Abstract
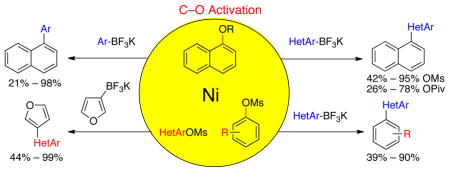
A general method based on nickel-catalyzed C–O activation of various phenol derivatives with potassium (hetero)aryltrifluoroborates has been developed. A large number of heterobiaryls can be easily obtained with yields up to 99% using methanesulfonate cross-coupling partners.
Transition metal-catalyzed cross-coupling reactions are among the most powerful transformations available to create carbon–carbon or carbon–heteroatom bonds. In particular, the Suzuki–Miyaura reaction represents one of the most common and reliable methods because of its tolerance of a wide range of functional groups, as well as the commercial availability and stability of its reagents.1 Usually, aryl halides and triflates serve as electrophilic coupling partners but there often remain some limitations of these reaction partners because of their toxicity, their cost or their inaccessibility.
An alternative is to use phenol derivatives bearing more environmentally sound, less expensive, and easier to handle nucleofuges. Palladium catalysts have shown promising results for the activation of the C–O bond when sulfonates such as tosylates2 or mesylates3 were used as leaving groups (LGs) but have been completely ineffective with the corresponding esters.4 More reactive nickel catalysts have been used in cross-couplings of diverse phenol derivatives. Relatively unreactive tosylate5 and mesylate5b,6 groups were first engaged with success with arylboronic acid derivatives. Recently ester and amide type nucleofuges (pivaloyl, acetyl, benzoyl, carbonate, sulfamate, carbamate)4a,7 as well as methyl ethers 8 have also been successfully used, emphasizing the importance of nickel as a general catalyst in C–O activation processes. Nearly all of these protocols employ a large excess (2–5 equiv) of the organoboron coupling partner.
Despite the significant advances in the pool of available electrophiles and their cross-coupling, much effort remains to generalize the transformation. To date, very few examples of palladium catalyzed C-O activation have been described with heteroarylboronic acids. Buchwald first reported a very efficient palladium cross-coupling of tosylated2c and mesylated3c phenols with an excess of the boron reagent (2 equiv). With the same kind of catalytic system, but decreasing the loading of the boron species to 1.1 equiv, Wu observed a low yield in the cross-coupling of tosylated phenol with potassium thiophen-2-yltrifluoroborate.2d During the course of our investigation, Kwong reported a palladium-catalyzed C–O activation of heteroaryl mesylates with a single potassium heteroaryltrifluoroborate in excess (2 equiv) to afford heterobiaryls with yields up to 82%.3d Moreover, all nickel-catalyzed systems have been limited to arylboronic acid derivatives. In view of the ubiquity of heterobiaryls in natural products, polyaromatic molecules, pharmaceuticals and other useful materials, it is of interest to develop general protocols to forge C(heteroaryl)-C(aryl or heteroaryl) bonds.
Potassium organotrifluoroborates have emerged as alternative coupling partners that provide some advantages to sensitive boronic acid derivatives, which more easily undergo protodeboronation.9 Herein, we disclose the first nickel-catalyzed Suzuki cross-coupling of phenol derivatives with potassium heteroaryltrifluoroborates. The developed reaction proceeds with exceptional heterocycle compatibility and generally high yields.
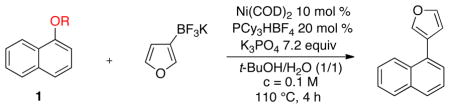
We initially chose the pivaloyl-protected naphthol as the substrate for the C–O activation. An extensive screening of various reaction parameters (e.g., choice of solvent, nickel catalyst, ligand, base, etc.) was carried out10 and revealed the essential role of Ni(COD)2 as a catalyst, while other Ni(II) and Ni(0) species failed. This reaction unusually occurs in the presence of a large amount of water in a protic solvent (tert-butanol/H2O in a 1/1 ratio).7a,c Moreover, we were able to reduce the loading of potassium organotrifluoroborate to 1.3 equiv.
