Abstract
20S-hydroxyvitamin D3 (20S-(OH)D3), an in vitro product of vitamin D3 metabolism by the cytochrome P450scc, was recently isolated, identified and shown to possess antiproliferative activity without inducing hypercalcemia. The enzymatic production of 20S-(OH)D3 is tedious, expensive, and cannot meet the requirements for extensive chemical and biological studies. Here we report for the first time the chemical synthesis of 20S-(OH)D3 which exhibited biological properties characteristic of the P450scc-generated compound. Specifically, it was hydroxylated to 20,23-dihydroxyvitamin D3 and 17,20-dihydroxyvitamin D3 by P450scc and was converted to 1α,20-dihydroxyvitamin D3 by CYP27B1. It inhibited proliferation of human epidermal keratinocytes with lower potency than 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3) in normal epidermal human keratinocytes, but with equal potency in immortalized HaCaT keratinocytes. It also stimulated VDR gene expression with similar potency to 1,25(OH)2D3, and stimulated involucrin (a marker of differentiation) and CYP24 gene expression, showing a lower potency for the latter gene than 1,25(OH)2D3. Testing performed with hamster melanoma cells demonstrated a dose-dependent inhibition of cell proliferation and colony forming capabilities similar or more pronounced than those of 1,25(OH)2D3. Thus, we have developed a chemical method for the synthesis of 20S-(OH)D3, which will allow the preparation of a series of 20S-(OH)D3 analogs to study structure-activity relationships to further optimize this class of compound for therapeutic use.
Keywords: 20S-hydroxyvitamin D3, melanoma, chemical synthesis, antiproliferative activity
Introduction
Besides its critical role in regulating bone mineralization and calcium homeostasis [1], it is well documented that the active form of vitamin D3 also regulates cell growth, differentiation, proliferation, apoptosis, and immune responses through the activation of the nuclear vitamin D receptor (VDR) [2–4]. However, clinical applications of pharmacological doses of its active form, 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3), are hampered by its toxic side effect of hypercalcemia [5–7]. Excessive elevated calcium concentration in serum leads to calcium deposition in many tissues, including crucial organs such as heart or kidney. The search for vitamin D3 analogs displaying minimal or no hypercalcemic side effects has been intense in the past decades. As a result, several vitamin D analogs that exhibit reduced hypercalcemia are entering clinical trials and are showing great promise as potential therapeutic agents for the treatment of cancer and other diseases [2, 4, 8].
Recent studies have revealed that a crucial enzyme in steroidogenesis, mammalian cytochrome P450scc, not only cleaves the side chain of cholesterol but also metabolizes 7-dehydrocholesterol (7DHC) to 7-dehydropregnenolone (7DHP) in vitro, in isolated mitochondria and in incubated ex-vivo adrenal glands through sequential hydroxylation of carbon atoms C-20 and C-22 and subsequent cleavage of the side chain [9–11]. Importantly, P450scc also hydroxylates the side chain of vitamin D3 to 20-hydroxyvitamin D3 (20S-(OH)D3), with subsequent transformation to di-, and tri-hydroxy metabolites [9, 12–15]. Similarly, 20-hydroxyvitamin D2 is a major product of P450scc action on vitamin D2, and 1α,20-dihydroxyvitamin D3 is formed from 1α-hydroxyvitamin D3 [12, 16, 17]. Thus, novel in vitro pathways have been identified that can generate hydroxylated forms of vitamin D with 20-hydroxy derivatives predominating.
Our recent studies have demonstrated that 20S-(OH)D3 acts as a potent inducer of keratinocyte differentiation with strong antiproliferative effects and NFκB inhibitory activity [18–20]. We have also found that 20S-(OH)D3 has significant anti-cancer activities in vitro, while being non-calcemic [20]. Collectively, these results strongly indicate that 20S-(OH)D3 or its analogs can serve as a therapeutic agent(s) with potentially lower toxicity in comparison to many synthetic xenobiotics. To further assess its biological activity and efficacy in animal models larger quantities of 20S-(OH)D3 are required. The current enzymatic generation of 20S-(OH)D3 by P450scc has substantial limitations with regard to scale-up, cost and time needed for production of more than milligram quantities of metabolites. The isolation and purification of metabolites is very tedious and expensive. To provide larger quantities of 20S-(OH)D3 for in vivo biological studies and further chemical modifications, we developed an efficient synthetic route for this compound. The conformational analysis during the course of the nucleophilic addition of Grignard reagent to 7-dehydropregnenolone acetate and analysis of NMR spectra indicated the formation of the 20S-epimer of 20-(OH)D3 exclusively. Subsequent studies confirmed that 20S-(OH)D3 can be enzymatically metabolized by P450scc and CYP27B1 to the expected dihydroxy products. These have potent antiproliferative and pro-differentiation activities in cultured keratinocytes and melanoma cells that are similar to those reported previously for 20(OH)D3 generated through the action of P450scc [10, 18].
Experimental
Generation, isolation and purification of biologically generated 20(OH)D3
The enzymatic production of 20S-(OH)D3 has been reported previously [12, 13]. Briefly, cytochrome P450scc purified from bovine adrenal glands was used to generate 20S-(OH)D3 from D3 followed by isolation with preparative thin layer chromatography (TLC). The metabolite was further purified using a C18 column in an RP-HPLC system equipped with a diode array detector (Waters, Milford, MA). Fractions with 20S-(OH)D3 of at least 99% purity were combined, dried under nitrogen, and stored at −70°C.
Chemical Synthesis
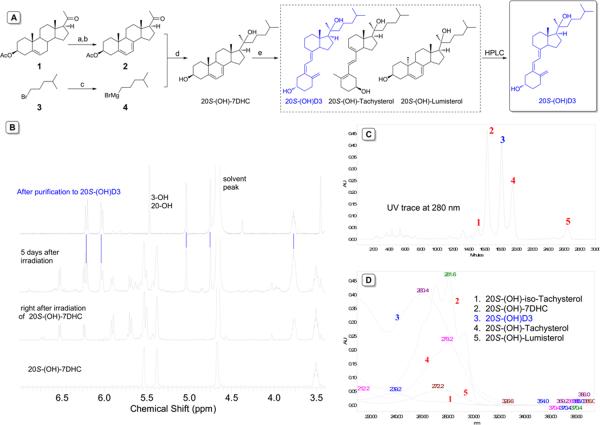
All reagents for the synthesis were purchased from commercial sources and were used without further purification. Moisture-sensitive reactions were carried out under an argon atmosphere. Routine TLC was performed on aluminum backed Uniplates (Analtech, Newark, DE). NMR spectra were obtained on a Bruker ARX-300 MHz (Billerica, MA) or a Varian Inova-500 MHz spectrometer (Varian NMR Inc., Palo Alto, CA). Chemical shifts are reported as parts per million (ppm) relative to the residue signals in methanol-d4 (3.31 ppm for proton and 49.15 ppm for carbon). Temperature was regulated with a general accuracy of ±0.1°C. Mass spectral data was collected on a Bruker ESQUIRE-LC/MS system equipped with an ESI source. The scheme for the synthesis of 20S-(OH)D3 is shown in Figure 1A.
