Abstract
Neutrophils rely on exocytosis to mobilize receptors and adhesion molecules and to release microbicidal factors. This process should be strictly regulated because uncontrolled release of toxic proteins would be injurious to the host. In vivo studies showed that the small GTPase Rab27a regulates azurophilic granule exocytosis. Using mouse neutrophils deficient in Rab27a (Rab27aash/ash), Rab27b (Rab27b knockout) or both (Rab27a/b double KO), we investigated the role of the Rab27 isoforms in neutrophils. We found that both Rab27a and Rab27b deficiencies impaired azurophilic granule exocytosis. Rab27aash/ash neutrophils showed upregulation of Rab27b expression which did not compensate for the secretory defects observed in Rab27a-deficient cells suggesting that Rab27 isoforms play independent roles in neutrophil exocytosis. Total internal reflection fluorescence microscopy analysis showed that Rab27aash/ash and Rab27b knockout neutrophils have a decreased number of azurophilic granules near the plasma membrane. The effect was exacerbated in Rab27a/b double KO neutrophils. Rab27-deficient neutrophils, showed impaired activation of the NADPH oxidase at the plasma membrane although intraphagosomal ROS production was not affected. Exocytosis of secretory vesicles in Rab27-deficient neutrophils was functional, suggesting that Rab27 GTPases selectively control the exocytosis of neutrophil granules.
INTRODUCTION
The cargo and membrane proteins stored in neutrophil secretory organelles participate in the execution of many neutrophil functions including adhesion, chemotaxis, phagocytosis and killing (1). Besides their role in the innate immune response, neutrophil granular proteins have been involved in other physiological and pathological processes including angiogenesis (2), vascular inflammatory disorders (3), tumor progression and metastasis (4). Due to the importance of the exocytic process in neutrophils and the potential deleterious effect of the toxic content of neutrophil granules, neutrophil degranulation requires precise mechanisms of control. Despite the significance of this cellular process, the molecular components of the machinery that regulates the mobilization of neutrophil secretory organelles have not been completely characterized.
Based on their protein content, density and tendency to undergo exocytosis, four types of granules and vesicles have been described in human neutrophils (1). This includes the azurophilic (primary) granules which contain myeloperoxidase, elastase and α-defensins as well as other antimicrobial factors (5), the peroxidase-negative specific (secondary) granules, gelatinase (tertiary) granules and secretory vesicles. Two other types of mobilizable neutrophil organelles have been described: multivesicular bodies (MVB) and multilaminar components, both enriched in the glycoproteins LAMP-1 (lysosome-associated membrane protein 1) and LAMP-2 (6), but not in LAMP-3 which localizes at azurophilic granule membranes (7). Although murine neutrophil secretory organelles are not as well characterized, it is generally accepted that they contain azurophilic granules (8), specific granules (9) and secretory vesicles (10).
During the phagocytosis of microorganisms, azurophilic granules fuse with the phagosome where their antibacterial proteins and peptides play a central role in bacterial killing (11). In response to soluble and particulate stimuli, azurophilic granules also undergo exocytosis (10, 12) and their cargo proteins participate in extracellular microorganism killing (13). Similarly, specific granules can mobilize to the phagosome or release their content to the extracellular milieu. Specific granules contain modulators of the inflammatory response including lactoferrin and matrix metalloproteinase 9 (MMP-9) (14, 15). Gelatinase granules also contain MMP-9 but lack lactoferrin (16). Both specific and gelatinase granules are the main storage organelles of the membrane associated subunit of the NADPH oxidase, the cytochrome b558. Secretory vesicles are readily releasable organelles that undergo exocytosis in response to weak stimulation (16). They play a fundamental role in the response of neutrophils to infections. They are enriched in β2 integrins (CD11b/CD18) as well as in membrane receptors including complement receptor 1, the receptor for formylated peptides and the TLR complex molecule CD14.
Neutrophil exocytosis is dependent on GTP availability (17). The participation of small GTPases in the regulation of exocytosis in granulocytes has been also suggested (18). Rab27a, a small GTPase known to play a general role in exocytosis, is expressed in neutrophils (19, 20). Patients with genetic defects in the rab27a gene (type 2 Griscelli syndrome, GS) develop an immunodeficiency disorder characterized by malfunctioning cytotoxic T-lymphocytes and by impaired natural killer cell function (21, 22). Two case reports suggested that patients with GS may have defects in the function of their granulocytes (22, 23). However, further analysis is necessary to clarify the role of this GTPase in neutrophil function. We have previously shown that Rab27a-deficient neutrophils form extracellular traps (NETs) (24). We have also demonstrated that Rab27a-deficient mice present with impaired secretion of myeloperoxidase when challenged with lipopolysaccharide in vivo (19). At the molecular level, Rab27a regulates exocytosis through interaction with specific effector molecules; eleven Rab27a effector molecules have been identified to date (25). Two of these effectors, JFC1/Slp1 and Munc13-4 are expressed in neutrophils and were demonstrated by our group to regulate exocytosis in these cells (10, 19). In those studies, using human neutrophils, Rab27a effector-downregulated granulocytes and Munc13-4-deficient murine neutrophils, we demonstrated that Munc13-4 function is important for the exocytic mechanism of a variety of structurally and functionally different secretory organelles in neutrophils while JFC1 plays a more specific role in regulating azurophilic granule exocytosis (10, 19).
Rab27b shares 72% homology with Rab27a at the amino acid level (26) and can partially compensate for the absence of Rab27a in some cellular systems (27). Although Rab27b has the ability to bind to Rab27a effectors in overexpression experiments, only Granuphilin (Slp4), MyRIP (Slac2-c) and Noc 2 have been shown to bind to both Rab27 proteins at the endogenous protein level (reviewed by Izumi (28)). Despite these similarities, the expression of Rab27b is thought to be far more restricted than that of Rab27a. Thus, while Rab27a is widely expressed and has been demonstrated to play a role in the regulation of exocytosis in many tissues with secretory function (29), Rab27b has been shown to play a significant role in exocytosis in a limited number of cellular systems including platelets (30), pituitary gland (31), pancreatic acinar cells (32), parotid acinar cells (33) and mast cells (34). However, using a knockin mouse model expressing LacZ downstream of the endogenous Rab27b promoter, the expression of Rab27b in a wider range of tissues was predicted (35). So far, a possible role of Rab27b in neutrophil exocytosis is currently unknown. In this work, using unique genetically modified mouse models, we analyze the role of Rab27a and Rab27b in neutrophil function and demonstrate that they play key, yet different, roles in the regulation of neutrophil granule exocytosis.
RESULTS
Rab27b is expressed in neutrophils and its expression is upregulated in the absence of Rab27a
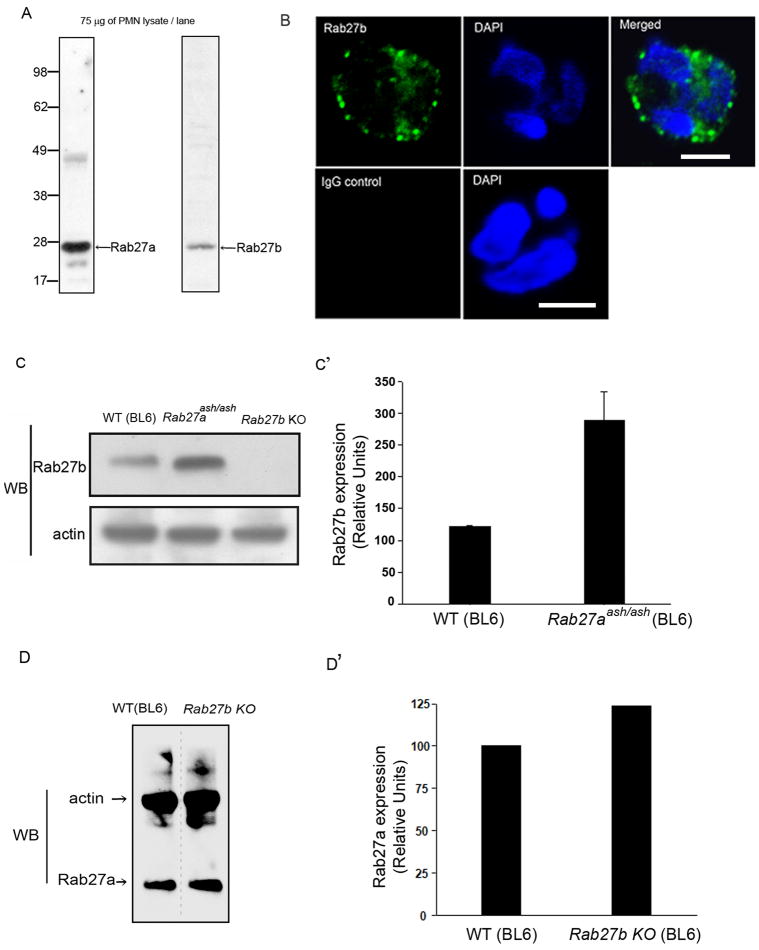
Rab27b is 72% homologous to Rab27a, ((26) and Supplementary Fig. S1) binds to Rab27a effectors and plays an important role in the regulation of many secretory mechanisms (28). Although its expression is more restricted than that of Rab27a, in some cellular systems Rab27a and Rab27b coexist (30, 34). Despite their close homology, Rab27a and Rab27b seem to play rather different roles in some cellular systems (30, 34). Therefore, we sought to elucidate whether Rab27b was expressed in neutrophils and if it plays a significant role in the exocytic mechanisms in these cells. To this end, we first analyzed the expression of Rab27b in human neutrophils by Western blot using an antibody raised against a carboxyterminal peptide that is specific for Rab27b. In Figure 1A, we present evidence that this antibody detects Rab27b expression in human neutrophils showing a single band at around 28 kDa. Next, by immunofluorescence analysis, we show that neutrophils display a punctate distribution of Rab27b that resembles the distribution of secretory organelles (Fig. 1B). Western blot analysis of Rab27b expression in murine neutrophils confirmed that this small GTPase is expressed in these cells (Fig. 1C). Strikingly, we found that neutrophils from BL6-Rab27aash/ash mice have a marked upregulation of the expression of Rab27b (Fig. 1C). The upregulation of Rab27b was also observed in neutrophils from the C3H-Rab27aash/ash colony and in neutrophils from a different mouse model of Rab27a-deficiency, Rab27acct/cct concrete), (data not shown). These results suggest that the expression of Rab27b is transcriptionally or translationally linked to Rab27a expression levels. Contrarily, normal Rab27a expression levels were observed in Rab27b KO neutrophils (Fig. 1D).
