Abstract
α-Synuclein (SNCA) plays an important role in the regulation of dopaminergic neurotransmission and neurodegeneration in Parkinson disease. We investigated reward and punishment learning in asymptomatic carriers of a rare SNCA gene duplication who were healthy siblings of patients with Parkinson disease. Results revealed that healthy SNCA duplication carriers displayed impaired reward and intact punishment learning compared with noncarriers. These results demonstrate that a copy number variation of the SNCA gene is associated with selective impairments on reinforcement learning in asymptomatic carriers without the motor symptoms of Parkinson disease.
Keywords: Parkinson disease, reinforcement learning, dopamine, basal ganglia
It has been demonstrated that the copy number variation of the α-synuclein gene (SNCA), which encodes a protein regulating dopamine turnover in the presynaptic terminal, causes Parkinson disease (1). The multiplication of the SNCA locus is very rare; beyond the few families reported in the literature (2), Ahn et al. (3) identified three patients with duplication from 1,106 screened individuals with parkinsonism. Each patient had biological relatives who were asymptomatic carriers. However, the penetrance of SNCA duplication is not exactly defined, and its behavioral consequences in asymptomatic carriers are unknown (4).
The protein encoded by the SNCA gene may serve as a regulator of the mesolimbic dopaminergic system and hence might play an important role in reward learning. Oksman et al. (5) showed that the absence of SNCA resulting from a spontaneous mutation in C57BL/6J mice enhanced operant behavior during intracranial self-stimulation, suggesting that the lack of SNCA sensitized the reward system of the brain. Transgenic mice overexpressing A30P-mutated SNCA exhibit reduced locomotor activity and smaller evoked dopamine release in the striatum (6). Evidence from a positron emission tomography study in humans suggests that high dopamine synthesis in the striatum results in better reversal learning from reward than from punishment, whereas low baseline dopamine synthesis is associated with the opposite pattern of performance (7).
In this study, we aimed to investigate the possible reinforcement learning phenotype of SNCA multiplication and overexpression in humans. We assessed seven asymptomatic healthy carriers of SNCA duplication who later developed Parkinson disease and 10 noncarrier comparison participants on a reward- and punishment-guided A/B probabilistic classification learning task. Participants could win or lose imaginary quarter dollars by deciding whether abstract images belong to category A or B (Fig. 1A). On reward trials, correct responses were followed by a 25-point gain. On punishment trials, incorrect responses were followed by a 25-point loss. Some images belonged to category A with 80% probability and to category B with 20% probability, whereas others had the opposite category probability. On some trials, feedback was not provided. Reward learning was also assessed with an independent probability learning task in which participants cannot lose points or avoid feedback (8).
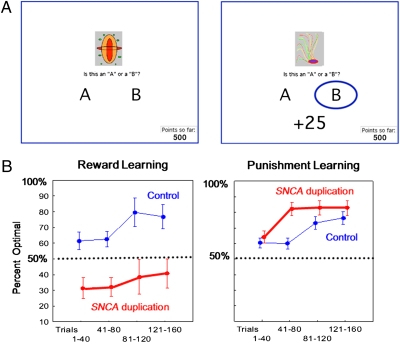
Fig. 1.
(A) Layout of probabilistic A/B classification learning task. Participants were asked to indicate whether an image belonged to category A or B by pressing different keys. (B) Performance on classification learning task. SNCA duplication carriers (n = 7) displayed selective impairment on reward learning task relative to noncarriers (n = 10). Error bars indicate SE.
Based on animal data suggesting the role of SNCA in reward learning (5, 6), we hypothesized, and subsequently confirmed, that healthy asymptomatic individuals with an extra copy of the SNCA gene show an analogous, selective deficit in reward learning.
Results
The expression of the SNCA protein [ratio of SNCA protein (ng/mL) and total protein (g/mL)] in peripheral mononuclear blood cells was increased in the asymptomatic carriers compared with that of the noncarrier participants [carriers: 37.3 (SD = 3.5), noncarriers: 23.9 (SD = 3.8), t(15) = 7.42, P < 0.0001].
The percentage of optimal category decisions in the A/B categorization task is shown in Fig. 1B. ANOVA revealed a significant difference between SNCA duplication carriers and noncarriers [F(1,15) = 8.8, P = 0.01]. Crucially, this difference was confined to reward learning, as revealed by the two-way interaction between carrier status (SNCA duplication carriers vs. noncarriers) and trial type (reward vs. punishment) [F(1,15) = 14.7, P = 0.002]. A Scheffé post hoc test performed on this two-way interaction confirmed that SNCA duplication carriers displayed an impaired performance on the reward learning task relative to noncarriers (P = 0.0007), whereas their performance was spared on the punishment task (P = 0.7) (Fig. 1B). Indeed, in the reward learning task, the performance of the SNCA duplication carriers remained well below the chance level (50%) on all trial blocks. ANOVA also revealed a significant main effect of task phase (four blocks of 20 trials) [F(1,15) = 11.2, P < 0.0001]. However, the interactions between carrier status and task phase, trial type, and task phase, as well as among carrier status, trial type, and task phase, were not significant (P > 0.2).