Figure 1.
Synthesis and characterization of 20(OH)D3. (A) Reagents and conditions: (a) Dibromantin, 2,2'-azobisisobutyronitrile, benzene/hexane (1:1), 100°C, reflux; (b) Bu4NBr, Bu4NF, THF, room temperature; (c) Mg, THF, 45°C; (d) THF, 0°C – RT; (e) UVB irradiation for 5 min followed by 3 days in solution at room temperature. Only major products from the UVB irradiation are shown. (B) Monitoring reactions by NMR from the starting material 20S-(OH)-7DHC to 20S-(OH)D3 after preparative HPLC purifications, only fingerprint regions are shown. (C) HPLC trace of product mixture detected at 280 nm of UV absorption, peak 3 is the desired 20(OH)D3 product. (D) Extracted UV spectra from the diode-array detector of each HPLC peak. 20(OH)D3 is separated with high purity from this preparative HPLC operation, and the purified product is further confirmed by 1H NMR and mass spectrometry.
Synthesis of compound 2
To a solution of compound 1 (3.58 g, 10.0 mmol) in benzene–hexane (200 ml, 1:1 in volume) was added dibromantin (1.72 g, 6.0 mmol) and 2,2'-azobisisobutyronitrile (68 mg, 0.4 mmol). The mixture was refluxed for 20 min in a preheated oil bath (100°C) and then placed in an ice bath to cool. Insoluble material was removed by filtration and the filtrate was concentrated to yield a yellow-brown solid. To a solution of this yellow-brown solid in tetrahydrofuran (50 ml) was added tetrabutylammonium bromide (0.8 g, 2.5 mmol) and stirred for 75 min at room temperature. To this reaction mixture was added tetrabutylammonium fluoride (20 ml of 1.0 M solution in tetrahydrofuran, 20 mmol) and the resulting solution was stirred for 50 min. Water was added and the mixture was extracted by ethyl acetate. The organic layer was dried and concentrated. The residual was subjected to flash chromatography (eluted with hexane–ethyl acetate 2:1) to give a white solid. Yield: 36%. 1H NMR (500 MHz, CDCl3): δ 5.60 (dd, J = 12 Hz, 4.0 Hz, 1 H), 5.44-5.56 (m, 1 H), 4.73-4.75 (m, 1 H), 2.66 (t, J = 10 Hz, 1 H), 2.53-2.55 (m, 1 H), 2.38-2.41 (m, 1 H), 2.23-2.26 (m, 1 H), 2.17 (s, 3 H), 2.13-2.16 (m, 1 H), 2.06 (s, 3 H), 1.72-1.96 (m, 8 H), 1.52-1.62 (m, 3 H), 1.40 (dt, J = 30 Hz, 5 Hz, 1 H), 0.97 (s, 3 H), 0.60 (s, 3 H). ESI-MS: calculated for C23H32O3, 356.2, found 379.3 [M+Na]+.
Synthesis of compound 4
Compound 3 (1-Bromo-4-Methyl-Pentane, 3.3g, 20.0 mmol) in dry THF (50mL) was added dropwise to Mg (735 mg, 30.0 mmol, 1.5 eq) in an argon-purged flask and then stirred for 2 h at 45 °C. The resulting solution was cooled to room temperature and used for next step without further purification.
Synthesis of 20S-(OH)-7DHC
Compound 2 (712 mg, 2.0 mmol) was added to a solution of compound 4 (excess, 20–30 eq) in dry THF at 0°C under argon. The solution was allowed to warm to room temperature and was stirred overnight. The reaction mixture was quenched with aq. NH4Cl (sat.), extracted with EtOAc, the organic layer was washed with brine and water, dried by MgSO4 and concentrated. The crude material was subject to column chromatography (hexane:ethyl acetate 10:1) to give a white solid. Yield: 75%. 1H NMR (500 MHz, CD3OD, See Table 1 for full assignments): δ 5.54-5.57 (m, 1 H), 5.39-5.42 (m, 1 H), 3.49-3.58 (m, 1 H), 2.39-2.45 (m, 1 H), 2.24-2.30 (m, 1 H), 2.17-2.24 (m, 1 H), 1.29-2.00 (m, 18 H), 1.27 (s, 3 H), 1.14-1.23 (m, 3 H), 0.96 (s, 3 H), 0.91 (d, J = 6.5 Hz, 6 H), 0.81 (s, 3 H). ESI-MS: calculated for C27H44O3, 400.3, found 423.3 [M+Na]+.
Table 1.
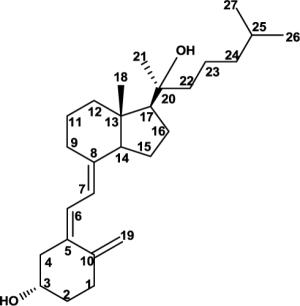
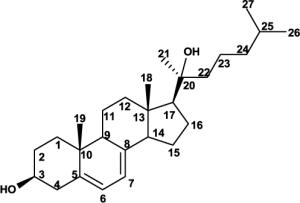
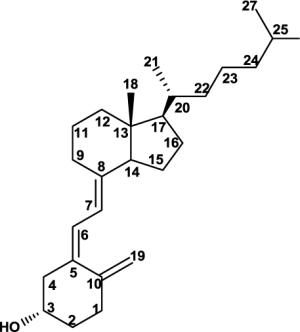
Full structural assignment of D3, 20S-(OH)D3 and its precursor, 20S-(OH)-7DHC. NMR chemical shift assignments for 20S-(OH)-7DHC, 20S-(OH)D3, and D3 by analysis of their 2D NMR. (Solvent: CD3OD for D3 and 20S-(OH)D3; CDCl3 for 20S-(OH)-7DHC). CDCl3 was used for 20S-(OH)-7DHC in order to compare its proton chemical shifts with reported values for 20R- and 20S-(OH)-cholesterol and to assign the absolute configuration at C20. The S-configuration at C20 is established based on the large downfield shift for 21-Me based on well-established literature. 1D and 2D spectra for 20S-(OH)-7DHC and 20S-(OH)D3 are shown in the supplementary materials.