Figure 1. Rab27b expression is upregulated in Rab27a deficient neutrophils.
A, Western blot analysis of the expression of Rab27a and Rab27b in human neutrophils. B, Immunofluorescence analysis of the subcellular distribution of Rab27b in wild type murine neutrophils. Scale bar = 5 μm. The data depicted in A and B are representative of at least three similar experiments. C, Western blot analysis of the expression of Rab27b in ashen (Rab27aash/ash) or wild type (WT) neutrophils from the BL6 mouse strain. Thirty five μg of protein from the indicated neutrophil lysates were loaded in each lane. C′, The bands were quantified by densitometry analysis. The data represent the mean ± SD from two independent experiments. D, Western blot analysis of the expression of Rab27a in Rab27b KO or wild type (WT) neutrophils from the BL6 mouse strain was performed as described above. In this assay, we used the monoclonal antibody 4B12 that specifically detects Rab27a but does not react with Rab27b (30). Quantification by densitometry is shown in D′.
Rab27b localizes at low density secretory organelles
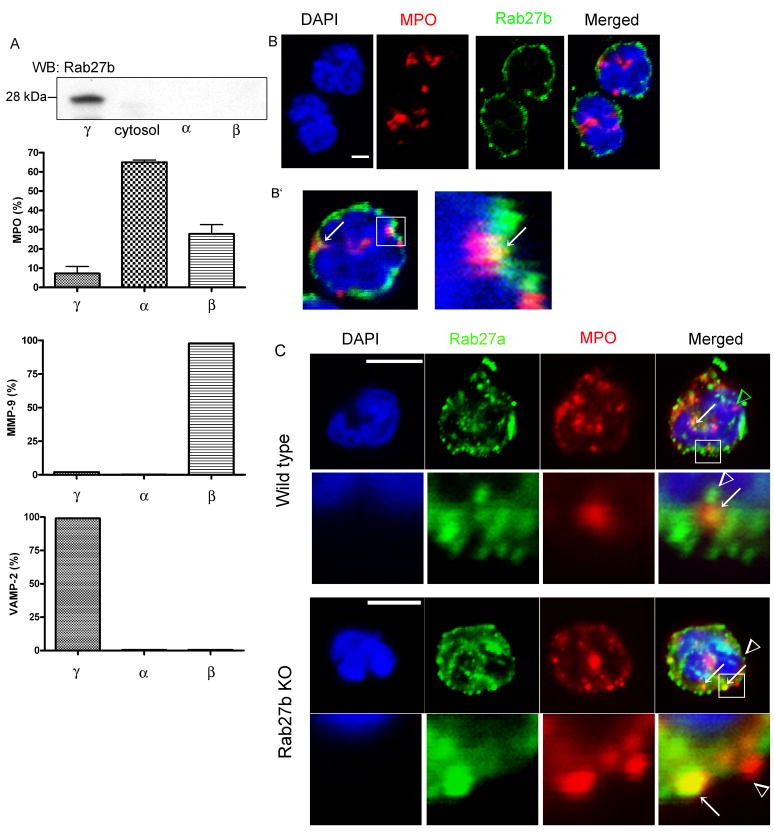
To better understand the function of Rab27b, we next performed fractionation studies in human neutrophils and detected the subcellular distribution of Rab27b by Western blot. The results indicate that Rab27b is distributed in organelles of very low density. This fraction is also enriched in the secretory protein VAMP-2 (Fig. 2A). This differs from the distribution of Rab27a which localizes not only at organelles of low density but also at those of intermediate density (19). Next, we investigated the subcellular distribution of Rab27b related to the azurophilic granule marker MPO by immunofluorescence analysis. In Figure 2B, we show that Rab27b has a peripheral distribution and shows relatively low colocalization with MPO. Rab27a-containing granules, which have a more homogenous distribution (Fig. 2C), also show only partial colocalization with azurophilic granules (Fig. 2C and (19)). Importantly, no differences in Rab27a subcellular localization were detected between Rab27b KO and wild type neutrophils (Fig. 2C).
Figure 2. Subcellular distribution of Rab27a and Rab27b in neutrophils.
A, Western blot analysis of the distribution of Rab27b in human neutrophils. The cells were lysed by nitrogen cavitation and organelles were separated using a two-layer Percoll gradient. Granule markers in the various fractions were identified by Western blot and quantified by densitometry. B, Immunofluorescence staining of endogenous Rab27b (green) and myeloperoxidase (red) in murine neutrophils. B′ shows a different layer from the z-series obtained from the upper right cell showed in B and the magnification of the selected area indicating partial colocalization of Rab27b and MPO (arrow). Scale bar = 2.5 μm. C, Immunofluorescence analysis of the distribution of Rab27a in wild type (upper panels) or Rab27b KO neutrophils (lower panels). Arrows indicate areas of colocalization of Rab27a with MPO. Arrowheads indicate granules that were positive for one marker but not for the other. Scale bar = 5 μm.
Rab27a and Rab27b control the exocytosis of azurophilic granules by independent mechanisms
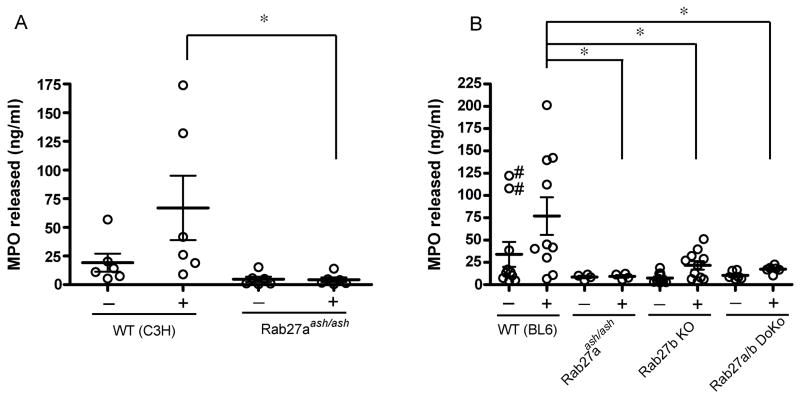
We demonstrated that myeloperoxidase secretion is impaired in Rab27a-deficient mice in vivo (19). In this work, to analyze a putative role for Rab27a in the secretion of azurophilic granules at the cellular level, we studied myeloperoxidase secretion in neutrophils from C3H-Rab27aash/ash mice, a very well-characterized mouse model of Rab27a-deficiency (36). To this end, we first evaluated azurophilic granule exocytosis in neutrophils isolated from blood obtained by cardiac puncture. The cells were stimulated with the phorbol ester PMA. PMA has been shown to stimulate myeloperoxidase release (37–39) through the mobilization of 1/3 of the azurophilic granules (39), and in our hands triggers MPO exocytosis from peripheral but not peritoneal murine neutrophils. We found that MPO secretion is dramatically impaired in the absence of Rab27a (Fig. 3A). Secondly, we evaluated MPO secretion in response to physiological stimuli. To this end, we used peritoneal neutrophils and heat-killed Listeria monocytogenes as stimulus. Again, we found that neutrophils from Rab27aash/ash mice have a severe defect in the secretion of MPO (Supplementary Fig. S2). We conclude that Rab27a plays a key role in the control of exocytosis of azurophilic granules.
Figure 3. Neutrophils deficient in GTPases from the Rab27 family have impaired azurophilic granule secretion.
A and B, Peripheral neutrophils from Rab27aash/ash, Rab27b knockout (KO), Rab27a/b double knockout (DoKo) or wild type (WT) control mice were resuspended in serum-free RPMI, stimulated with 0.1 μg/ml PMA (+) or left untreated (−) and incubated for 1h at 37 °C. MPO release was measured using a murine MPO-specific enzyme-linked immunosorbent assay. Results are mean ± SEM of data from 6 to 10 mice.*, p<0.03. #, statistical outliers.
