Abstract
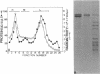
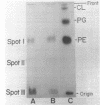
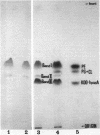
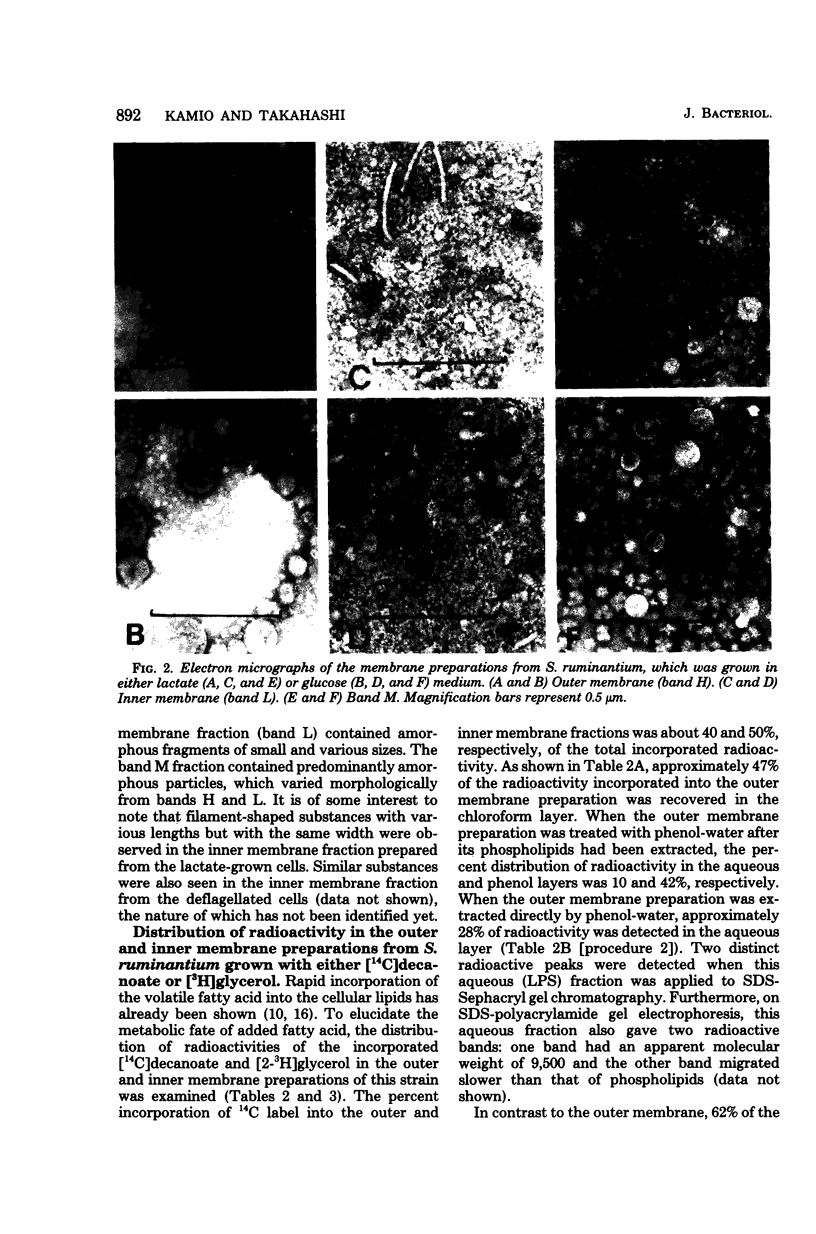
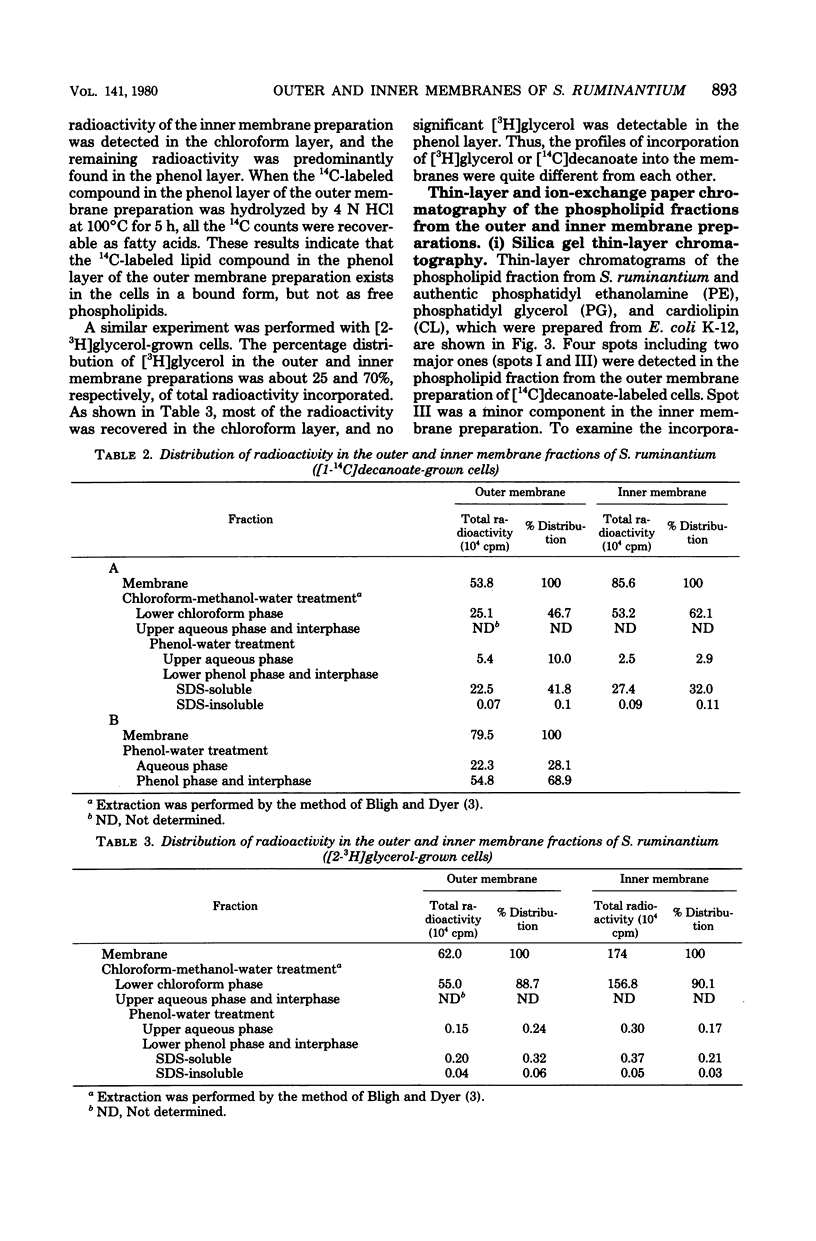
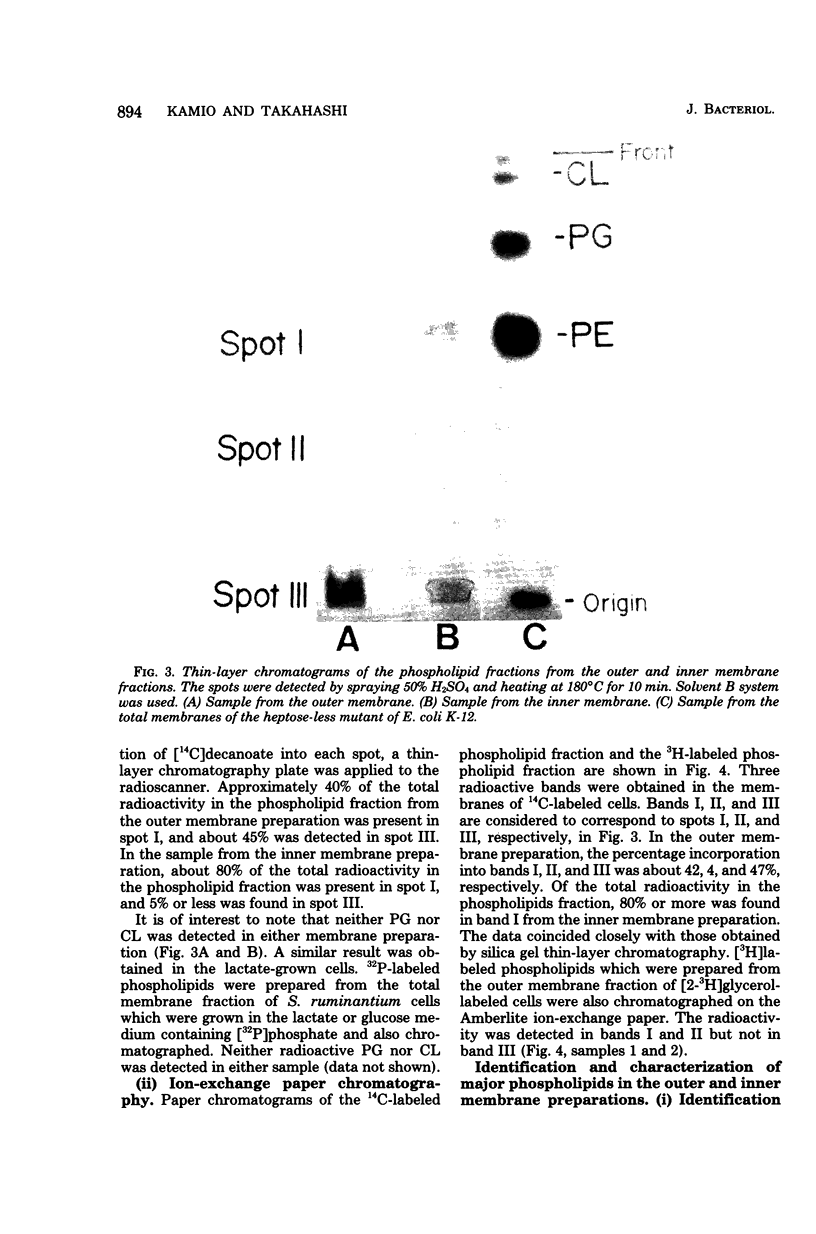
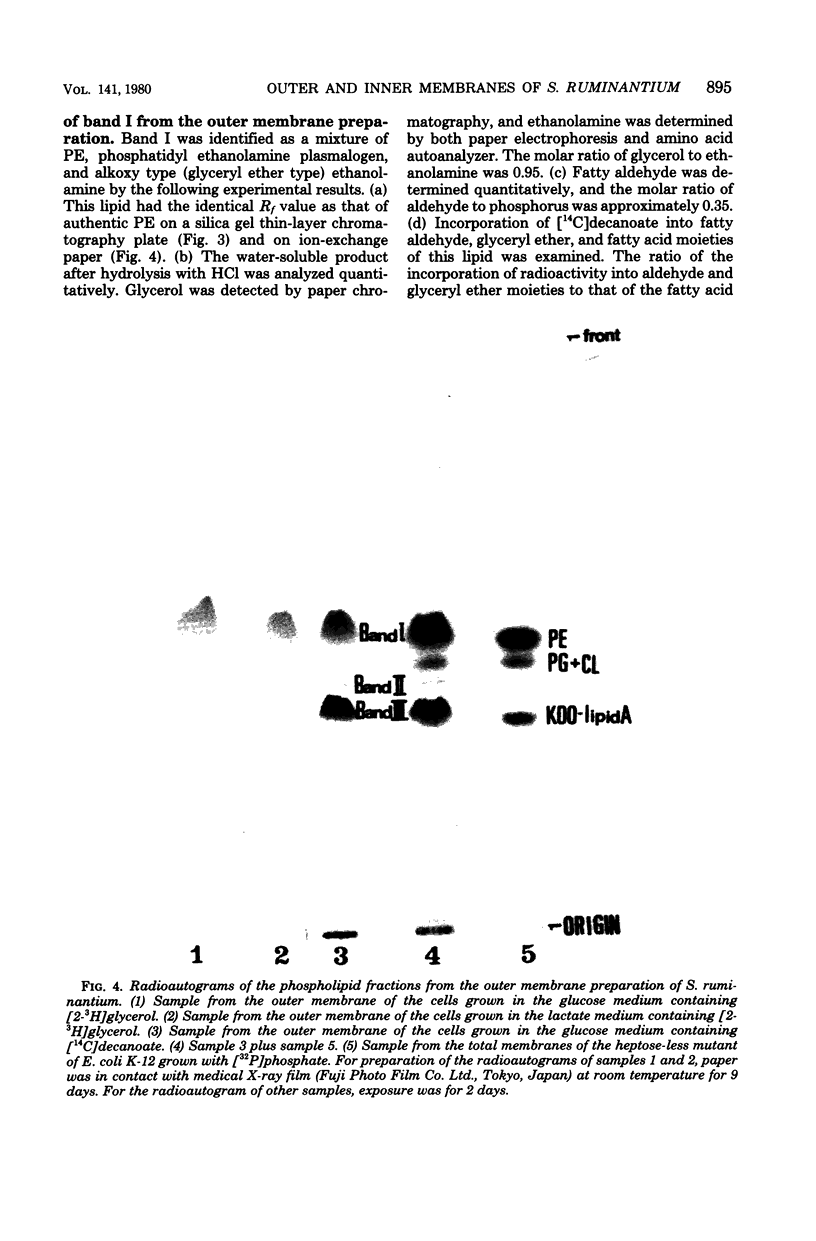
The isolation procedure and characterization of the outer and inner membranes from Selenomonas ruminatium cells, a strictly anaerobic bacterium, are described. The metabolic fate of [14C]decanoate incorporated into the outer and inner membranes was examined. The percent distribution of radioactivities in the outer and inner membranes was about 40 and 50% of the total incorporated activity, respectively. Approximately 47% of the radioactivity incorporated into the outer membrane was recovered in the phospholipid fraction, and the remaining radioactivity was found in both aqueous and phenol layers when the outer membrane was treated with phenol-water. In contrast to [14C]decanoate, the percent distribution of [3H]glycerol in the outer and inner membranes was about 25 and 70% of the total incorporated activity, respectively. Most of the assimilated 3H was located in the phospholipid fraction of both membranes. However, no significant label was