| Atom | 20S-(OH)D3 | 20S-(OH)-7DHC | D3 | |||
|---|---|---|---|---|---|---|
| 1H | 13C | 1H | 13C | 1H | 13C | |
| 1 | 2.11α, 2.40β | 32.2 | 1.30α, 1.89β | 38.5 | 2.12α, 2.40β | 33.8 |
| 2 | 1.97α, 1.53β | 35.2 | 1.88α, 1.48β | 32.0 | 1.98α, 1.54β | 36.8 |
| 3 | 3.76 | 69.2 | 3.64 | 70.5 | 3.76 | 70.7 |
| 4 | 2.53α, 2.19β | 45.6 | 2.47α, 2.27β | 40.8 | 2.53α, 2.19β | 47.2 |
| 5 | NA | 136.3 | NA | 141.4 | NA | 137.5 |
| 6 | 6.21 | 121.2 | 5.57 | 119.6 | 6.22 | 122.8 |
| 7 | 6.02 | 118.0 | 5.40 | 116.7 | 6.04 | 119.1 |
| 8 | NA | 141.0 | NA | 140.4 | NA | 142.7 |
| 9 | 1.68α, 2.85β | 28.4 | 1.96 | 46.1 | 1.69α, 2.86β | 30.1 |
| 10 | NA | 145.6 | NA | 37.1 | NA | 147.1 |
| 11 | 1.56α, 1.66β | 23.1 | 1.61α, 1.74β | 21.1 | 1.55α, 1.67β | 24.7 |
| 12 | 1.37α, 2.07β | 40.9 | 1.28α, 2.16β | 39.6 | 1.33α, 2.03β | 42.1 |
| 13 | NA | 45.6 | NA | 43.3 | NA | 47.1 |
| 14 | 2.00 | 56.4 | 1.88 | 54.7 | 2.02 | 57.7 |
| 15 | 1.51 | 21.7 | 1.75α, 1.25β | 22.4 | 1.46 | 23.4 |
| 16 | 1.69α, 1.78β | 21.7 | 1.45α,1.75β | 22.6 | 1.31α, 1.91β | 28.9 |
| 17 | 1.67 | 58.5 | 1.58 | 57.9 | 1.30 | 58.1 |
| 18 | 0.69 | 12.8 | 0.79 | 13.7 | 0.56 | 12.5 |
| 19 | 4.74, 5.04 | 111.3 | 0.94 | 16.4 | 4.75, 5.04 | 112.8 |
| 20 | NA | 74.5 | NA | 75.4 | 1.40 | 37.6 |
| 20-OH | 5.49 | NA | * | NA | NA | NA |
| 21 | 1.23 | 24.8 | 1.29 | 26.6 | 0.95 | 19.6 |
| 22 | 1.32, 1.46 | 43.9 | 1.32, 1.44 | 43.9 | 1.04, 1.39 | 37.5 |
| 23 | 1.33 | 21.7 | 1.28 | 22.0 | 1.19, 1.39 | 25.1 |
| 24 | 1.16 | 39.6 | 1.15 | 39.8 | 1.16 | 40.8 |
| 25 | 1.55 | 27.8 | 1.54 | 28.0 | 1.54 | 29.3 |
| 26, 27 | 0.89 | 21.7 | 0.87 | 22.7 | 0.89 | 23.2 |
|
| ||||||
|
|
|
||||
NA – Not applicable (ternary carbons);
chemical shift was not observable at this position.
Synthesis of 20S-(OH)D3
The UV conversion of 20S-(OH)-7DHC to 20S-(OH)D3 was conducted using a UVB light source (4.8 ± 0.2 mW cm−2) with a maximum emission spectrum in the range of 280–320 nm, as described previously [21]. Briefly, a solution of 20S-(OH)-7DHC (10 mg, 1 mg/mL in methanol) was subjected to UV irradiation for 5 min in a quartz cuvette, using a Biorad UV Transilluminator 2000 (Biorad, Hercules, CA). The reaction mixture was incubated at room temperature (25°C) for 3 days and the product was separated by RP-HPLC chromatography using a Waters HPLC-system equipped with a diode-array detector (Waters Associates, Milford, MA). The reaction mixture was injected by an autosampler into an Atlantis C18 column (Waters, IL) with mobile phase as 70/30 methanol/water and a flow rate of 1.5 mL/min. Fractions were collected every 15 seconds and were reanalyzed by RP-HPLC. Fraction containing >95% of pure compound were dried. The product was identified based on the retention time, UV absorption spectra, MS and NMR measurement (See Table 1 for full NMR assignments)
Molecular modeling
Molecular modeling studies were performed using Schrodinger Molecular Modeling Suite 2009 (Schrodinger Inc., New York, NY). Molecules were constructed and systematic conformation search were performed using Macromodel and OPLS-2005 forcefield within the software. Lowest energy conformers for molecules were selected and their geometry were further optimized with density functional theory (DFT) using the B3LYP hybrid functional [22, 23] together with Pople's 6-31G(d,p) basis [24, 25] for final calculations (655 basis functions).
Metabolism of 20S-(OH)D3 by cytochrome P450scc and CYP27B1
Incorporation of 20S-(OH)D3 into phospholipid vesicles and measurement of its metabolism by P450scc was carried out as described previously [15]. The incubation mixture comprised 510 μM phospholipid vesicles containing 20S-(OH)D3 at a ratio to phospholipid of 0.1 mol/mol, 2 μM bovine cytochrome P450scc, 15 μM adrenodoxin, 0.4 μM adrenodoxin reductase, 2 mM glucose 6-phosphate, 2 U/ml glucose 6-phosphate dehydrogenase and 50 μM NADPH. Samples were pre-incubated for 8 min, reactions started by the addition of NADPH and incubations carried out at 37°C with shaking for 6 min. After extraction with dichloromethane samples were analyzed by HPLC as described before using a C18 column (Grace 15 cm × 4.6 mm, particle size 7 μm) [15]. Metabolism of 20S-(OH)D3 by CYP27B1 (25-hydroxyvitamin D3 1α-hydroxylase) was measured by a similar procedure as described before [26].
Cell culture
Normal human keratinocytes (HEKn) were isolated from neonatal foreskin of African American donors using 2.5 UI/ ml dispase and grown in keratinocyte basal medium (KBM) supplemented with keratinocytes growth factors (KGF) (Lonza) on collagen coated plates [19]. For experiments, cells in their third passage were used.
Hamster AbC1 melanoma cells were cultured in F10 media (Sigma-Aldrich, St. Louis, MO), while immortalized human epidermal keratinocytes (HaCaT) were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 5% fetal bovine serum (FBS) and 1% penicillin/streptomycin/amphotericin antibiotic solution (Sigma-Aldrich, St. Louis, MO) [12]. Prior to treatment with vitamin D3 metabolites, cells were serum deprived for 24 h and further incubated in F10 medium containing 5% charcoal-treated FBS (ctFBS) (HyClone, Logan, UT). All cultures were performed at 37°C in 5% CO2.
Cell proliferation assays
1. MTS test
HEKn keratinocytes were plated in 96-well plates, 10,000 cells/ well. After overnight incubation of cells, 1,25(OH)2D3 or chemically synthesized 20S-(OH)D3, initially dissolved in ethanol and then diluted in KGM medium containing 0.5% BSA, was added to the medium to achieve final concentrations of 0.01 μM or 1 μM, while in control cultures, the final concentration of ethanol vehicle was 0.1%. After 20 h of incubation with these compounds, 20μl of MTS/ PMS solution (Promega, Madison, WI) was added to the cells. Four hours later, absorbance was recorded at 490 nm using an ELISA plate reader. The number of viable cells was measured in six replicates.
