez J. P., Mercenier A., Simon J. P., Broman K., Wiame J. M. Structure and function of ornithine carbamoyltransferases. Eur J Biochem. 1977 Nov 1;80(2):401–409. doi: 10.1111/j.1432-1033.1977.tb11895.x. [DOI] [PubMed] [Google Scholar]
- Legrain C., Stalon V. Ornithine carbamoyltransferase from Escherichia coli W. Purification, structure and steady-state kinetic analysis. Eur J Biochem. 1976 Mar 16;63(1):289–301. doi: 10.1111/j.1432-1033.1976.tb10230.x. [DOI] [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Ornithine transcarbamylase from Streptococcus faecalis and bovine liver. I. Isolation and subunit structure. J Biol Chem. 1972 Mar 25;247(6):1641–1653. [PubMed] [Google Scholar]
- Marshall M., Cohen P. P. Ornithine transcarbamylase from Streptococcus faecalis and bovine liver. II. Multiple binding sites for carbamyl-P and L-norvaline, correlation with steady state kinetics. J Biol Chem. 1972 Mar 25;247(6):1654–1668. [PubMed] [Google Scholar]
- Messenguy F., Penninckx M., Wiame J. M. Interaction between arginase and ornithine carbamoyltransferase in Saccharomyces cerevisiae. The regulatory site for ornithine. Eur J Biochem. 1971 Sep 24;22(2):277–286. doi: 10.1111/j.1432-1033.1971.tb01542.x. [DOI] [PubMed] [Google Scholar]
- Penninckx M., Simon J. P., Wiame J. M. Interaction between arginase and L-ornithine carbamoyltransferase in Saccharomyces cerevisiae. Purification of S. cerevisiae enzymes and evidence that these enzymes as well as rat-liver arginase are trimers. Eur J Biochem. 1974 Nov 15;49(2):429–442. doi: 10.1111/j.1432-1033.1974.tb03848.x. [DOI] [PubMed] [Google Scholar]
- Pierson D. L., Cox S. L., Gilbert B. E. Human ornithine transcarbamylase. Purification and characterization of the enzyme from normal liver and the liver of a Reye's syndrome patient. J Biol Chem. 1977 Sep 25;252(18):6464–6469. [PubMed] [Google Scholar]
- Prescott L. M., Jones M. E. Modified methods for the determination of carbamyl aspartate. Anal Biochem. 1969 Dec;32(3):408–419. doi: 10.1016/s0003-2697(69)80008-4. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Berlin C. M., Sweeney E. W., Carroll W. R. The generation of energy by the arginine dihydrolase pathway in Mycoplasma hominis 07. J Biol Chem. 1966 May 25;241(10):2228–2236. [PubMed] [Google Scholar]
- Shinners E. N., Catlin B. W. Arginine biosynthesis in Neisseria gonorrhoeae: enzymes catalyzing the formation of ornithine and citrulline. J Bacteriol. 1978 Oct;136(1):131–135. doi: 10.1128/jb.136.1.131-135.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalon V., Ramos F., Piérard A., Wiame J. M. Regulation of the catabolic ornithine carbamoyltransferase of Pseudomonas fluorescens. A comparison with the anabolic transferase and with a mutationally modified catabolic transferase. Eur J Biochem. 1972 Aug 18;29(1):25–35. doi: 10.1111/j.1432-1033.1972.tb01953.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]