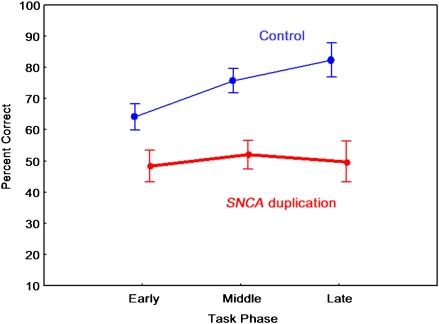
The percentage of correct choices in the reward-related probability learning task is shown in Fig. 2. ANOVA revealed significant main effects of group [(F(1,15) = 82.44, P < 0.001] and task phase (early, middle, and late trials) [F(2,30) = 17.01, P < 0.001]. The two-way interaction between group and task phase was significant [F(2,30) = 11.31, P < 0.001]. Scheffé tests indicated that SNCA duplication carriers exhibited lower performances on each phase of the task relative to controls (P < 0.005). The performance of the carriers was not significantly different from the chance level (Fig. 2).
Fig. 2.
Performance on reward-related probability learning task. SNCA duplication carriers (n = 7) displayed impaired performance relative to noncarriers (n = 10) in each phase of the task. Error bars indicate SE.
Discussion
Our results suggest that the duplication of the SNCA locus is associated with robustly impaired reward learning in asymptomatic carriers without the motor symptoms of Parkinson disease. In addition, carriers exhibited a slightly elevated performance on punishment learning relative to noncarriers. These behavioral alterations were present in the asymptomatic premotor stage of Parkinson disease. However, all carriers developed motor symptoms during a 5-y follow-up period after behavioral testing.
The finding that carriers performed below chance on reward learning indicates that they have learned the discrimination rule here but were responding consistently with the wrong answer. A possible explanation is that they were so punishment averse that, having learned to choose the outcome that leads to no feedback for the punishment-trained cues (which is the correct response), they overgeneralize this to the reward-trained cues so as to get the no-feedback response (which is the wrong answer). Another possibility is that the pattern of performance can be attributed to the initial choice; if the initial choice was no feedback, the participant did not change this strategy. However, if there had been an initial positive feedback, the participant may have maintained this strategy, resulting in an average below-chance performance. To test these confounding factors and to confirm our results using an independent paradigm, we administered a reward-related probability learning task without punishment signals (no point loss) and without the possibility of choosing a no-feedback strategy (8). Results revealed that SNCA duplication carriers exhibited a chance-level performance without any evidence of learning during the task, which is consistent with the hypothesis that these individuals exhibit impaired reward learning.
These results are similar to the findings that we obtained from young, nonmedicated patients with Parkinson disease (9) and to the predictions of computational models of cognitive reinforcement learning in parkinsonism (10). Although elderly patients with sporadic Parkinson disease who are temporarily withdrawn from dopaminergic medications do not show profound reward learning impairments (11), the case can be substantially different in young, never-medicated patients with early-onset disease (9) and in persons with genetic alterations. We speculate that reward-coding neurons in the substantia nigra (12, 13) may be affected in these individuals. Strikingly, none of the carriers in our study exhibited Parkinson disease at the time of behavioral testing, as they were in the premotor stage of the disease, which indicates that reward learning deficits can be dissociated from motor symptoms and can precede the onset of motor deficits. Impaired reward learning can be due to subtle neurodegenerative processes, but it is also possible that increased expression of SNCA reduced dopamine release in the nigrostriatal synapses (14). Although the [123I]β-CIT SPECT examination revealed no marked presynaptic dopaminergic abnormalities, more subtle alterations may have occurred, in addition to the possibility that the sample size was too small to detect significant changes. Nemani et al. (14) demonstrated that a slight overexpression of SNCA in the synaptic terminal, which is similar to the estimated effect of gene duplication in the absence of overt toxicity, significantly inhibits neurotransmitter release. Reduced synaptic vesicle density at the active zone and a deficient clustering of vesicles may explain how increased SNCA expression affects neurotransmitter release that precedes neuropathological changes (14).