2. DNA synthesis
Testing of DNA synthesis was carried out as described previously [12, 21]. Cells were inoculated into 24-well plates at 5,000 – 25,000 cells/well, depending on cell type. After overnight incubation at 37°C, the cultures were placed in serum free media to synchronize cells at the G0/G1 phase of the cell cycle. After 24 h 20S-(OH)D3 was added with fresh media containing growth supplements and incubated for additional 72 h. After a defined period of time, [3H]-thymidine (specific activity 88.0 Ci/mmol; Amersham Biosciences, Piscataway, NY, USA) was added to a final concentration of 0.5 μCi/mL in medium. After 4 h of incubation at 37°C, media were discarded, cells precipitated in 10% TCA in PBS (phosphate-buffered saline) for 30 min, washed twice with 1 mL PBS and then incubated with 1 N NaOH/ 1% SDS (250 μL/well) for 30 min at 37°C. The extracts were collected in scintillation vials and 5 mL of scintillation cocktail was added to each. 3H-radioactivity incorporated into DNA was measured with a beta counter (Direct Beta-Counter Matrix 9600; Packard).
3. Colony forming assay
The assay followed our standard methodology, as described previously [12, 21]. Briefly, cells were plated in six-well plates at a density of 192 cells/well in medium containing 5% ctFBS (Charcoal-treated fetal bovine serum), 1% antibiotic solution and 20S-(OH)D3 at graded concentrations or ethanol (vehicle control). Cells were cultured at 37°C for 7 days with media being changed every 3 days. At the end of incubation, the colonies were fixed with 4% paraformaldehyde in PBS overnight at 4°C, washed, stained with 5% crystal violet in PBS for 30 min, rinsed, and air-dried. The number and size of the colonies were measured using an ARTEK counter 880 (Dynex Technologies Inc., Chantilly, VA). Colony forming units were calculated by dividing the number of colonies by the number of cells plated and then multiplying by 100.
4. ApoTox-Glo Triplex Assay
Melanoma cells were plated in 96- well plates, 10,000 cells/ well. After overnight incubation of cells, 1,25(OH)2D3 or 20S-(OH)D3 was added to the medium as described above. After 24 h of incubation with these compounds, 20 μl of Viability/Cytotoxicity Reagent containing both GF-AFC Substrate and bis-AAF-R110 Substrate (Promega, Madison, WI) was added to the cells. After 30 min of incubation at 37°C, fluorescence was recorded at 400 nm excitation / 505 nm emission for viability and 485 nm excitation / 520 nm emission for cytotoxicity using a microplate plate reader for fluorescence and luminescence (Turner). One hundred μl of Caspase-Glo 3/7 Reagent (Promega) was further added to the cells, and after 30 min of incubation at room temperature, luminescence was recorded. Numbers of viable, cytotoxic and apoptotic cells were measured in four replicates.
Real-time RT PCR
The RNA from HEKn keratinocytes treated with 20S-(OH)D3 or 1,25(OH)2D3, was isolated using the Absolutely RNA Miniprep Kit (Stratagen). Reverse transcription (1 μg RNA/reaction) was performed using the Transcriptor First Strand cDNA Synthesis Kit (Roche). Real-time PCR was performed using cDNA diluted 5-fold in sterile water and a TaqMan PCR Master Mix. Reactions (in triplicate) were performed at 95°C for 5 min and then 45 cycles of 95°C for 10 sec, 60°C for 30 sec and 72°C for 30 sec. The primers and probes were designed with the universal probe library (Roche). Data were collected on a Roche Light Cycler 480. The amount of amplified product for each gene was compared to that for Cyclophilin B using a comparative CT method. A list of the primers used for RTPCR DNA amplification is shown in Table 2.
Table 2.
Primers used for RT-PCR DNA amplification.
| Gene | Primer sequence (left and right) |
|---|---|
| CYCLOPHILIN B1 | L 5`-TGTGGTGTTTGGCAAAGTTC-3` |
| R 5`-GTTTATCCCGGCTGTCTGTC-3` | |
| VDR | L 5`-CTTACCTGCCCCCTGCTC-3` |
| R 5`-AGGGTCAGGCAGGGAAGT-3` | |
| INVOLUCRIN | L 5`-TGCCTCAGCCTTACTGTGAGT-3` |
| R 5`-TCATTTGCTCCTGATGGGTA-3` | |
| CYP24 | L 5`-CATCATGGCCATCAAAACAAT-3` |
| R 5`-GCAGCTCGACTGGAGTGAC-3` |
Statistical analyses
Data are presented as mean ± SEM or SD as indicated in the figure legends and were analyzed with a Student's t-test (for two groups) or ANOVA using Prism 4.00 (GraphPad, San Diego, CA).
Results
Synthesis of 20S-hydroxyvitamin D3 (20S-(OH)D3)
As shown in Figure 1A, pregnenolone acetate 1 was brominated at the C-7 followed by dehydrobromination to generate 7-dehydropregnenolone acetate 2 [27]. Compound 2 was then reacted with freshly prepared 4-methylpentylmagnesium bromide 4 generated from 4-methylpentyl bromide 3, to afford 20S-(OH)-7DHC [28–30]. UVB irradiation of 20S-(OH)-7DHC followed by 3~4 days incubation at room temperature in methanol solution produced a mixture of 20S-(OH)D3, 20S-(OH)-tachysterol, 20S-(OH)-lumisterol, and other minor 20S-(OH)-steroids (Figure 1A). Reaction progress was readily monitored by 1H-NMR based on characteristic peaks of each product (Figure 1B). The reaction mixture was subsequently separated by preparative HPLC (Figure 1C) and each product was identified by its distinct UV absorption profile (Figure 1D) using a diode-array detector (Waters Corporation, Milford, MA). Although we could isolate different 20S-hydroxy products, at this stage we focused our attention on 20S-(OH)D3, since this is the P450scc generated metabolite that shows very promising biological activities. The isolated 20S-(OH)D3 was dried under a gentle stream of nitrogen, and the structure was confirmed by analysis of its NMR spectra (Figure 1B) and mass spectrometry. 20S-(OH)D3 with at least 97% purity was stored at −70°C until further use.
Interestingly, while in theory both 20R-(OH)-7DHC and 20S-(OH)-7DHC should be produced from the Grignard reaction (after step d in Figure 1A), only one epimer of 20-(OH)-7DHC and hence only the corresponding single epimer of 20-(OH)D3 was obtained. This strongly suggested that even though the acetyl side chain of compound 2 is not very bulky, the attack of Grignard agent is highly stereo-selective at the side chain. The same course of the reaction was observed in the synthesis of 20S-hydroxycholesterol and other 20-hydroxysteroids [28–30].