To elucidate the role of Rab27b in the secretory function of neutrophils, we comparatively evaluated the exocytosis of azurophilic granules in Rab27aash/ash, Rab27b KO and Rab27a/b double KO neutrophils. First, we show that BL6-Rab27aash/ash neutrophils have impaired exocytosis of azurophilic granules similar to that observed in neutrophils from the C3H strain (Fig. 3B). These results indicate that the defect observed in Rab27aash/ash neutrophils is independent of the background strain. Second, our results show that Rab27b KO mice also have a marked impairment in the exocytosis of azurophilic granules when compared to wild type cells (Fig. 3B). However, Rab27b KO but not Rab27aash/ash peripheral neutrophils significantly responded to stimuli (unstimulated Rab27b KO vs stimulated Rab27b KO neutrophils, p<0.03) suggesting that the mechanisms regulated by these small GTPases are different. Importantly, the total myeloperoxidase content in neutrophils from Rab27-deficient mice was not significantly different from wild type controls (Supplementary Fig. S3). As expected, Rab27a/b double KO neutrophils also showed a very significant decrease in the secretion of MPO when compared to wild type cells (Fig. 3B). Altogether, our results indicate that both Rab27a and Rab27b are important secretory components of the molecular machinery that controls the exocytosis of azurophilic granules. However, the observations that the absence of either of these components impairs MPO secretion and that the secretion of MPO is impaired in the absence of Rab27a despite an increase in Rab27b expression suggest that these small GTPases play different roles in the regulation of neutrophil exocytosis.
The non-stimulated secretion of the wild type BL6 control showed an unusually high mean. From the internal distribution of the MPO secretion data for this group, became apparent that two samples gave abnormally high values (Fig 3B). Statistical analysis determined that these two samples behave as experimental outliers (Fig 3B). Importantly, the nature of the population distribution for this experimental sample is known a priori from previous MPO secretion experiments in which no bimodal behavior was detected regarding the basal secretion of MPO in non-stimulated neutrophils from BL6 wild type mice(10, 19, 40). Similarly, no bimodal behavior was observed for the data corresponding to the C3H colony or any of the colonies in the BL6 background (other than the aforementioned BL6 unstimulated wild type control) under any experimental conditions (Fig. 3). Therefore, it is reasonable to conclude that the two odd data points with unusually high values are indeed outliers and that the basal secretion of MPO in the wild type control is not different from the basal secretion in the Rab27-deficient colonies. It should be highlighted too, that the stimulus-induced secretion in the wild type control would still be significantly higher than the stimulated secretion of MPO in the Rab27-deficient colonies even if the corresponding stimulated pairs were removed.
No gross morphological abnormalities are present in Rab27aash/ash, Rab27b knockout or Rab27a/b double knockout neutrophils
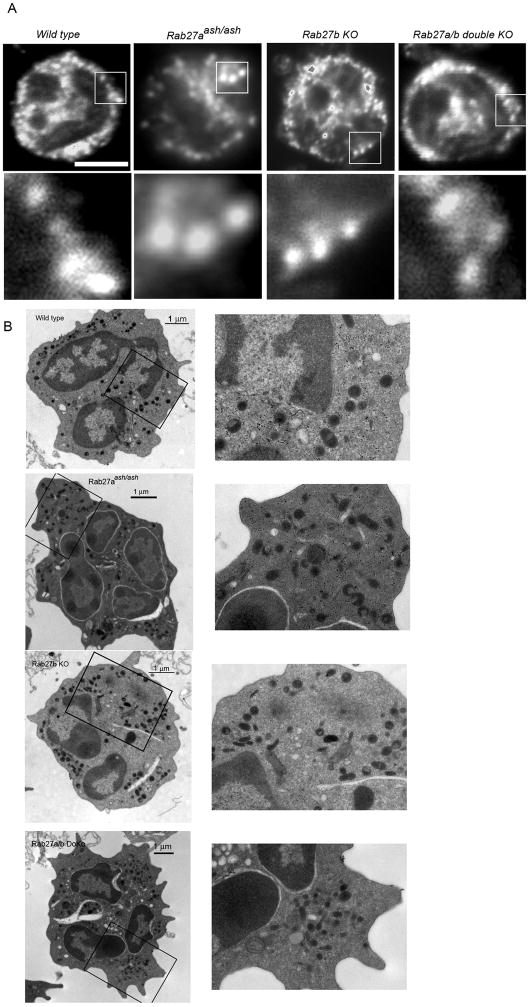
To explore whether morphological abnormalities in neutrophil granules could explain the poor secretion shown by Rab27-deficient cells, we first analyzed the distribution of MPO in these cells by immunofluorescence and confocal microscopy analysis. In Figure 4A, we show that endogenous myeloperoxidase is detected in punctate structures resembling secretory organelles in neutrophils from all colonies evaluated. These results suggest that compartmentalization of MPO proceeds independently of the Rab27 family of GTPases. To explore granular morphology of Rab27-deficient cells in further detail, we analyzed neutrophils using transmission electron microscopy. Our results indicate that no gross morphological abnormalities are present in neutrophil granules from the Rab27aash/ash colonies when compared to wild type controls independently of the background strain (Fig. 4B (BL6) and Supplementary Fig. S4 (C3H)). Moreover, no apparent differences in granule composition and heterogeneity were observed between neutrophils from Rab27b knockout or Rab27a/b double knockout and wild type mice (Fig. 4B).
Figure 4. No gross morphological abnormalities are present in the granules from Rab27aash/ash, Rab27b knockout or Rab27a/b double knockout neutrophils.
A, Peripheral neutrophils from Rab27aash/ash, Rab27b knockout (KO) and Rab27a/b double knockout (DoKo) or wild type (WT) mice were seeded on poly-L-lysine-coated 1.5 coverglass, fixed, stained for the visualization of endogenous myeloperoxidase and analyzed by immunofluorescence confocal microscopy. A representative image of each type is shown in the upper panels. Magnifications of the indicated areas are shown in the lower panels. At least 15 cells of each type were analyzed. Scale bar = 5 μm. B, Transmission electron microscopy analysis of Rab27aash/ash, Rab27b KO and Rab27a/b double knockout neutrophils. The panels on the right are magnifications of the corresponding areas selected in the left panels. Similar results were observed in Rab27aash/ash neutrophils from the C3H strain (Supplementary Fig. S4).
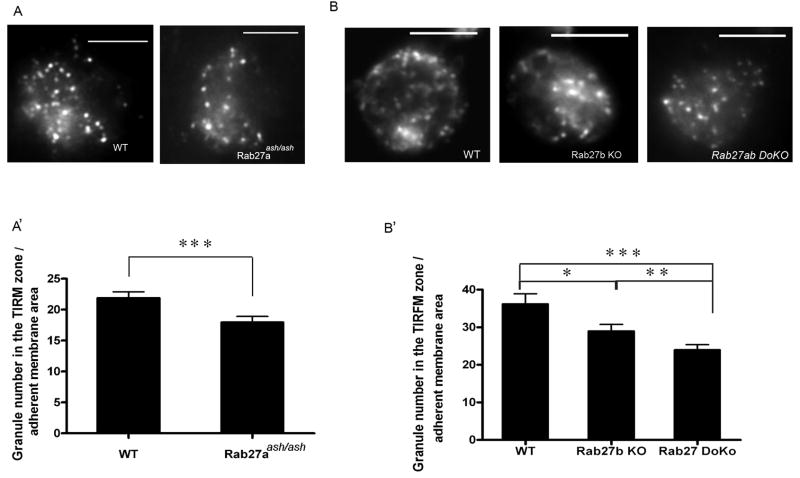
Rab27 KO neutrophils have a decreased number of azurophilic granules in proximity to the plasma membrane
To further understand the mechanism underlying azurophilic granule exocytosis, we utilized total internal reflection fluorescent microscopy (TIRFM) to analyze azurophilic granule distribution in Rab27-deficient neutrophils. To this end, neutrophils were isolated from Rab27aash/ash, Rab27b KO and Rab27a/b double KO mice or control mice, adhered to fibrinogen-coated coverslips and the distribution of endogenous myeloperoxidase-containing granules was analyzed by TIRFM. In TIRFM, imaging is confined to the cell surface such that it detects events within 100 nm of the coverslip allowing the analysis of events that take place in close proximity to the plasma membrane (41). Here, we show that Rab27aash/ash and Rab27b KO granulocytes have a significant decrease in the number of azurophilic granules in the TIRFM zone (Fig. 5). This was further manifested in Rab27a/b double KO neutrophils. This data supports the hypothesis that Rab27a and Rab27b regulate azurophilic granule exocytosis by independent, non-redundant mechanisms.
Figure 5. Rab27-deficient neutrophils have a decreased number of azurophilic granules in proximity to the plasma membrane.