SNCA multiplication is a rare cause of Parkinson disease (1–4), and so far only very few asymptomatic carriers have been reported with negative phenotypes for motor symptoms, olfaction, and rapid eye movement sleep behavior (3, 4). Here, we show, in this rare nonelderly sample, that carriers display reward learning deficits that may precede the development of clinical motor symptoms, providing a unique opportunity to further explore the nature of the premotor stage of Parkinson disease and to develop early biomarkers.
Methods
Participants.
Volunteers were seven siblings (five male and two female) of three Parkinson disease patients with SNCA duplication. All participants were of Caucasian origin. The siblings were asymptomatic carriers of SNCA duplication at the time of behavioral assessment. During a subsequent follow-up period, all carriers developed Parkinson disease and a marked cognitive decline, as revealed by the Mini-Mental State Examination (15) [baseline: 30.0 (SD = 0), follow-up: 22.4 (SD = 2.1]. The comparison group included 10 healthy volunteers without SNCA duplication. The carriers and the controls were matched for age, education, IQ (16), and Hollingshead's socioeconomic status (17) (Table 1). All participants were screened with the Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (18) and underwent a detailed neurological examination including routine head MRI and [123I]β-CIT SPECT. These assessments revealed no psychiatric disorders, neurological signs and symptoms, neuropsychological deficits, and dopamine transporter abnormalities in the participants at the time of behavioral testing (a neuropsychological characterization is provided in Table S1). All participants gave written informed consent, and the study was approved by the local ethics board.
Table 1.
Demographic characteristics of the participants
| Asymptomatic carriers | Controls | |
| Age (y) | 47.7 (8.6) | 45.6 (8.2) |
| Education (y) | 13.0 (3.5) | 12.2 (3.6) |
| IQ | 107.6 (14.2) | 109.3 (11.2) |
| Socioeconomic status | 37.6 (5.3) | 36.7 (5.6) |
Data are mean (SD). Student's t tests (two-tailed) revealed no significant differences between carriers and controls (P > 0.6).
Behavioral Testing.
A/B classification learning.
The A/B probabilistic classification task was administered according to the procedure of Bódi et al. (9). Stimuli were presented on a Macintosh iBook. On each trial, participants viewed one of four images (S1–S4), and were asked to guess whether it belonged to category A or B. On any given trial, stimuli S1 and S3 belonged to category A with 80% probability and to category B with 20% probability, whereas stimuli S2 and S4 belonged to category B with 80% probability and to category A with 20% probability. Stimuli S1 and S2 were used in the reward learning task. Thus, if the participant correctly guessed category membership on a trial, a reward of +25 points was received. If the participant guessed incorrectly, no feedback appeared. Stimuli S3 and S4 were used in the punishment learning task. If the participant guessed incorrectly on a trial with either of these stimuli, a punishment of –25 was received, and correct guesses received no feedback. Reward and punishment trials were intermixed so that no-feedback trials were potentially ambiguous. The ±25 points symbolized quarter dollars to win and lose, but no real payments were used in this study.
Reward-related probability learning.
The reward-related probability learning task was administered after the adaptation and modification of the procedure of Delgado et al. (8). Stimuli were presented on a personal computer. On a trial, participants saw a card depicting one of five cues (star, square, triangle, circle, or diamond) for 12 s. The task was to guess if the value of a card was higher or lower than the number 5. Each trial represented a different card or value. Each cue represented the probability (67%) that the card was higher or lower than the number 5. After the presentation of the card with a cue, a question mark appeared on the screen to prompt guessing by pressing up or down arrows on the computer keyboard (up, higher than five; down, lower than five). After the choice, the outcome (value of the card) was shown and correct choices were followed by a reward feedback (dollar signs and a smiling cartoon face appearing on the screen). The aim of the game was to maximize rewards by learning the probabilities over time. There were 120 trials followed by a reward feedback (24 presentations for each cue). If the participant's guess was wrong, the outcome was presented in the absence of loss. Participants were told that although the outcome of their guess was not right, they would not be punished in this game and that they could not lose money. Therefore, the task was biased toward the processing of reward feedback (“punishment” was only the absence of reward in wrong guesses). In the analysis, the 120 reward trials were included. The trials for each cue were divided into three phases. The early learning phase consisted of the first eight presentations of a cue; the middle learning phase consisted of the next eight presentations of a cue; and the late learning phase consisted of the last eight presentations of a cue (8).
Genetic Analysis and Protein Detection.
FISH and Affymetrix 250k SNP microarrays were used for genetic analysis. FISH was performed according to the protocol of Singleton et al. (1). SNCA PAC 27M07 (146 kb; AF163864) was labeled using fluorescein isothiocyanate, and SNCA promoter and intron four fragments (13 and 21 kb) were labeled with rhodamine. Samples were considered duplicated if they had three FISH probe signals in greater than 20% of interphase cells from 100 nuclei examined. The duplication status was confirmed using Affymetrix 250k SNP microarrays. Copy number was estimated using the dChipSNP software, as described previously (2). SNCA protein levels were measured from peripheral blood mononuclear cells using ELISA (Human Alpha-Synuclein Immunoassay Kit, Biosource Europe). The ratio of SNCA protein (ng/mL) and total protein (g/mL) was calculated to compare duplication carriers and noncarriers (19).