Extensive efforts have been made to determine the absolute configuration of the 20(OH)-7DHC epimer in order to unambiguously assign the absolute configuration of the resultant 20(OH)D3. However, both Mosher's method (tertiary alcohol, failed to form Mosher's ester) and crystallization for X-ray crystallography were not successful. NMR and HPLC analyses have clearly indicated that both 20(OH)-7DHC and the resultant 20(OH)D3 are pure compounds, not a mixture of epimers.
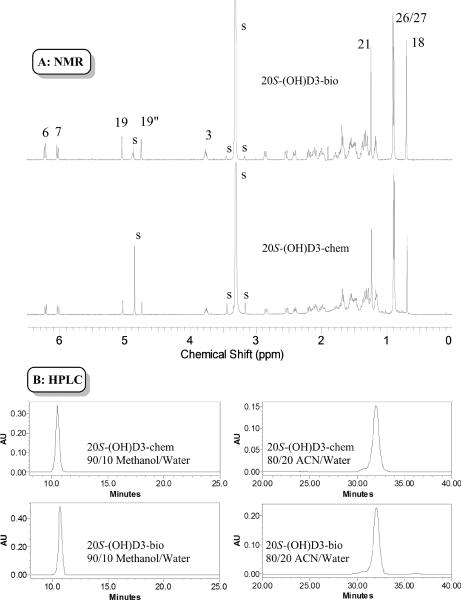
Since the goal of this work was to synthesize 20S-(OH)D3 that would be identical with the biologically potent one produced by P450scc, we compared the NMR spectra and HPLC retention times of the biologically generated 20S-(OH)D3 and the synthetic product (Figure 2). To our satisfaction, both NMR spectra and HPLC retention time in different solvent compositions were identical for the two compounds, confirming that the chemically synthesized 20(OH)D3 isomer is the same as the enzymatically generated one and we currently propose it to be the S-epimer, 20S-(OH)D3. Complete 1H and 13C assignment of 20S-(OH)D3 is shown in Table 1. The S-configuration is confirmed based on well-established literature [28, 29, 31, 32]. First, condensation of pregnenolone acetate and the Grignard reagent (isohexylmagnesium bromide) resulted exclusively in 20S-OH-cholesterol [28, 31], very similar to the current study (Figure 1A, the only difference being the presence of an extra double bond in the Bring). Second and most importantly, it is well-established that in the pregnane series, the 1H NMR chemical shift of 21-Me in 20S-OH isomers was downfield relative to 20R-OH isomers [28]. They have distinct values and are the bases used to assign the absolute configuration at C20 of these two epimers [28–32]. The 1H NMR chemical shift for 21-Me in 20S-OH-cholesterol is 1.17 ppm to 1.28 ppm [30], and 20R-OH-cholesterol is 1.00 ppm to 1.12 ppm, depending on NMR solvents [30]. The 1H NMR chemical shift for 21-Me in synthesized 20-(OH)-7DHC is 1.29 ppm which is almost identical to that in 20S-OH-cholesterol. Since the sidechain and the nearby rings have exactly the same structure, the absolute configuration most likely is 20S-(OH)-7DHC. Subsequent photolysis will not affect the sidechain. Consistent with this analysis, the 1H NMR chemical shifts for 21-Me are 1.23 ppm for both enzymatically generated and chemically synthesized 20(OH)D3 (Table 1). Collectively, these results strongly suggest that our compound is 20S-(OH)D3, confirming previous analysis [13]. We are currently in the process of making different derivatives in order to obtain satisfactory crystals to further confirm this result. For comparison, complete assignments for D3 and 20S-(OH)-7DHC are also shown in this table.
Figure 2.
The chemically synthesized compound (20S(OH)D3-chem) is identical to the enzymatically generated metabolite (20S(OH)D3-bio), confirmed by both NMR (A) and HPLC (B). The S-configuration is established based on the chemical shift of 21-Me for similar structures and the stereospecificity of enzymatic reactions. “s” denotes residue methanol solvent peaks, and the peak at 4.81 ppm (HDO) was suppressed with pre-saturation.
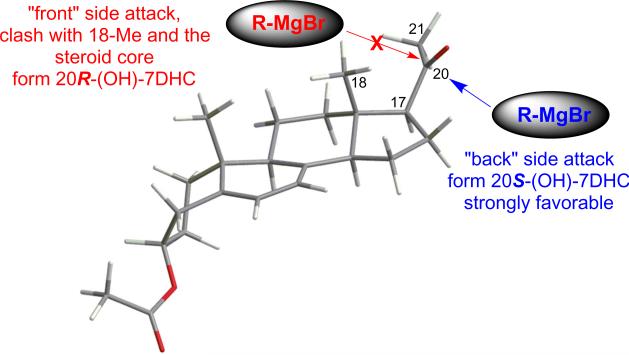
To understand the possible reason why only 20S-(OH)-7DHC was formed synthetically, we performed quantum mechanical calculations employing density functional theory (DFT) using the Jaguar module (version 7.6) in Schrodinger Molecular Modeling Suite 2009. The geometrically optimized structure for the precursor to 20(OH)-7DHC (compound 2 in Figure 1) is shown in Figure 3. The calculation results clearly indicate that there is a preference for the orientation of the acetyl group for compound 2. It is conceivable that this conformational preference dictated the outcome of the bulky side chain addition to form 20-(OH)-7DHC (Figure 1A). As shown in Figure 3, the attack of the nucleophilic Grignard reagent takes place predominantly from the less hindered “back” side of the carbonyl group to form 20S-(OH)-7DHC, while the attack from the “front” side to form 20R-(OH)-7DHC is sterically prohibited.
Figure 3.
Preferred conformation of the precursor (compound 2) to 20(OH)-7DHC calculated with density function theory with 6-31G** baseset. “Front” side attack by the bulky Grignard agent to form 20R isomer is prohibited due to the steric hindrance from the steroid core, while the “back” side attack to form 20S isomer is strongly favored.
Theoretically detailed quantum mechanics calculations of various transition states from compound 2 to the two epimers of 20-(OH)-7DHC may be more suitable to explain the product selectivity, but such calculations, especially involving the metal containing Grignard reagents, are beyond the scope of the present study. While such calculations for the transition state energies are difficult and may not be reliable, comparing energies of the starting compound 2 and the products may provide some insight in the product selectivity. At the DFT/6-31G** level and in aqueous solution, the energy for the preferred orientation (Figure 3, energy= −1211.32927 hartree) of compound 2, which will result in the preferred formation of 20S-(OH)-7DHC, is 3.44 kJ/mol lower than the alternative conformation (energy=−1121.32796 hartree) that exposes the other face of the carbonyl group to attack by the Grignard reagent. Hence, the preferred conformation dominates compound 2. In addition, the product 20S-(OH)-7DHC (energy = −1205.86798 hartree) is slightly more stable (ΔE=0.97 kJ/mol) than 20R-OH-7DHC (energy = −1205.86761 hartree). Thermodynamically, the product formation of 20S-(OH)-7DHC is also expected to be favored. Thus these theoretical calculations support our designation of the product as the S-epimer.