Neutrophils from Rab27aash/ash (A) or Rab27b knockout (KO) and Rab27a/b double knockout (DoKo) (B) mice and their appropriate wild type (WT) controls were seeded on coverglass and the distribution of endogenous myeloperoxidase-containing granules was analyzed by total internal reflection fluorescence microscopy (TIRFM). Representative images of each type are shown in the upper panels. The number of azurophilic granules identified in the TIRFM zone (calibrated at 108 nm from the coverslip) per adherent membrane area unit for wild type or Rab27-deficient neutrophils is shown in the lower panels. The data depicted in A′ and B′ are represented as the mean ± SEM. Statistical analysis was performed using the non-parametric Mann-Whitney test.***, p<0.01; **, p<0.02*, p<0.05. Twenty to 40 cells from each group were analyzed. Rab27aash/ash and wild type neutrophils from the C3H mouse strain were used in A and neutrophils from BL6 mice were used in B. Scale bar = 5 μm
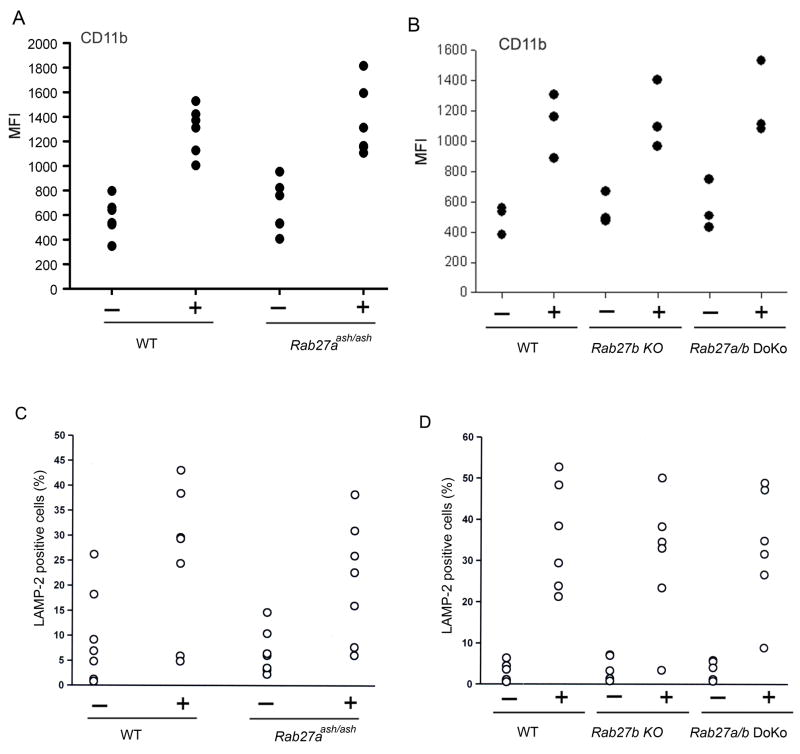
Mobilization of CD11b-containing secretory organelles proceeds independently of Rab27a and Rab27b
To explore a possible role for Rab27a in the secretion of readily mobilizable secretory organelles, we analyzed the mobilization of organelles that contain the β2 integrin subunit CD11b, a molecule that plays an important role in neutrophil adhesion to the activated endothelium during the innate immune response. To this end, we followed the plasma membrane expression of CD11b in peripheral neutrophils from Rab27aash/ash and wild type mice by flow cytometry. We observed that Rab27aash/ash neutrophils mobilize CD11b-containing organelles as efficiently as wild type control cells (Fig. 6A). These results suggest that neutrophils are able to upregulate plasma membrane expression of β2-integrins in a Rab27a-independent manner.
Figure 6. Rab27a and Rab27b are not essential components of the secretory machinery that regulates mobilization of CD11b or LAMP-2-containing organelles in neutrophils.
Exocytosis of readily mobilizable organelles was analyzed by following the surface expression of CD11b in neutrophils from wild type (WT) and ashen mice (Rab27aash/ash) (A) or Rab27b knockout (KO) and Rab27a/b double knockout (DoKo) mice (B). Neutrophils were stimulated with 0.1 μg/ml PMA (+) or left untreated (−), double labeled for the granulocyte marker Gr-1 and for the surface expression of the β2 integrin subunit CD11b. The samples were analyzed by flow cytometry. Each symbol represents the result obtained using an individual mouse. No significant differences were detected between the populations analyzed (unpaired Student’s t-test). C and D, Wild type (WT), Rab27a ash/ash, Rab27b KO or Rab27a/b double KO neutrophils were stimulated with 0.1 μg/ml PMA (+) or left untreated (−), double labeled for the granulocyte marker Gr-1 and for the surface expression of the membrane protein LAMP-2. The samples were analyzed by flow cytometry. Each symbol represents the value obtained using an individual mouse. No significant differences were observed between stimulated Rab27-deficient neutrophils and wild type cells (unpaired Student’s t-test). Neutrophils from the C3H and the BL6 mouse strains were used in A and C, and B and D, respectively.
Next, we performed similar studies in Rab27b KO and Rab27a/b double KO neutrophils. No significant differences were observed in the mobilization of CD11b between Rab27b KO or Rab27a/b double KO mice and wild type controls when stimulated with PMA (Fig. 6B) or LPS (data not shown). Together with the observations that Munc13-4-deficient neutrophils present a normal mobilization of CD11b-containing vesicles in response to stimuli (10), these results suggest that this mechanism is not regulated by the Rab27 family of proteins or effectors.
Mobilization of LAMP-2-containing secretory organelles proceeds independently of Rab27a and Rab27b
To further analyze the role of the Rab27 family members in the regulation of exocytosis in neutrophils, we focused on a different granule marker, the membrane protein lysosome-associate membrane protein-2 (LAMP-2), previously described to localize at multivesicular bodies (MVB) in human neutrophils (6). We observed that Rab27aash/ash, Rab27b knockout and Rab27a/b double knockout neutrophils have normal mobilization of this cellular marker in response to stimuli (Figs. 6C and D). Similar results were obtained when the mobilization of the closely related membrane protein lysosome-associate membrane protein-1 (LAMP-1) was analyzed (data not shown). These results rule out regulatory roles for Rab27a or Rab27b in the control of MVB mobilization in neutrophils.
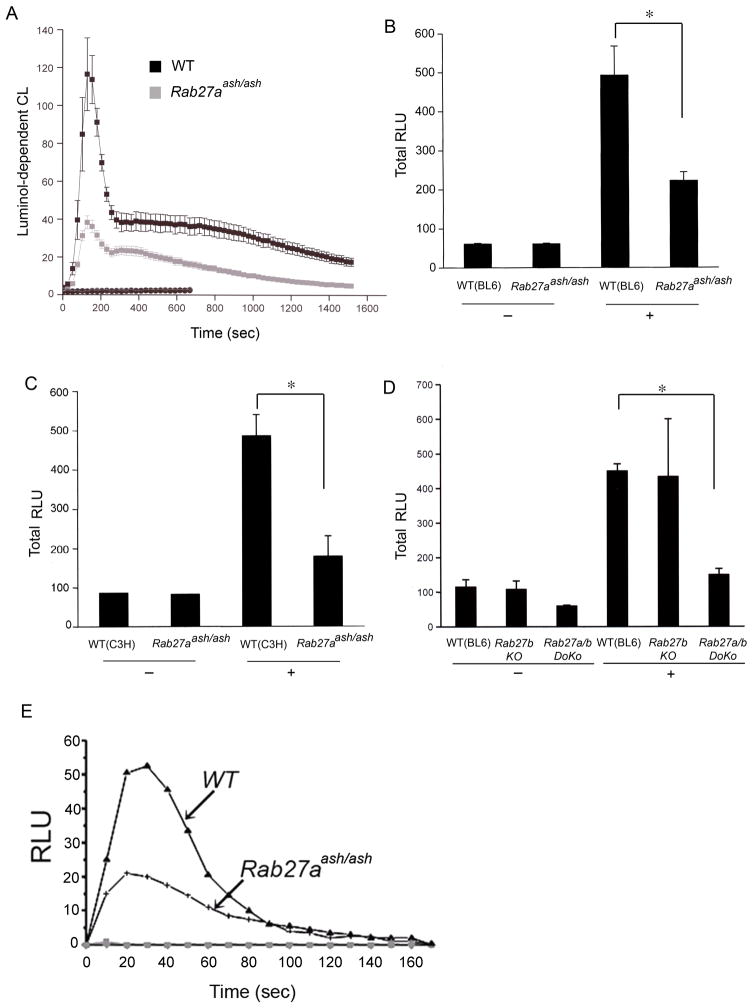
Reactive oxygen species (ROS) production is impaired in Rab27-deficient neutrophils
The production of ROS by the NADPH oxidase complex and myeloperoxidase is an essential antimicrobial mechanism in neutrophils. To evaluate a possible role of Rab27 proteins in the regulation of the neutrophil oxidative response, we first analyzed ROS production using the cell permeant reagent luminol, which detects both extracellular and intracellular ROS produced by the NADPH oxidase and myeloperoxidase. In Figure 7A–D, we show that Rab27aash/ash and Rab27a/b double KO cells but not Rab27b KO neutrophils have an impaired luminol-detected oxidative response to phorbol ester. In Figure 7E, we also show that neutrophils from Rab27aash/ash mice have an impaired oxidative response to the chemotactic factor fMLP.
Figure 7. Global ROS production is impaired in Rab27aash/ash and Rab27a/b double knockout mice.