With the optimized conditions established for the pivaloyl LG 1d, we next attempted to extend the process to various LGs on naphthol in the Suzuki–Miyaura cross-coupling with potassium furan-3-yltrifluoroborate (Table 1). Thus, the sulfonate LGs gave the best results, with mesylate 1a (which afforded the desired compound in quantitative GC yield) functioning better than the tosylate 1b and the sulfamate 1c. Both ester (Piv and Boc) and amide (CONEt2) type LGs, even though they are less reactive, are also well-tolerated. The benzoyl group proved much less efficient, and the methyl ether is not suitable under these conditions.
Table 1.
Influence of the Nucleofuge on Naphthol for the Nickel-catalyzed C–O Activation
 | |||
|---|---|---|---|
| entry | OR | yield (%)a | |
| 1 | OMs | 1a | 100 |
| 2 | OTs | 1b | 85 |
| 3 | OSO2NMe2 | 1c | 85 |
| 4 | OPiv | 1d | 68 |
| 5 | OBoc | 1e | 59 |
| 6 | OCONEt2 | 1f | 55 |
| 7 | OBz | 1g | 37 |
| 8 | OMe | 1h | / |
Relative GC yield determined using dodecane as the internal standard.
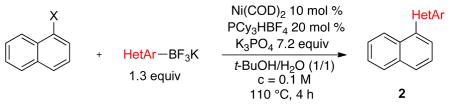
Encouraged by these results, we pursued our study by varying the potassium heteroaryltrifluoroborates with two classes of derivatives: mesylates (1a) and pivaloylates (1d) (Table 2). We synthesized six heterobiaryls 2a–2f from these derivatized naphthols (Table 2, entries 1–6), and the same tendency as previously described was observed: the mesylated compounds always afforded the heteroaryls with higher yields than the pivaloyl compounds.11 Although the yields are moderate with the pivaloyl substrate, the current method complements and even improves upon previous methods in its use of reduced quantities of the organoboron reagent,4a and is the first metal-catalyzed cross-coupling reaction between a phenolic pivaloylate and a heteroarylboron reagent.
Table 2.
Scope of Potassium Heteroaryltrifluoroborates
 | ||||
|---|---|---|---|---|
| entry | HetAr-BF3K | yield (%) | ||
| 1 | X = OMs | 2a | 93 | |
| X = OPiv | 78 | |||
| 2 | X = OMs | 2b | 90 | |
| X = OPiv | 75a | |||
| 3 | X = OMs | 2c | 64 | |
| X = OPiv | 26a, b | |||
| 4 | 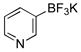 |
X = OMs | 2d | 82 |
| X = OPiv | 60 | |||
| 5 | X = OMs | 2e | 69 | |
| X = OPiv | 32b | |||
| 6 | 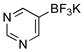 |
X = OMs | 2f | 83 |
| X = OPiv | 26b | |||
| 7 | X = OMs | 2g | 79 | |
| 8 | 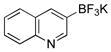 |
X = OMs | 2h | 95 |
| 9 | X = OMs | 2i | 42 | |
| 10 | X = OMs | 2j | 81 | |
| 11 | 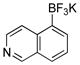 |
X = OMs | 2k | 91 |
| 12 | 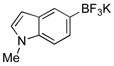 |
X = OMs | 2l | 74 |
| 13 | 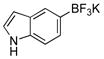 |
X = OMs | 2m | 90c |
Dioxane/H2O (1/1), c = 0.05 and t-BuPCy2.
Relative GC yield determined using dodecane as an internal standard.
With 10% of impurities that cannot be separated.
Five-membered, six-membered and benzannulated heterocycles have successfully been engaged with a mesylated naphthol with yields up to 95% (Table 2, entry 8). 3-Furanyl and 3-thiophenyl derivatives were coupled with very high yields, 93% and 90%, respectively (Table 2, entries 1 and 3). Although the 2-boronated counterparts usually suffer from facile protodeboronation, the desired products 2c and 2g were obtained with good yields. Pyridine derivatives, known as difficult cross-coupling partners owing to their low stability and electron deficiency,12 react efficiently to afford the desired compounds 2d and 2e in good yields (Table 2, entries 4 and 5). Pyrimidine, quinoline, benzofuran, isoquinoline and indole derivatives are also very good nucleophilic partners, leading to the desired compounds 2f, 2h and 2j–l with yields ranging from 74% to 95%. Even indole derivative 2m was obtained in 90% yield. Only the 2-benzothiophene was more sensitive to protodeboronation, affording 2i in a yield of 42%.