Chemically synthesized 20-S(OH)D3 is metabolized byP450scc and CYP27B1
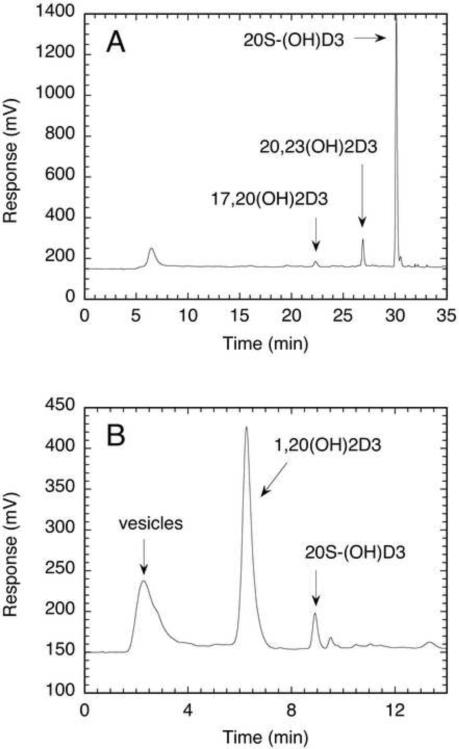
To test enzymatic metabolism, chemically synthesized 20S-(OH)D3 was incorporated into phospholipid vesicles prepared by sonication and incubated with bovine P450scc or mouse CYP27B1, plus adrenodoxin and adrenodoxin reductase. The 20S-(OH)D3 was hydroxylated by P450scc identically to the enzymatically generated compound [15] with the expected 20,23-dihydroxyvitamin D3 and 17,20-dihydroxyvitamin D3 appearing as products (Figure 4A). The chemically synthesized 20S-(OH)D3 was 1α-hydroxylated by CYP27B1 with greater than 90% of it being converted to 1α,20-dihydroxyvitamin D3 during the incubation (Figure 4B), in agreement with the reported in vitro metabolism of enzymatically synthesized compound [26].
Figure 4.
Chromatograms showing metabolism of chemically synthesized 20S-(OH)D3 by P450scc and CYP27B1. 20S-(OH)D3 in phospholipid vesicles was incubated with P450scc for 6 min at 37°C (Fig. 4A) or with CYP27B1 for 1 h at 25°C (Fig. 4B) and products analyzed by reverse phase HPLC. Products 20,23-dihydroxyvitamin D3 (20,23(OH)2D3), 17,20-dihydroxyvitamin D3 (17,20(OH)2D3) and 1α,20-dihydroxyvitamin D3 (1,20(OH)2D3) were identified from their identical retention times to authentic standards. Control chromatograms (not shown) confirmed that no products were present in the absence of enzyme.
Chemically synthesized 20S-(OH)D3 is biologically active
The biological activity of 20S-(OH)D3, measured in human normal epidermal keratinocytes, immortalized HaCaT keratinocytes and hamster AbC1 melanoma cells, showed similar effects to those reported for P450scc generated compound [18, 19].
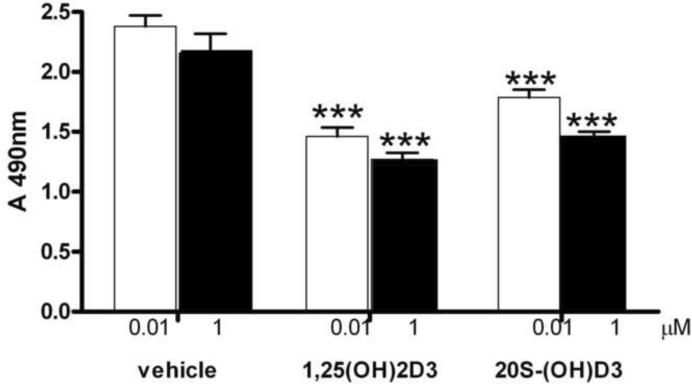
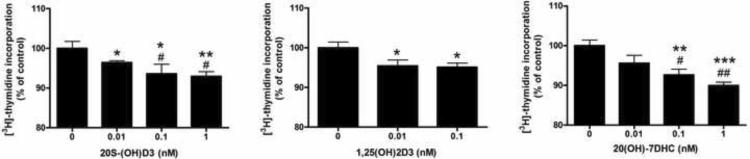
First, we tested the effect on keratinocytes proliferation and found that treatment of normal human epidermal keratinocytes with 20S-(OH)D3 and 1,25(OH)2D3 at concentrations of 10−8 and 10−6 M for 24 h led to the inhibition of cell proliferation when compared to control (vehicle treated) cells (Figure 5). Similarly, 20S-(OH)D3 inhibited proliferation (as measured by 3H-thymidine incorporation into DNA) of HaCaT immortalized epidermal keratinocytes in a dose-dependent manner (Figure 6). The inhibitory effect was similar to that exerted by 1,25(OH)2D3, being seen at a concentration as low as 10−11 M (Figure 6). Interestingly, the precursor compound, 20S-(OH)-7DHC, also inhibited DNA synthesis, albeit at higher concentrations (≥10−10 M).
Figure 5.
20S-(OH)D3 inhibits growth of normal epidermal keratinocytes.
Keratinocytes were treated with 10−8 M (black bar) or 10−6 M (open bar) of chemically synthesized 20S-(OH)D3 or 1α,25(OH)2D3 (1,25(OH)2D3). Corresponding concentrations of 0.001 and 0.1% ethanol were used as negative (vehicle) controls. Relative number of MTS positive cells is presented as a change in absorbance at 490 nm. The experiment was repeated twice. Data are presented as mean ±SD (*p<0.05, **p<0.01, ***p<0.001).
Figure 6.
20S-(OH)D3 inhibits DNA synthesis in HaCaT keratinocytes.
HaCaT cells were incubated with drugs for 72 h. [3H]-thymidine was added for last 4 h of incubation. DNA synthesis was measured by counting the radioactivity incorporated into TCA precipitable material. The experiment was repeated twice. Data are presented as means ± SEM. The dose dependent inhibition was analyzed by one-way ANOVA with #, P < 0.05 and ##, P < 0.01. The differences between control and treatments were also analyzed with student's t-test where *, P < 0.05; **, P < 0.01; ***, P < 0.001.