A, Neutrophils isolated from ashen (Rab27aash/ash) or wild type mice (C57BL/6 background) were stimulated with PMA (0.1μg/ml) and the luminol-dependent chemiluminescence was continuously monitored for 25 min at 37°C. The results show the mean ± SEM of samples from three individual mice. Black and grey squares correspond to stimulated WT or Rab27aash/ash neutrophils, respectively. Circles correspond to unstimulated cells. B, The integrated chemiluminescence response of WT and Rab27aash/ash neutrophils is expressed as relative light units (RLU). C, The results show the chemiluminescence response of neutrophils from WT or Rab27aash/ash (C3H) mice. D, Chemiluminescence response of cells from WT, Rab27b knockout (KO) or Rab27a/b double knockout (DoKo) from C57BL/6 mice. Results are the mean ± SEM from data obtained using samples from three individual mice for each colony. *, p< 0.01. E, Luminol-dependent chemiluminescence of wild type or ashen neutrophils after incubation with LPS (10 ng/ml) for 30 min and activation with 1μM fMLP (▲ and +) or untreated cells (● and ▼) is shown.
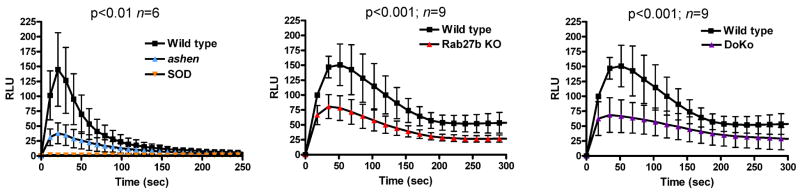
NADPH oxidase-dependent extracellular ROS production is impaired but not abolished in the absence of Rab27 GTPases
In principle, the deficiency in non-phagosomal ROS production observed in Rab27aash/ash neutrophils could be explained by lack of myeloperoxidase exocytosis in these cells. However, since the exocytosis of tertiary and specific granules, which contain the membrane associated subunit of the NADPH oxidase, might be impaired in the absence of Rab27a (10), we next evaluated whether the deficient oxidative response could be further explained by the lack of mobilization of cytochrome b558 to the plasma membrane. To this end, we evaluated the contribution of the NADPH oxidase on extracellular ROS generation using cell-impermeant isoluminol and carrying out the reactions in the presence of sodium azide to inhibit endogenous MPO activity. Horse-radish peroxidase was added to amplify the reaction as described (42). First, we confirmed that the chemiluminescence response depends on extracellular NADPH oxidase-derived ROS as it was abolished by superoxide dismutase which is cell-impermeant (Fig. 8). We found that the absence of either Rab27a or Rab27b significantly impaired NADPH oxidase activity (Fig. 8). However, a significant response was detected even when both GTPases were absent supporting the idea that Rab27-dependent and independent mechanisms cooperate to assemble the NADPH oxidase at the plasma membrane.
Figure 8. Plasma membrane-associated NADPH oxidase activity is impaired in neutrophils from Rab27-deficient mice.
Peripheral neutrophils from ashen (Rab27aash/ash), Rab27b knockout (KO), Rab27a/b double knockout (DoKo) or wild type (WT) mice were incubated in the presence of 10 μg/ml cytochalasin D for 10 min and stimulated with 10 μM fMLP or left untreated. NADPH oxidase-dependent extracellular chemiluminescence was measured by inhibiting endogenous MPO (sodium azide) and using cell-impermeant isoluminol. Wild type neutrophil kinetics are shown with black squares (■) and those from Rab27-deficient cells using triangles (▲). Inverted triangles in the left panel (▼) indicate the negligible chemiluminescence response obtained from wild type or Rab27a-deficient neutrophils in the presence of superoxide dismutase. Data is presented as mean ± SEM.
Rab27a and Rab27b are not involved in granule translocation to the phagosome in neutrophils
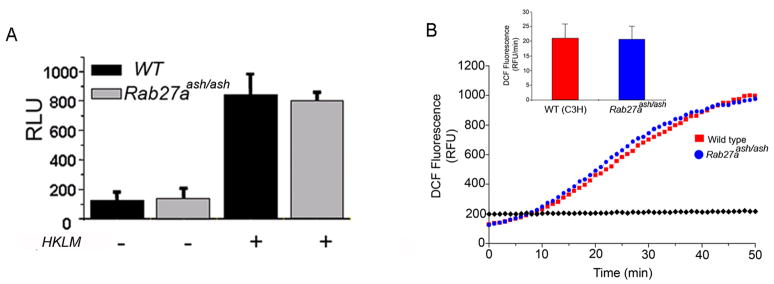
Next, we evaluated whether the intraphagosomal oxidative response is regulated by GTPases of the Rab27 family. First, we found that the luminol-detected oxidative response triggered by phagocytosis of opsonized bacteria is not impaired in the absence of Rab27a expression (Fig. 9A) supporting the idea that Rab27a regulates the non-phagosomal but not the intraphagosomal oxidative response. To further support this observation, we evaluated the neutrophil oxidative response using Fc OxyBurst, a dichlorodihydrofluorescein immune-complex conjugate that detects the kinetics of the activation of the NADPH oxidase within the phagosome (43). In Figure 9B, we show that ROS production in the phagosome is not impaired in Rab27aash/ash neutrophils. These results indicate that, similar to that shown for azurophilic granules (19), Rab27a is not involved in neutrophil specific granule trafficking to the phagosome.
Figure 9. Rab27-deficient neutrophils are characterized by normal intraphagosomal ROS production.
A, Murine neutrophils were isolated from peripheral blood obtained by cardiac puncture. The cells were stimulated with opsonized heat-killed Listeria monocytogenes (HKLM) and luminol-dependent chemiluminescence was continuously monitored at 37°C. Results represent the mean ± SEM (n=3). B, Measurement of ROS release in the phagovacuoles of immune complex-stimulated wild type or Rab27aash/ash neutrophils using Fc OxyBurst. The base line represents the dichlorodihydrofluorescein (DCF) fluorescence obtained in the absences of neutrophils (◆). The inset shows the mean ± SEM of three experiments performed with cells from three individual mice.
DISCUSSION
In this work, we demonstrate that Rab27a and Rab27b play key, independent roles in the regulation of azurophilic granule exocytosis and suggest that Rab27a and Rab27b regulates vesicular transport to the plasma membrane in neutrophils. We also demonstrate that Rab27-deficiency leads to a defect in the production of extracellular ROS although the intra-phagosomal oxidative response remains unaffected. Furthermore, we show that the mobilization of LAMP-2 and readily-releasable CD11b-containing vesicles proceeds independently from Rab27a or Rab27b function. Altogether, our data suggest central, selective, yet different, roles for these Rab27 isoforms in the regulation of neutrophil functions associated with the process of exocytosis.
Rab27a and Rab27b share 72 % homology at the amino acid level and are known to regulate exocytosis in many cellular systems; however, their roles in the mechanisms that control the exocytosis of neutrophil granules are not fully characterized. Here, we show that the absence of Rab27a or Rab27b impairs azurophilic granule secretion. However, Rab27b knockout but not Rab27aash/ash neutrophils significantly secreted MPO in response to stimuli suggesting that Rab27a can partially compensate for the absence of Rab27b. Although counterintuitive, double knockout neutrophils slightly responded to stimuli probably suggesting that another Rab protein may compensate for the defect only when both Rab27 isoforms are absent. Neutrophils deficient in Rab27a or Rab27b showed impaired NADPH oxidase activity. These results, together with the observations that the expression of Rab27b is upregulated in Rab27aash/ash neutrophils but does not compensate for the lack of Rab27a function and that the expression of Rab27a in Rab27b KO cells is similar to that in wild type neutrophils, support the idea that these GTPases play important yet different roles during neutrophil granule exocytosis. Similar to that observed here, Rab27a and Rab27b have been shown to play different roles in other cellular systems in which they coexist. In platelets for example, absence of Rab27b leads to a defect in the secretion of dense granules (30). Rab27a also participates in secretion of dense granules (30). However, its expression only partially compensates for the secretory defects observed in the absence of Rab27b. Similarly, Rab27a and Rab27b play different roles in mast cells (34). Our data concur with that from platelets and mast cells suggesting that Rab27a and Rab27b play complementary but different roles in neutrophils.
Azurophilic granule secretion was abolished in the absence of Rab27a but only partially impaired in the absence of Rab27b. Furthermore, immunofluorescence analysis shows that Rab27b mainly localizes on peripherally distributed structures and has poor spatial agreement with azurophilic granules in unstimulated cells (Fig, 2), while Rab27a better colocalizes with azurophilic granules and is present in granules that are homogenously distributed in neutrophils (19). The data suggest that Rab27b would play a downstream role in azurophilic granule secretion in relationship to the role played by Rab27a. Moreover, the poor colocalization observed between Rab27b and MPO-associated granules together with the secretion data showing a role for Rab27b in azurophilic granule secretion suggests that azurophilic granules may undergo a maturation process during regulated exocytosis. Similar mechanisms have been described for other lysosome-related organelles (44). For example, in CTLs, Rab27a-associated vesicles do not colocalize with cytotoxic granules containing perforin and granzyme B and the granules acquire secretory ability later during activation in a process that is dependent on Rab GTPases and effectors (44). Considering the toxic properties of azurophilic granule luminal proteins, it would not be unlikely that a maturation step would act as checkpoint during granule secretion. Current studies are being performed in our laboratory to better characterize this process.