detected in either the protein or cell wall fraction. The following observations were made concerning lipid compositions in the outer and inner membranes by chemical and isotopic analyses. (i) The outer and inner membranes contained no detectable phosphatidyl glycerol or cardiolipin. (ii) A prominent radioactive compound, designated band III lipid, was found mainly in the outer membrane as a major radioactive spot when cells were grown with [14C]decanoate. This lipid contained phosphorus, 2-keto-3-deoxyoctulosonic acid and 3-OH fatty acid but no detectable glycerol. This lipid was identified tentatively to be 2-keto-3-deoxyoctulosonic acid-lipid A. (iii) Although the ubiquity of phosphatidyl ethanolamine plasmalogen in both outer and inner membranes was confirmed, the occurrence of the molecular species of phosphatidyl ethanolamine plasmalogen was quite different in the outer and inner membranes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Lee F. D., Durston W. E. An improved bacterial test system for the detection and classification of mutagens and carcinogens. Proc Natl Acad Sci U S A. 1973 Mar;70(3):782–786. doi: 10.1073/pnas.70.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay P. K., Lai J. S., Wu H. C. Incorporation of phosphatidylglycerol into murein lipoprotein in intact cells of Salmonella typhimurium by phospholipid vesicle fusion. J Bacteriol. 1979 Jan;137(1):309–312. doi: 10.1128/jb.137.1.309-312.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay P. K., Wu H. C. Biosynthesis of the covalently linked diglyceride in murein lipoprotein of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5318–5322. doi: 10.1073/pnas.74.12.5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium. Identification of proteins exposed on cell surface. Biochim Biophys Acta. 1977 Feb 4;464(3):589–601. doi: 10.1016/0005-2736(77)90033-5. [DOI] [PubMed] [Google Scholar]
- Kamio Y., Takahashi H. Chemical structure of lipid A of Selenomonas ruminantium. J Biochem. 1971 Jul;70(1):187–191. doi: 10.1093/oxfordjournals.jbchem.a129619. [DOI] [PubMed] [Google Scholar]
- Kamio Y., Takahashi H. Outer membrane proteins and cell surface structure of Selenomonas ruminantium. J Bacteriol. 1980 Feb;141(2):899–907. doi: 10.1128/jb.141.2.899-907.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanegasaki S., Takahashi H. Function of growth factors for rumen microorganisms. I. Nutritional characteristics of Selenomonas ruminantium. J Bacteriol. 