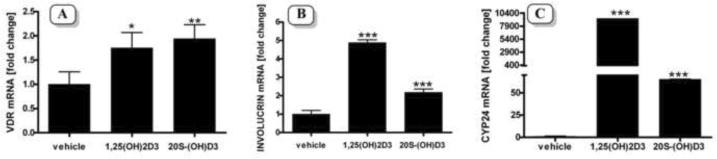
Next we tested the effect of 20S-(OH)D3 on the vitamin D receptor (VDR), involucrin and CYP24 gene-expression and found that 20S-(OH)D3 stimulated expression of VDR at comparable levels to 1,25(OH)2D3 (Figure 7A). However, it was less potent in the stimulation of the expression of involucrin (2 vs 5 fold) and CYP24 (60 vs 10,000 fold) in comparison to 1,25(OH)2D3 (Figure 7B and 7C). The more than 100 times difference between 1,25(OH)2D3 and 20S-(OH)D3 on the induction of CYP 24 expression is similar to that reported previously for biochemically synthesized 20S-(OH)D3 [18, 19, 33], and suggests that 20S-(OH)D3 will have only a minor influence on the inactivation (24 hydroxylation) of active forms of vitamin D3.
Figure 7.
20S-(OH)D3 stimulates expression of VDR (A), involucrin (B), and CYP24 (C) genes in cultured human epidermal keratinocytes.
Keratinocytes were treated with 10−7 M of chemically synthesized 20S-(OH)D3, 1α,25(OH)2D3, or 0.01% ethanol (vehicle control) for 24 h. mRNA was isolated and subjected to RTPCR. Data are presented as mean ±SD from 3 assays (*p<0.05, **p<0.01, ***p<0.001).
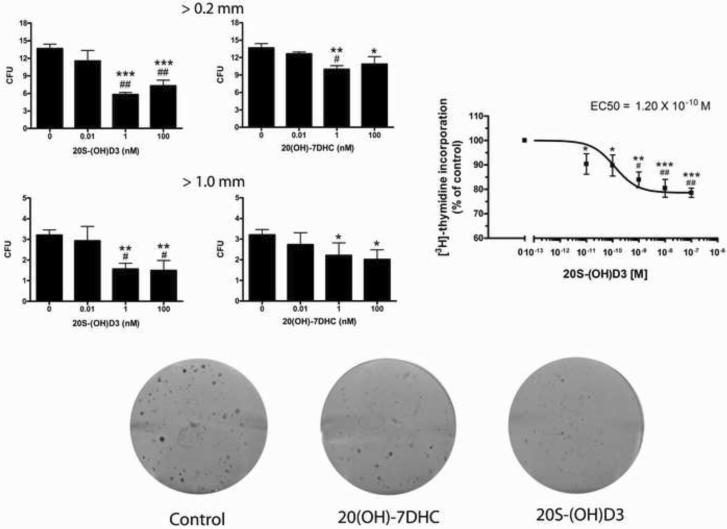
We also tested the effect of chemically synthesized 20S-(OH)D3 on cultured hamster AbC1 melanoma cells. As shown in Figure 8, 20S-(OH)D3 inhibited cell proliferation in a dose-dependent manner with an IC50 value of 1.2 × 10−10 M. Importantly, 20S-(OH)D3 also inhibited colony formation being more potent than its precursor 20-(OH)-7DHC (Figure 8) in inhibition of all (> 0.2 mm) or larger (> 1mm) colonies. Interestingly, 1,25(OH)2D3 had either minimal or no effect on colony formation (data not shown). Moreover, testing using ApoTox-Glo Triplex Assay showed lack of measureable pro-apoptotic or cytotoxic effects of the vitamin D hydroxyl derivatives against melanoma cells in comparison to ethanol controls (data not shown). This indicates that the antiproliferative effect is connected with slowed cell cycling as described previously for biochemically generated 20S-(OH)D3 [18, 33].
Figure 8.
20S-(OH)D3 inhibits DNA synthesis and colony formation in hamster AbC1 melanoma cells. For colony forming assays (left panel), 4 independent plates per each concentration were incubated with drugs for 7 days and colonies stained with crystal violet. The number of colony units (CFU) formed was counted for all colonies (>0.2 mm) and large colonies (>1 mm). To measure DNA synthesis (right panel), AbC1 cells plated into 4 independent cultures were incubated with drugs for 72 h, and [3H]-thymidine was added for the last 4 h of incubation. DNA synthesis was measured by counting the radioactivity incorporated into TCA precipitable material. Data are presented as means ± SEM for DNA synthesis and the colony forming assay (n = 4). *, P < 0.05; **, P < 0.01; ***, P < 0.001 in student's t-test with the differences between control and treatments. The dose-dependent inhibition was analyzed by one-way ANOVA with #, P < 0.05 and ##, P < 0.01. Lower panel shows representative plates with AbC1 colony formations that were treated with vehicle (control) or test compound at a concentration of 10−9 M.
Discussion
The ability to chemically synthesize highly active and non-hypercalcemic 20S-(OH)D3 is very important, because it enables large scale production of this compound as well as serving as the basis for further structural modification and extensive structure-activity relationship studies for this novel class of vitamin D derivatives. Interestingly, the synthetic method shown in Figure 1 stereo-specifically produces only 20S-(OH)D3, instead of both epimers. This is most likely due to the preferred conformation of the reactant and the associated strong steric preference for the formation of this isomer. While we have not been successful in obtaining good crystals for X-ray crystallography determination, detailed spectral comparison of the chemically synthesized compound with the enzymatically generated metabolite revealed identical structures. The stereospecificity of enzymatic metabolism by P450scc at C20 and the NMR chemical shifts for 21-Me confirms the 20S-configuration in the chemically synthesized compound.
As expected, the chemically synthesized 20S-(OH)D3 undergoes enzymatic reactions similar to that of biochemically generated metabolite and is biologically active. Its activity is similar to that of 1,25(OH)2D3 in immortalized keratinocytes and melanoma, but has lower potency in primary cultures of normal keratinocytes. The differences in activity against immortalized and normal keratinocytes may suggest a certain degree of selectivity against immortalized or malignant cell lines. In addition, 20S-(OH)D3 has lower potency compared to 1,25(OH)2D3 in activating CYP24 which catalyses the major bio-deactivation pathway for vitamin D analogs [18, 19, 33]. This may provide an advantage in using 20S-(OH)D3 analogs as therapeutic agents because their deactivation would be slow, which could effectively increase their life time during circulation for potential reduced dose intervals.
In conclusion, we have chemically synthesized 20S-(OH)D3 that has biological activity similar to that of 20S-(OH)D3 generated through the enzymatic action of P450scc on vitamin D3 [18, 19, 33]. Analytical procedures have confirmed that both compounds have identical structures and stereochemistry. Synthesis of larger quantities of material for further extensive in vivo biological studies can now take place. Furthermore, the developed synthetic method will provide a means for chemical modifications of 20S-(OH)D3 to generate the structure-activity relationship data necessary to deliver the most promising therapeutic agents for treating hyper-proliferative disorders.
Supplementary Material
Acknowledgement
The project described was supported by Grant Number AR052190 from NIH/NIAMS to AS and 1R15CA125623 from NIH/NCI to WL. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. We thank Edith Tang for performing the activity assay with CYP27B1.