Statistical Analysis.
The dependent measure in the A/B categorization task was the percentage of optimal choices in four phases (20 trials each) of reward and punishment learning, which was entered into an ANOVA. The between-subject factor was the carrier status of the participants (SNCA duplication carriers vs. noncarrier comparison participants), and the within-subject factors were trial type (reward vs. punishment) and task phase (four blocks of 20 trials). In the reward-related probability learning task, the dependent measure was the percentage of correct choices. The between-subject factor was the gene duplication carrier status, and the within-subject factor was the task phase (early, middle, and late). Scheffé tests were used for post hoc comparisons. The SNCA protein levels were compared with Student t tests (two-tailed). The level of statistical significance was P < 0.05.
Supplementary Material
Acknowledgments
We thank Nathaniel Daw for his contribution to the design of the behavioral task. This work was supported by grants from the Bachman-Strauss Dystonia and Parkinson Foundation's Dekker Foundation Award (to M.A.G.) and by grants from the National Institutes of Health–National Institute of Neurological Disorders and Stroke (Grant R01 NS047434-02) and National Science Foundation Cognitive Neuroscience Program (Grant 0718153). S.K. is supported by the Hungarian Research Fund (OTKA NF72488).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006068107/-/DCSupplemental.
References
- 1.Singleton AB, et al. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 2.Ross OA, et al. Genomic investigation of alpha-synuclein multiplication and parkinsonism. Ann Neurol. 2008;63:743–750. doi: 10.1002/ana.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn TB, et al. alpha-Synuclein gene duplication is present in sporadic Parkinson disease. Neurology. 2008;70:43–49. doi: 10.1212/01.wnl.0000271080.53272.c7. [DOI] [PubMed] [Google Scholar]
- 4.Nishioka K, et al. Expanding the clinical phenotype of SNCA duplication carriers. Mov Disord. 2009;24:1811–1819. doi: 10.1002/mds.22682. [DOI] [PubMed] [Google Scholar]
- 5.Oksman M, Tanila H, Yavich L. Brain reward in the absence of alpha-synuclein. Neuroreport. 2006;17:1191–1194. doi: 10.1097/01.wnr.0000230507.70843.51. [DOI] [PubMed] [Google Scholar]
- 6.Yavich L, et al. Locomotor activity and evoked dopamine release are reduced in mice overexpressing A30P-mutated human alpha-synuclein. Neurobiol Dis. 2005;20:303–313. doi: 10.1016/j.nbd.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Cools R, et al. Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. J Neurosci. 2009;29:1538–1543. doi: 10.1523/JNEUROSCI.4467-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delgado MR, Miller MM, Inati S, Phelps EA. An fMRI study of reward-related probability learning. Neuroimage. 2005;24:862–873. doi: 10.1016/j.neuroimage.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Bódi N, et al. Reward-learning and the novelty-seeking personality: A between- and within-subjects study of the effects of dopamine agonists on young Parkinson's patients. Brain. 2009;132:2385–2395. doi: 10.1093/brain/awp094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank MJ, Seeberger LC, O'reilly RC. By carrot or by stick: Cognitive reinforcement learning in parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- 11.Cools R, Altamirano L, D'Esposito M. Reversal learning in Parkinson's disease depends on medication status and outcome valence. Neuropsychologia. 2006;44:1663–1673. doi: 10.1016/j.neuropsychologia.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaghloul KA, et al. Human substantia nigra neurons encode unexpected financial rewards. Science. 2009;323:1496–1499. doi: 10.1126/science.1167342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nemani VM, et al. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010;65:66–79. doi: 10.1016/j.neuron.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Wechsler D. Wechsler Adult Intelligence Scale—Revised Manual. New York: Psychological Corporation; 1981. [Google Scholar]
- 17.Cirino PT, et al. Measuring socioeconomic status: Reliability and preliminary validity for different approaches. Assessment. 2002;9:145–155. doi: 10.1177/10791102009002005. [DOI] [PubMed] [Google Scholar]
- 18.First MB, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington, DC: American Psychiatric Press; 1996. [Google Scholar]
- 19.Fuchs J, et al. Genetic variability in the SNCA gene influences alpha-synuclein levels in the blood and brain. FASEB J. 2008;22:1327–1334. doi: 10.1096/fj.07-9348com. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.