1967 Jan;93(1):456–463. doi: 10.1128/jb.93.1.456-463.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mizushima S., Nakarmura K. [Assembly of E coli outer membrane (author's transl)]. Tanpakushitsu Kakusan Koso. 1977;22(1):21–30. [PubMed] [Google Scholar]
- Mizushima S., Yamada H. Isolation and characterization of two outer membrane preparations from Escherichia coli. Biochim Biophys Acta. 1975 Jan 14;375(1):44–53. doi: 10.1016/0005-2736(75)90071-1. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J Bacteriol. 1975 Nov;124(2):942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wober W., Alaupović P. Studies on the protein moiety of endotoxin from gram-negative bacteria. Characterization of the protein moiety isolated by acetic acid hydrolysis of endotoxin from Serratia marcescens 08. Eur J Biochem. 1971 Apr;19(3):357–367. doi: 10.1111/j.1432-1033.1971.tb01324.x. [DOI] [PubMed] [Google Scholar]
- Wober W., Alaupović P. Studies on the protein moiety of endotoxin from gram-negative bacteria. Characterization of the protein moiety isolated by phenol treatment of endotoxin from Serratia marcescens 08 and Escherichia coli 0 141:K85(B). Eur J Biochem. 1971 Apr;19(3):340–356. doi: 10.1111/j.1432-1033.1971.tb01323.x. [DOI] [PubMed] [Google Scholar]