Abbreviations
- D3
Vitamin D3
- 20S-(OH)D3
20S-hydroxyvitamin D3
- 1,25(OH)2D3
1α,25-dihydroxyvitamin D3
- ESI-MS
electrospray ionization Mass Spectroscopy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Pinette KV, et al. Vitamin D receptor as a drug discovery target. Mini Rev Med Chem. 2003;3(3):193–204. doi: 10.2174/1389557033488204. [DOI] [PubMed] [Google Scholar]
- 3.Masuda S, Jones G. Promise of vitamin D analogues in the treatment of hyperproliferative conditions. Mol Cancer Ther. 2006;5(4):797–808. doi: 10.1158/1535-7163.MCT-05-0539. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi T, Morikawa K. Vitamin D receptor agonists: opportunities and challenges in drug discovery. Curr Top Med Chem. 2006;6(12):1303–16. doi: 10.2174/156802606777864917. [DOI] [PubMed] [Google Scholar]
- 5.Beer TM, Myrthue A. Calcitriol in the treatment of prostate cancer. Anticancer Res. 2006;26(4A):2647–51. [PubMed] [Google Scholar]
- 6.Eelen G, et al. Mechanism and potential of the growth-inhibitory actions of vitamin D and ana-logs. Curr Med Chem. 2007;14(17):1893–910. doi: 10.2174/092986707781058823. [DOI] [PubMed] [Google Scholar]
- 7.Vieth R. Vitamin D and cancer mini-symposium: the risk of additional vitamin D. Ann Epidemiol. 2009;19(7):441–5. doi: 10.1016/j.annepidem.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz GG. Vitamin D and intervention trials in prostate cancer: from theory to therapy. Ann Epidemiol. 2009;19(2):96–102. doi: 10.1016/j.annepidem.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Guryev O, et al. A pathway for the metabolism of vitamin D3: unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1) Proc Natl Acad Sci U S A. 2003;100(25):14754–9. doi: 10.1073/pnas.2336107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slominski A, et al. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem. 2004;271(21):4178–88. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slominski AT, et al. Sequential metabolism of 7-dehydrocholesterol to steroidal 5,7-dienes in adrenal glands and its biological implication in the skin. PLoS One. 2009;4(2):e4309. doi: 10.1371/journal.pone.0004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slominski A, et al. An alternative pathway of vitamin D metabolism. Cytochrome P450scc (CYP11A1)-mediated conversion to 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2. FEBS J. 2006;273(13):2891–901. doi: 10.1111/j.1742-4658.2006.05302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slominski A, et al. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J. 2005;272(16):4080–90. doi: 10.1111/j.1742-4658.2005.04819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuckey RC, et al. Metabolism of 1alfa-hydroxyvitamin D3 by cytochrome P450scc to biologically active 1alfa,20-dihydroxyvitamin D3. J. Steroid Biochem. Molec. Biol. 2008;112:213–219. doi: 10.1016/j.jsbmb.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuckey RC, et al. Pathways and products for the metabolism of vitamin D3 by cytochrome P450scc. FEBS J. 2008;275(10):2585–96. doi: 10.1111/j.1742-4658.2008.06406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen MN, et al. Metabolism of vitamin D2 to 17,20,24-trihydroxyvitamin D2 by cytochrome P450scc (CYP11A1) Drug Metab. Dispos. 2009;37:761–767. doi: 10.1124/dmd.108.025619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuckey RC, Nguyen MN, Slominski A. Kinetics of vitamin D3 metabolism by cytochrome P450scc (CYP11A1) in phospholipid vesicles and cyclodextrin. Int J Biochem Cell Biol. 2008;40(11):2619–26. doi: 10.1016/j.biocel.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zbytek B, et al. 20-Hydroxyvitamin D3, a product of vitamin D3 hydroxylation by cytochrome P450scc, stimulates keratinocyte differentiation. J Invest Dermatol. 2008;128(9):2271–80. doi: 10.1038/jid.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janjetovic Z, et al. 20-Hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF-kappaB activity by increasing IkappaB alpha levels in human keratinocytes. PLoS One. 2009;4(6):e5988. doi: 10.1371/journal.pone.0005988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slominski AT, et al. Products of Vitamin D3 or 7-Dehydrocholesterol Metabolism by Cytochrome P450scc Show Anti-leukemia Effects, Having Low or Absent Calcemic Activity. PLoS One. 2010;5(3):e9907. doi: 10.1371/journal.pone.0009907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim TK, et al. A new steroidal 5,7-diene derivative, 3beta-hydroxyandrosta-5,7-diene-17beta-carboxylic acid, shows potent anti-proliferative activity. Steroids. 2010;75(3):230–239. doi: 10.1016/j.steroids.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becke AD. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993;98(7):5648–5652. [Google Scholar]
- 23.Lee C, Yang W, Parr RG. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B. 1988;37(2):785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 24.Francl MM, et al. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 1982;77(7):3654–3665. [Google Scholar]
- 25.Hariharan PC, Pople JA. Influence of polarization functions on MO hydrogenation energies. Theo. Chim. Acta. 1973;28(3):213–222. [Google Scholar]
- 26.Tang EKY, et al. Metabolism of substrates incorporated into phospholipid vesicles by mouse 25-hydroxyvitamin D3 1alfa-hydroxylase (CYP27B1) J. Steroid Biochem. Molec. Biol. 2010;119:171–179. doi: 10.1016/j.jsbmb.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 27.Guo LW, et al. Chemical synthesis of 7- and 8-dehydro derivatives of pregnane-3,17alpha,20-triols, potential steroid metabolites in Smith-Lemli-Opitz syndrome. Steroids. 2003;68(1):31–42. doi: 10.1016/s0039-128x(02)00113-7. [DOI] [PubMed] [Google Scholar]
- 28.Mijares A, et al. Studies on the C-20 epimers of 20-hydroxycholesterol. J Org Chem. 1967;32(3):810–2. doi: 10.1021/jo01278a066. [DOI] [PubMed] [Google Scholar]
- 29.Nes WR, Varkey TE. Conformational analysis of the 17(20) bond of 20-keto steroids. J Org Chem. 1976;41(9):1652–3. doi: 10.1021/jo00871a041. [DOI] [PubMed] [Google Scholar]
- 30.Honda M, Komori T. Structures of thornasterols A and B (biologically active glycosides from asteroidia, XI) Tetrahedron Letters. 1986;27(29):3369–3372. [Google Scholar]
- 31.Burstein SH, Peron FG, Williamson E. Reactions of 20-hydroxylated steroids with bovine adrenal tissue preparations. Steroids. 1969;13(3):399–412. doi: 10.1016/0039-128x(69)90047-6. [DOI] [PubMed] [Google Scholar]
- 32.Corey EJ, Virgil SC, Sarshar S. New mechanistic and stereochemical insights on the biosynthesis of sterols from 2,3-oxidosqualene. J Am Chem Soc. 1991;113(21):8171–8172. [Google Scholar]
- 33.Slominski AT, et al. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PLoS One. 2010;5(3):e9907. doi: 10.1371/journal.pone.0009907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.