Another possible explanation for the independent mechanisms mediated by Rab27a and Rab27b is that they interact with different Rab effectors in vivo. In a previous work, we showed that endogenous Rab27a interacts with the effector Slp1/JFC1 in human neutrophils (19). Both Slp1/JFC1 and Rab27a colocalize at azurophilic granules and regulate their exocytosis (10, 19). Downregulation of Slp1/JFC1 increases the number of fast moving granules in granulocytes suggesting that the effector might regulate the process of tethering or docking (10). Here, we found that Rab27a or Rab27b-deficiencies decrease the number of azurophilic granules in close proximity to the plasma membrane thus supporting a role for Rab27 GTPases in the delivery of granules to the plasma membrane or in the docking process. The observation that Rab27b distribution is more peripheral than Rab27a suggests that Rab27a is likely to regulate an upstream step in the secretory process while Rab27b may act at a late stage in exocytosis. Furthermore, the observation that Rab27b-deficient neutrophils show a significant exocytic response while exocytosis is abolished in Rab27a-deficient neutrophils suggests that Rab27b function is perhaps more redundant. The different roles played by the Rab27 isoforms in neutrophils may be explained by their interaction with different effector molecules. Thus, although Rab27a and Rab27b are able to interact with the same set of molecular effectors in overexpression systems, and Slp1/JFC1 interacts with Rab27b in pancreatic acinar cells (45), it is not clear yet whether Rab27b interacts with Slp1/JFC1 in neutrophils. It is also uncertain whether Rab27b interacts with Munc13-4, a Rab27a effector shown by our group to regulate exocytosis of azurophilic and gelatinase granules in neutrophils (10). Another possible candidate is Slp3, a Rab27 effector recently shown to be expressed in these cells (46). The mechanism of exocytosis regulation mediated by Rab27b in neutrophils and its possible interaction with these effectors is currently under investigation in our laboratory.
In this work, we use neutrophils from Rab27aash/ash and Rab27b KO mice to demonstrate that these GTPases are essential for azurophilic granule exocytosis. Furthermore, previous data from our laboratory showing that Rab27a-deficient mice have impaired secretion of myeloperoxidase in vivo and that endogenous Rab27a and its effector Slp1/JFC1 colocalize with a minor pool of azurophilic granules in neutrophils further support a role for Rab27a in azurophilic granule exocytosis (19). Contrarily, a recent study by Mollinedo and collaborators (47) stated that Rab27a is not involved in the secretion of this set of neutrophil granules. The lack of evidence of Rab27a involvement in azurophilic granule exocytosis in Mollinedo’s work is most probably explained by the use of electropermeabilization and calcium stimulation. Since even gentle electroporation stimulates the mobilization of granule markers in human neutrophils (48), it is very likely that MPO secretion was primed or activated in their assays by the electric pulse itself, by way of a Rab27a-independent mechanism. Furthermore, as demonstrated for lysosomes, Ca2+-triggered exocytosis induces secretion of those granules already in close proximity to the plasma membrane but does not lead to a significant increase in the recruitment of granules to the vicinity of the plasma membrane (49). Thus, the experimental conditions used in reference (47) would likely neglect pre-fusion events. As these events are regulated by Rab27a and its effectors (10), in our view, those experimental conditions are not ideal for studying Rab27a function. Supporting our observations, previous analysis using SLO-permeabilized human neutrophils and physiological stimulation instead of electroporation demonstrated that molecular interference using anti-Rab27a antibodies completely blocks azurophilic granule exocytosis (10).
Although most azurophilic granules are of high density, previous works showed that a minor peak of azurophilic granule markers co-fractionates with membranes of intermediate and low density in neutrophil gradients (20, 39). Our observation that Rab27a co-fractionates with MPO-positive granules of low density together with immunofluorescence analysis showing localization of endogenous Rab27a at some but not all azurophilic granules (19) strongly suggest that Rab27a colocalizes with a subpopulation of low-density azurophilic granules. Immunoelectron microscopy analysis showing that 20% of MPO-positive granules contain Rab27a (47) and that a subpopulation of Rab27a-positive granules that co-fractionate with ~20% of MPO-positive granules (fraction 7 in reference (47)) is mobilized in response to stimuli further supports our previous results. Surprisingly, Mollinedo and col (47) found Rab27a even in the most dense neutrophil membrane fraction. This is probably explained by the use of hypotonic lysis and dounce homogenization in the preparation of neutrophil membranes, a method that has been criticized due to its low efficiency and to the membrane damage inflicted by the prolonged hypotonic shock (39). Altogether, fractionation and microscopy data demonstrate that a small population of azurophilic granules contains Rab27a. As only a small fraction of azurophilic granules are mobilizable (39) and Rab27a deficiency impairs azurophilic granule exocytosis ((19) and this work), our data suggest that only the Rab27a-containing subpopulation of azurophilic granules can undergo regulated secretion.
Neither Rab27a nor Rab27b seem to regulate the secretion of readily releasable CD11b-containing vesicles or LAMP-2-positive organelles in neutrophils. On the other hand, Rab27a and Rab27b control exocytosis of not only azurophilic granules but also cytochrome b558-containing granules ((10) and this work). Although CD11b is present in secretory vesicles and specific granules in human neutrophils, the observation that stimulated Rab27aash/ash cells mobilize CD11b but have impaired specific granule mobilization suggest that putative CD11b molecules from specific granules are by far outnumbered by translocation from secretory vesicles in murine neutrophils. An important consequence of the lack of mobilization of secretory organelles in Rab27-deficient neutrophils is the accompanying defect in their oxidative response. Interestingly, marked defects in plasma membrane-associated NADPH oxidase activity were evident in Rab27 deficiency but substantial ROS production was detected even when both Rab27 GTPases were absent. This probably indicates that different populations of cytochrome b558-contatining granules utilize diverse trafficking mechanism to assemble the oxidase at the plasma membrane.
Different from that shown for dendritic cells, (50) extracellular but not intraphagosomal ROS production is defective in Rab27a-deficient neutrophils. This may reflect the different roles played by the NADPH oxidase complex in these cells (i.e. antigen processing in dendritic cells (50) and oxidant-mediated killing (51) or as an electron pump for protease-mediated killing (11) in neutrophils). The abnormality in the production of ROS in Rab27aash/ash neutrophils is most likely caused by both impaired mobilization of cytochrome b558 to the plasma membrane and deficient MPO secretion. The poor mobilization of the membrane associated subunit of the NADPH oxidase to the plasma membrane might help explain previous case reports showing that neutrophils from some patients with GS have a deficient oxidative response. Consequently, the defects in the exocytic process and the abnormal extracellular ROS production are likely to contribute to a systemic defect in the innate immune response in patients with GS.
In conclusion, we report here that Rab27a plays a central role in the regulation of azurophilic granule exocytosis and a significant role in the activation of the NADPH oxidase at the plasma membrane thus regulating extracellular ROS production. We also show for the first time that Rab27b contributes to the regulation of azurophilic granule exocytosis and plasma membrane NADPH oxidase activation by a mechanism that is independent of Rab27a function. Our findings identify Rab27a and Rab27b as potential independent targets for therapeutic intervention for the treatment of inflammatory processes associated with uncontrolled release of neutrophil granule proteins.
Materials and Methods
Materials
LPS (E.coli, serotype R515) was obtained from Alexis Biochemicals. HKLM (heat-killed Listeria monocytogenes) was obtained from InvivoGen and Zymosan (S. cerevisiae) was from Invitrogen. Paraformaldehyde (PFA) was from Electron Microscopy Sciences.
Experimental animal models
Our experiments utilize ashen mice (C3H/HeSn-Rab27aash/ash) that contain a splicing mutation in Rab27a and their parental strain C3H/HeSn (C3H, The Jackson Laboratory). Where indicated, we also used C57BL/6-Rab27aash/ash and control C57BL/6 (BL6). Rab27aash/ash mice have been extensively utilized for the study of Rab27a deficiency and were described previously (36). The Rab27b knockout (KO) in a C57BL/6 background was generated using the conditional Cre–loxP system of site-specific recombination to delete exons 2 and 3 from the rab27b gene (30). Rab27b−/− mice do not show any general phenotype, such as developmental abnormalities, feeding, or other behavioral changes (30). Rab27a/b double KO were generated by crossing Rab27b KO mice with C57BL/6-Rab27aash/ash mice and subsequently crossing the heterozygous progeny as described (30). All studies were conducted according to NIH and institutional guidelines and with approval of the institutional animal review board.
Isolation of murine neutrophils
Peripheral murine neutrophils were obtained from blood collected by cardiac puncture and erythrocytes were removed by lysis using a solution consisting of 168 mM NH4Cl, 10 mM Tris/HCl, pH 7.4. Neutrophils were further isolated using a Percoll-gradient fractionation system as previously described (40). After isolation, cells were kept on ice until used. For some experiments, granulocytes were isolated using MACS® technology with anti-mouse Ly-6G (clone 1A8)-biotin, anti-biotin microbeads and MS MACS columns, as described by the manufacturer (Miltenyi Biotec). Peritoneal neutrophils were isolated exactly as described previously (40).
Mobilization of azurophilic granules in murine neutrophils
To follow azurophilic granule mobilization, murine neutrophils were resuspended in serum-free RPMI. Peritoneal neutrophils were activated using heat-killed Listeria monocytogenes (1×108 particles/ml) and peripheral neutrophils were activated with PMA (0.1μg/ml) for 1 h at 37 °C. Reactions were terminated by placing tubes on ice. The cells were immediately spun down and the supernatants and pellets were collected and stored at −20 °C for later analyses. The analysis of secreted MPO was performed using a murine MPO-specific enzyme-linked immunosorbent assay (HyCult Biotechnology) according to manufacturer instructions.