Notably, by increasing the scale of the reaction to 5.0 mmol, the Ni(COD)2 loading can be decreased to 2 mol % to obtain 2a with a yield of 86% (compared to 93% on a 0.25 mmol scale).13 Importantly, a comparable loading of the more expensive palladium catalyst is required for the same kind of C–O activation, emphasizing the role of nickel as an efficient and cost effective catalyst.14
We next evaluated the tolerance of the method by introducing functionalized potassium aryltrifluoroborates (Table 3). We were pleased to observe that both electron-donating (Table 3, entries 2–5) or electron-withdrawing groups (Table 3, entries 7, 9 and 10) on the aryl ring did not affect the reaction. The desired compounds 3a–e, g, i, j were obtained with yields above 71%. The position of the methoxy substituent on the aryl ring was also varied, and even in the more sterically hindered ortho-position, the desired compound 3e was obtained with a yield of 85%. The low yields observed with the aldehyde and ester substituents underscore the limits of the method. The aldehyde substrate afforded a complex mixture of products, whereas the ester substrate only proceeded to ~60% conversion, even after extended periods of time.
Table 3.
Scope of Potassium Aryltrifluoroborates
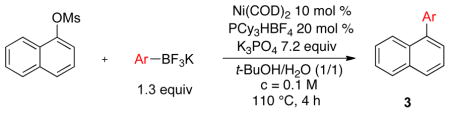 | |||
|---|---|---|---|
| entry | Ar-BF3K | yield (%) | |
| 1 | 3a | 71 | |
| 2 | 3b | 76 | |
| 3 | 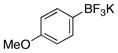 |
3c | 91 |
| 4 | 3d | 98 | |
| 5 | 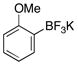 |
3e | 85 |
| 6 | 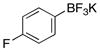 |
3f | 90 |
| 7 | 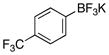 |
3g | 71 |
| 8 | 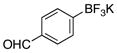 |
3h | 21 |
| 9 | 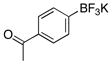 |
3i | 94 |
| 10 | 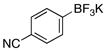 |
3j | 71 |
| 11 | 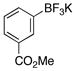 |
3k | 46 |
Various mesylated heteroaryls were also investigated with the potassium furan-3-yltrifluoroborate as coupling partners (Table 4). The cross-couplings proceeded in very high yields ranging from 70% to 99% for all the pyridine derivatives 5a–e (Table 4, entries 1–5). Benzothiazole 4f also appears to be a good electrophilic partner to obtain the desired heterobiaryl 5f in 77% yield. Only indole partner 4g showed a lower reactivity, affording the desired compound 5g in a moderate yield.
Table 4.
Scope of Heteroaryl Mesylates
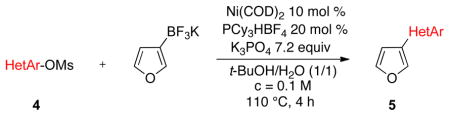 | ||||
|---|---|---|---|---|
| entry | HetAr-OMs | product | yield (%) | |
| 1 | 4a | 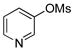 |
5a | 80 |
| 2 | 4b | 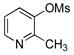 |
5b | 70 |
| 3 | 4c | 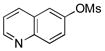 |
5c | 99 |
| 4 | 4d | 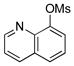 |
5d | 92 |
| 5 | 4e | 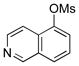 |
5e | 87 |
| 6 | 4f | 5f | 77 | |
| 7 | 4g | 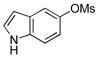 |
5g | 44 |
Finally, functionalized aryl mesylates were also tested with different potassium heteroaryltrifluoroborates (Table 5). The various electronic properties of the different substituents on the electrophilic counterpart do not seem to influence the reaction. Heteroaryls bearing a nitrile (6b), a methoxy group (6c), a ketone (6d) or an ester (6e) were obtained with good to excellent yields (Table 5, entries 1 to 5). Even a hindered, deactivated aryl mesylate (entry 6) reacts with potassium isoquinolin-5-yltrifluoroborate to generate the desired compound 6f with a yield of 39%.