TIRF microscopy analysis
Peripheral murine neutrophils were transferred to fibrinogen-coated Lab-Tek chambered coverglass #1.5 (Nunc). The cells were fixed with 3.7% paraformaldehyde, permeabilized with 0.01% saponin and blocked with a solution of 1% BSA in PBS. Samples were labeled with anti-murine MPO-specific antibody overnight at 4 °C and 488-Alexa-Fluor-conjugated secondary antibody (Molecular Probes). Cells were stored in Fluoromount-G (Southern Biotechnology, AL) until analyzed. TIRFM experiments were performed using a 100x 1.45 NA TIRF objective (Nikon) on a Nikon TE2000U microscope custom modified with a TIRF illumination module. Laser illumination (488 nm laser line) was adjusted to impinge on the coverslip at an angle to yield a calculated evanescent field depth of 108 nm. Images were acquired on a 14 bit, cooled CCD camera (Hamamatsu) controlled through Metamorph software (Molecular Devices Inc.). The images were recorded using 500 ms exposures. Images were analyzed using Image J software and vesicles were quantified using Quantity One analysis software (BioRad).
Human subjects
All procedures regarding human subjects have been reviewed and approved by the Human Subjects Committee at The Scripps Research Institute and were conducted in accordance with the requirements set forth by the mentioned Committee and the NIH. Experiments were performed according to the principles outlined by the Helsinki Declaration.
Statistical analysis
Unless otherwise indicated, results are presented as mean ± SEM. Statistical analysis was calculated using the Student’s t-test or the non-parametric Mann-Whitney test. A value of p<0.05 was considered as statistically significant. Peirce’s criterion and Grubb’s test were used to determine statistical outliers.
Supplementary Material
MPO secretion was evaluated using peritoneal neutrophils from WT or ashen (Rab27aash/ash) mice stimulated with heat-killed Listeria monocytogenes (HKLM). For the isolation of mouse peritoneal neutrophils, animals were injected intraperitoneally with 1 ml of a sterile 4% thioglycollate solution. Polymorphonuclear cells were harvested 4 hours after injection by lavage of the peritoneal cavity with RPMI medium. MPO secretion was analyzed by flow cytometry as described previously (Brzezinska et al, Immunology 2009, 127:388–397). Results represent the mean ± SEM from 7 individual mice of each group. *, p<0.01.
Neutrophils from the indicated colonies were isolate, lysed and the total MPO content was determined by ELISA.
The left panels show the electron microscopy analysis of wild type and Rab27aash/ash neutrophils (C3H background). The right panels show the magnification of the areas selected in the left panels.
Acknowledgments
Supported by U.S. Public Health Service Grants AI024227 and HL088256 to SDC. We thank Dr. Malcolm Wood for assistance with electron microscopy.
Footnotes
AUTHORSHIP
JLJ and AAB designed and performed experiments, and analyzed and interpreted data; DBM, BAE and HH performed experiments and analyzed data. TT and MCS designed and contributed animal models and interpreted data; SDC designed and performed research, analyzed and interpreted data and wrote the manuscript.
References
- 1.Borregaard N, Lollike K, Kjeldsen L, Sengelov H, Bastholm L, Nielsen MH, Bainton DF. Human neutrophil granules and secretory vesicles. Eur J Haematol. 1993;51:187–198. doi: 10.1111/j.1600-0609.1993.tb00629.x. [DOI] [PubMed] [Google Scholar]
- 2.Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci U S A. 2007;104:20262–20267. doi: 10.1073/pnas.0706438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergt C, Pennathur S, Fu X, Byun J, O’Brien K, McDonald TO, Singh P, Anantharamaiah GM, Chait A, Brunzell J, Geary RL, Oram JF, Heinecke JW. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci U S A. 2004;101:13032–13037. doi: 10.1073/pnas.0405292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tazawa H, Okada F, Kobayashi T, Tada M, Mori Y, Une Y, Sendo F, Kobayashi M, Hosokawa M. Infiltration of neutrophils is required for acquisition of metastatic phenotype of benign murine fibrosarcoma cells: implication of inflammation-associated carcinogenesis and tumor progression. Am J Pathol. 2003;163:2221–2232. doi: 10.1016/S0002-9440(10)63580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 6.Dahlgren C, Carlsson SR, Karlsson A, Lundqvist H, Sjolin C. The lysosomal membrane glycoproteins Lamp-1 and Lamp-2 are present in mobilizable organelles, but are absent from the azurophil granules of human neutrophils. Biochem J. 1995;311 (Pt 2):667–674. doi: 10.1042/bj3110667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuijpers TW, Tool AT, van der Schoot CE, Ginsel LA, Onderwater JJ, Roos D, Verhoeven AJ. Membrane surface antigen expression on neutrophils: a reappraisal of the use of surface markers for neutrophil activation. Blood. 1991;78:1105–1111. [PubMed] [Google Scholar]
- 8.Abdel-Latif D, Steward M, Macdonald DL, Francis GA, Dinauer MC, Lacy P. Rac2 is critical for neutrophil primary granule exocytosis. Blood. 2004;104:832–839. doi: 10.1182/blood-2003-07-2624. [DOI] [PubMed] [Google Scholar]
- 9.Pereira S, Lowell C. The Lyn tyrosine kinase negatively regulates neutrophil integrin signaling. J Immunol. 2003;171:1319–1327. doi: 10.4049/jimmunol.171.3.1319. [DOI] [PubMed] [Google Scholar]
- 10.Brzezinska AA, Johnson JL, Munafo DB, Crozat K, Beutler B, Kiosses WB, Ellis BA, Catz SD. The Rab27a Effectors JFC1/Slp1 and Munc13-4 Regulate Exocytosis of Neutrophil Granules. Traffic. 2008;9:2151–2164. doi: 10.1111/j.1600-0854.2008.00838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jog NR, Rane MJ, Lominadze G, Luerman GC, Ward RA, McLeish KR. The actin cytoskeleton regulates exocytosis of all neutrophil granule subsets. Am J Physiol Cell Physiol. 2007;292:C1690–C1700. doi: 10.1152/ajpcell.00384.2006. [DOI] [PubMed] [Google Scholar]
- 13.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 14.Legrand D, Elass E, Carpentier M, Mazurier J. Lactoferrin: a modulator of immune and inflammatory responses. Cell Mol Life Sci. 2005;62:2549–2559. doi: 10.1007/s00018-005-5370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakrabarti S, Patel KD. Regulation of matrix metalloproteinase-9 release from IL-8-stimulated human neutrophils. J Leukoc Biol. 2005;78:279–288. doi: 10.1189/jlb.1004612. [DOI] [PubMed] [Google Scholar]
- 16.Kjeldsen L, Sengelov H, Lollike K, Nielsen MH, Borregaard N. Isolation and characterization of gelatinase granules from human neutrophils. Blood. 1994;83:1640–1649. [PubMed] [Google Scholar]
- 17.Cockcroft S. Relationship between arachidonate release and exocytosis in permeabilized human neutrophils stimulated with formylmethionyl-leucyl-phenylalanine (fMetLeuPhe), guanosine 5′-[gamma-thio]triphosphate (GTP[S]) and Ca2+ Biochem J. 1991;275 (Pt 1):127–131. doi: 10.1042/bj2750127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Philips MR, Abramson SB, Kolasinski SL, Haines KA, Weissmann G, Rosenfeld MG. Low molecular weight GTP-binding proteins in human neutrophil granule membranes. J Biol Chem. 1991;266:1289–1298. [PubMed] [Google Scholar]
- 19.Munafo DB, Johnson JL, Ellis BA, Rutschmann S, Beutler B, Catz SD. Rab27a is a key component of the secretory machinery of azurophilic granules in granulocytes. Biochem J. 2007;402:229–239. doi: 10.1042/BJ20060950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lominadze G, Powell DW, Luerman GC, Link AJ, Ward RA, McLeish KR. Proteomic analysis of human neutrophil granules. Mol Cell Proteomics. 2005;4:1503–1521. doi: 10.1074/mcp.M500143-MCP200. [DOI] [PubMed] [Google Scholar]
- 21.Griscelli C, Durandy A, Guy-Grand D, Daguillard F, Herzog C, Prunieras M. A syndrome associating partial albinism and immunodeficiency. Am J Med. 1978;65:691–702. doi: 10.1016/0002-9343(78)90858-6. [DOI] [PubMed] [Google Scholar]
- 22.Klein C, Philippe N, Le Deist F, Fraitag S, Prost C, Durandy A, Fischer A, Griscelli C. Partial albinism with immunodeficiency (Griscelli syndrome) J Pediatr. 1994;125:886–895. doi: 10.1016/s0022-3476(05)82003-7. [DOI] [PubMed] [Google Scholar]
- 23.Harfi HA, Brismar J, Hainau B, Sabbah R. Partial albinism, immunodeficiency, and progressive white matter disease: a new primary immunodeficiency. Allergy Proc. 1992;13:321–328. doi: 10.2500/108854192778816933. [DOI] [PubMed] [Google Scholar]
- 24.Munafo DB, Johnson JL, Brzezinska AA, Ellis BA, Wood MR, Catz SD. DNase I Inhibits a Late Phase of Reactive Oxygen Species Production in Neutrophils. J Innate Immun. 2009;1:527–542. doi: 10.1159/000235860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukuda M. Versatile role of Rab27 in membrane trafficking: focus on the Rab27 effector families. J Biochem (Tokyo) 2005;137:9–16. doi: 10.1093/jb/mvi002. [DOI] [PubMed] [Google Scholar]
- 26.Ramalho JS, Tolmachova T, Hume AN, McGuigan A, Gregory-Evans CY, Huxley C, Seabra MC. Chromosomal mapping, gene structure and characterization of the human and murine RAB27B gene. BMC Genet. 2001;2:2. doi: 10.1186/1471-2156-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barral DC, Ramalho JS, Anders R, Hume AN, Knapton HJ, Tolmachova T, Collinson LM, Goulding D, Authi KS, Seabra MC. Functional redundancy of Rab27 proteins and the pathogenesis of Griscelli syndrome. J Clin Invest. 2002;110:247–257. doi: 10.1172/JCI15058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izumi T. Physiological roles of Rab27 effectors in regulated exocytosis. Endocr J. 2007;54:649–657. doi: 10.1507/endocrj.kr-78. [DOI] [PubMed] [Google Scholar]
- 29.Tolmachova T, Anders R, Stinchcombe J, Bossi G, Griffiths GM, Huxley C, Seabra MC. A general role for Rab27a in secretory cells. Mol Biol Cell. 2004;15:332–344. doi: 10.1091/mbc.E03-07-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tolmachova T, Abrink M, Futter CE, Authi KS, Seabra MC. Rab27b regulates number and secretion of platelet dense granules. Proc Natl Acad Sci U S A. 2007;104:5872–5877. doi: 10.1073/pnas.0609879104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao S, Torii S, Yokota-Hashimoto H, Takeuchi T, Izumi T. Involvement of Rab27b in the regulated secretion of pituitary hormones. Endocrinology. 2002;143:1817–1824. doi: 10.1210/endo.143.5.8823. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Li C, Izumi T, Ernst SA, Andrews PC, Williams JA. Rab27b localizes to zymogen granules and regulates pancreatic acinar exocytosis. Biochem Biophys Res Commun. 2004;323:1157–1162. doi: 10.1016/j.bbrc.2004.08.212. [DOI] [PubMed] [Google Scholar]
- 33.Imai A, Yoshie S, Nashida T, Shimomura H, Fukuda M. The small GTPase Rab27B regulates amylase release from rat parotid acinar cells. J Cell Sci. 2004;117:1945–1953. doi: 10.1242/jcs.01048. [DOI] [PubMed] [Google Scholar]
- 34.Mizuno K, Tolmachova T, Ushakov DS, Romao M, Abrink M, Ferenczi MA, Raposo G, Seabra MC. Rab27b regulates mast cell granule dynamics and secretion. Traffic. 2007;8:883–892. doi: 10.1111/j.1600-0854.2007.00571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomi H, Mori K, Itohara S, Izumi T. Rab27b is expressed in a wide range of exocytic cells and involved in the delivery of secretory granules near the plasma membrane. Mol Biol Cell. 2007;18:4377–4386. doi: 10.1091/mbc.E07-05-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson SM, Yip R, Swing DA, O’Sullivan TN, Zhang Y, Novak EK, Swank RT, Russell LB, Copeland NG, Jenkins NA. A mutation in Rab27a causes the vesicle transport defects observed in ashen mice. Proc Natl Acad Sci U S A. 2000;97:7933–7938. doi: 10.1073/pnas.140212797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bentwood BJ, Henson PM. The sequential release of granule constitutents from human neutrophils. J Immunol. 1980;124:855–862. [PubMed] [Google Scholar]
- 38.Ambruso DR, Knall C, Abell AN, Panepinto J, Kurkchubasche A, Thurman G, Gonzalez-Aller C, Hiester A, deBoer M, Harbeck RJ, Oyer R, Johnson GL, Roos D. Human neutrophil immunodeficiency syndrome is associated with an inhibitory Rac2 mutation. Proc Natl Acad Sci U S A. 2000;97:4654–4659. doi: 10.1073/pnas.080074897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kjeldsen L, Sengelov H, Borregaard N. Subcellular fractionation of human neutrophils on Percoll density gradients. J Immunol Methods. 1999;232:131–143. doi: 10.1016/s0022-1759(99)00171-4. [DOI] [PubMed] [Google Scholar]
- 40.Brzezinska AA, Johnson JL, Munafo DB, Ellis BA, Catz SD. Signalling mechanisms for Toll-like receptor-activated neutrophil exocytosis: key roles for interleukin-1-receptor-associated kinase-4 and phosphatidylinositol 3-kinase but not Toll/IL-1 receptor (TIR) domain-containing adaptor inducing IFN-beta (TRIF) Immunology. 2008 doi: 10.1111/j.1365-2567.2008.02980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steyer JA, Almers W. A real-time view of life within 100 nm of the plasma membrane. Nat Rev Mol Cell Biol. 2001;2:268–275. doi: 10.1038/35067069. [DOI] [PubMed] [Google Scholar]
- 42.Lundqvist H, Dahlgren C. Isoluminol-enhanced chemiluminescence: a sensitive method to study the release of superoxide anion from human neutrophils. Free Radic Biol Med. 1996;20:785–792. doi: 10.1016/0891-5849(95)02189-2. [DOI] [PubMed] [Google Scholar]
- 43.Ryan TC, Weil GJ, Newburger PE, Haugland R, Simons ER. Measurement of superoxide release in the phagovacuoles of immune complex-stimulated human neutrophils. J Immunol Methods. 1990;130:223–233. doi: 10.1016/0022-1759(90)90052-w. [DOI] [PubMed] [Google Scholar]
- 44.Menager MM, Menasche G, Romao M, Knapnougel P, Ho CH, Garfa M, Raposo G, Feldmann J, Fischer A, de Saint BG. Secretory cytotoxic granule maturation and exocytosis require the effector protein hMunc13-4. Nat Immunol. 2007;8:257–267. doi: 10.1038/ni1431. [DOI] [PubMed] [Google Scholar]
- 45.Saegusa C, Kanno E, Itohara S, Fukuda M. Expression of Rab27B-binding protein Slp1 in pancreatic acinar cells and its involvement in amylase secretion. Arch Biochem Biophys. 2008;475:87–92. doi: 10.1016/j.abb.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 46.Uriarte SM, Powell DW, Luerman GC, Merchant ML, Cummins TD, Jog NR, Ward RA, McLeish KR. Comparison of proteins expressed on secretory vesicle membranes and plasma membranes of human neutrophils. J Immunol. 2008;180:5575–5581. doi: 10.4049/jimmunol.180.8.5575. [DOI] [PubMed] [Google Scholar]
- 47.Herrero-Turrion MJ, Calafat J, Janssen H, Fukuda M, Mollinedo F. Rab27a regulates exocytosis of tertiary and specific granules in human neutrophils. J Immunol. 2008;181:3793–3803. doi: 10.4049/jimmunol.181.6.3793. [DOI] [PubMed] [Google Scholar]
- 48.Johnson JL, Ellis BA, Munafo DB, Brzezinska AA, Catz SD. Gene transfer and expression in human neutrophils. The phox homology domain of p47phox translocates to the plasma membrane but not to the membrane of mature phagosomes. BMC Immunol. 2006;7:28. doi: 10.1186/1471-2172-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaiswal JK, Andrews NW, Simon SM. Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells. J Cell Biol. 2002;159:625–635. doi: 10.1083/jcb.200208154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jancic C, Savina A, Wasmeier C, Tolmachova T, El-Benna J, Dang PM, Pascolo S, Gougerot-Pocidalo MA, Raposo G, Seabra MC, Amigorena S. Rab27a regulates phagosomal pH and NADPH oxidase recruitment to dendritic cell phagosomes. Nat Cell Biol. 2007;9:367–378. doi: 10.1038/ncb1552. [DOI] [PubMed] [Google Scholar]
- 51.Roos D, Winterbourn CC. Immunology. Lethal weapons Science. 2002;296:669–671. doi: 10.1126/science.1071271. [DOI] [PubMed] [Google Scholar]
- 52.Gilula NB, Epstein ML, Beers WH. Cell-to-cell communication and ovulation. A study of the cumulus-oocyte complex. J Cell Biol. 1978;78:58–75. doi: 10.1083/jcb.78.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MPO secretion was evaluated using peritoneal neutrophils from WT or ashen (Rab27aash/ash) mice stimulated with heat-killed Listeria monocytogenes (HKLM). For the isolation of mouse peritoneal neutrophils, animals were injected intraperitoneally with 1 ml of a sterile 4% thioglycollate solution. Polymorphonuclear cells were harvested 4 hours after injection by lavage of the peritoneal cavity with RPMI medium. MPO secretion was analyzed by flow cytometry as described previously (Brzezinska et al, Immunology 2009, 127:388–397). Results represent the mean ± SEM from 7 individual mice of each group. *, p<0.01.
Neutrophils from the indicated colonies were isolate, lysed and the total MPO content was determined by ELISA.
The left panels show the electron microscopy analysis of wild type and Rab27aash/ash neutrophils (C3H background). The right panels show the magnification of the areas selected in the left panels.