Table 5.
Scope of Functionalized Mesylated Phenols with Various Potassium Heteroaryltrifluoroborates
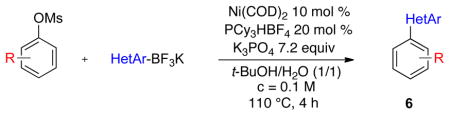 | ||||
|---|---|---|---|---|
| entry | Ar-OMs | HetAr-BF3K | product | yield (%) |
| 1 | 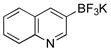 |
6a | 75 | |
| 2 | 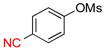 |
6b | 68 | |
| 3 | 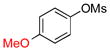 |
6c | 70 | |
| 4 | 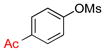 |
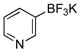 |
6d | 90 |
| 5 | 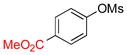 |
6e | 81 | |
| 6 | 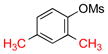 |
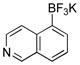 |
6f | 39 |
In conclusion, we have disclosed the first general nickel-catalyzed method for C-O activation with potassium heteroaryltrifluoroborates. Promising results were obtained when the pivaloyl moiety was used as the nucleofuge, and very good yields were achieved when the mesyl group was employed. The reaction is very tolerant of a large range of activated and deactivated nucleophilic potassium heteroaryl- and functionalized aryltrifluoroborates. Numerous electrophilic heteroaryl and functionalized aryl partners can also be introduced with high cross-coupling yields. Moreover, scaling up the reaction, the nickel catalyst loading can be decreased to 2 mol %, affording a very efficient and inexpensive method for C-O activation.
Supplementary Material
Acknowledgments
We thank the NIGMS (R01 GM035249) for their generous support. We acknowledge Frontier Scientific for its donation of heteroarylboronic acids. Dr. Rakesh Kohli (University of Pennsylvania) is acknowledged for obtaining HRMS data.
Footnotes
Supporting Information Available Experimental procedures and spectral data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Miyaura N, Suzuki A. Chem Rev. 1995;95:2457–2483. [Google Scholar]; (b) Suzuki A. J Organomet Chem. 1999;576:147–168. [Google Scholar]; (c) Martin R, Buchwald SL. Acc Chem Res. 2008;41:1461–1473. doi: 10.1021/ar800036s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.For Pd-catalyzed cross-coupling of aryl tosylates Roy AH, Hartwig JF. Organometallics. 2004;23:194–202.Petersen MD, Boye SV, Nielsen EH, Willumsen J, Sinning S, Wiborg O, Bols M. Bioorg Med Chem. 2007;15:4159–4174. doi: 10.1016/j.bmc.2007.03.069.Nguyen HN, Huang X, Buchwald SL. J Am Chem Soc. 2003;125:11818–11819. doi: 10.1021/ja036947t.Zhang LA, Meng TH, Wu J. J Org Chem. 2007;72:9346–9349. doi: 10.1021/jo7019064.So CM, Lau CP, Chan ASC, Kwong FY. J Org Chem. 2008;73:7731–7734. doi: 10.1021/jo8014819.
- 3.For Pd-catalyzed cross-coupling of aryl mesylates Percec V, Bae JY, Hill DH. J Org Chem. 1995;60:1060–1065.So CM, Lau CP, Kwong FY. Angew Chem Int Ed. 2008;47:8059–8063. doi: 10.1002/anie.200803193.Bhayana B, Fors BP, Buchwald SL. Org Lett. 2009;11:3954–3957. doi: 10.1021/ol9015892.Chow WK, So CM, Lau CP, Kwong FY. J Org Chem. 2010;75:5109–5112. doi: 10.1021/jo100846t.
- 4.(a) Quasdorf KW, Tian X, Garg NK. J Am Chem Soc. 2008;130:14422–14423. doi: 10.1021/ja806244b. [DOI] [PubMed] [Google Scholar]; (b) Li Z, Zhang SL, Fu Y, Guo QX, Liu L. J Am Chem Soc. 2009;131:8815–8823. doi: 10.1021/ja810157e. [DOI] [PubMed] [Google Scholar]
- 5.For recent examples of Ni-catalyzed Suzuki cross-coupling of aryl tosylates Zim D, Lando VR, Dupont J, Monteiro AL. Org Lett. 2001;3:3049–3051. doi: 10.1021/ol016526l.Percec V, Golding GM, Smidrkal J, Weichold O. J Org Chem. 2004;69:3447–3452. doi: 10.1021/jo049940i.Tang ZY, Hu QS. J Am Chem Soc. 2004;126:3058–3059. doi: 10.1021/ja038752r.Tang ZY, Spinella S, Hu QS. Tetrahedron Lett. 2006;47:2427–2430.Lipshutz BH, Butler T, Swift E. Org Lett. 2008;10:697–700. doi: 10.1021/ol702453q.
- 6.For examples of Ni-catalyzed Suzuki cross-coupling of aryl mesylates Percec V, Bae JY, Hill DH. J Org Chem. 1995;60:1060–1065.Ueda M, Saitoh A, Oh-tani S, Miyaura N. Tetrahedron. 1998;54:13079–13086.Kobayashi Y, William AD, Mizojiri R. J Organomet Chem. 2002;653:91–97.Kuroda JI, Inamoto K, Hiroya K, Doi T. Eur J Org Chem. 2009:2251–2261.
- 7.(a) Guan BT, Wang Y, Li BJ, Yu DG, Shi ZJ. J Am Chem Soc. 2008;130:14468–14470. doi: 10.1021/ja8056503. [DOI] [PubMed] [Google Scholar]; (b) Quasdorf KW, Riener M, Petrova KV, Garg NK. J Am Chem Soc. 2009;131:17748–17749. doi: 10.1021/ja906477r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Antoft-Finch A, Blackburn T, Snieckus V. J Am Chem Soc. 2009;131:17750–17752. doi: 10.1021/ja907700e. [DOI] [PubMed] [Google Scholar]; (d) Xu L, Li BJ, Wu ZH, Lu XY, Guan BT, Wang BQ, Zhao KQ, Shi ZJ. Org Lett. 2010;12:884–887. doi: 10.1021/ol9029534. [DOI] [PubMed] [Google Scholar]
- 8.Tobisu M, Shimasaki T, Chatani N. Angew Chem Int Ed. 2008;47:4866–4869. doi: 10.1002/anie.200801447. [DOI] [PubMed] [Google Scholar]
- 9.(a) Molander GA, Canturk B. Angew Chem Int Ed. 2009;48:9240–9261. doi: 10.1002/anie.200904306. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Molander GA, Canturk B, Kennedy LE. J Org Chem. 2009;74:973–980. doi: 10.1021/jo802590b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Knapp DM, Gillis EP, Burke MD. J Am Chem Soc. 2009;131:6961. doi: 10.1021/ja901416p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.See the Supporting Information for more details about the screening.
- 11.For potassium thiophen-2- and 3-yltrifluoroborates with pivaloyl protected naphthol, the best yields were obtained with slightly different optimized conditions: a mixture of dioxane/H2O (1/1), c = 0.05 and t-BuPCy2.
- 12.Barder TE, Walker SD, Martinelli JR, Buchwald SL. J Am Chem Soc. 2005;127:4685–4696. doi: 10.1021/ja042491j. [DOI] [PubMed] [Google Scholar]
- 13.1-NaphthOMs (1 equiv), furan-3-ylBF3K (1.3 equiv), Ni(COD)2 (2 mol %), PCy3HBF4 (4 mol %) and K3PO4 (7.2 equiv), 110 °C, 24 h.
- 14.Current prices for the catalysts are: bis(1,5-cyclooctadiene)nickel(0), $27/1 g; palladium(II) acetate, $59/1 g.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


