Abstract
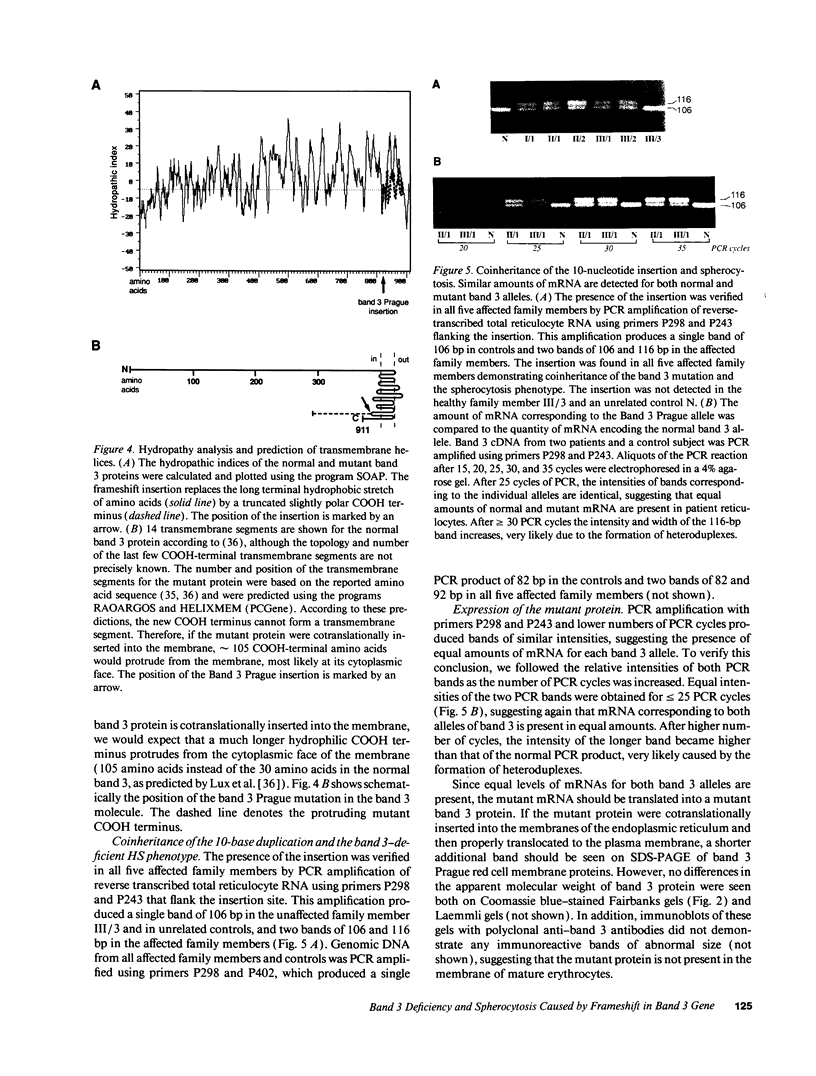
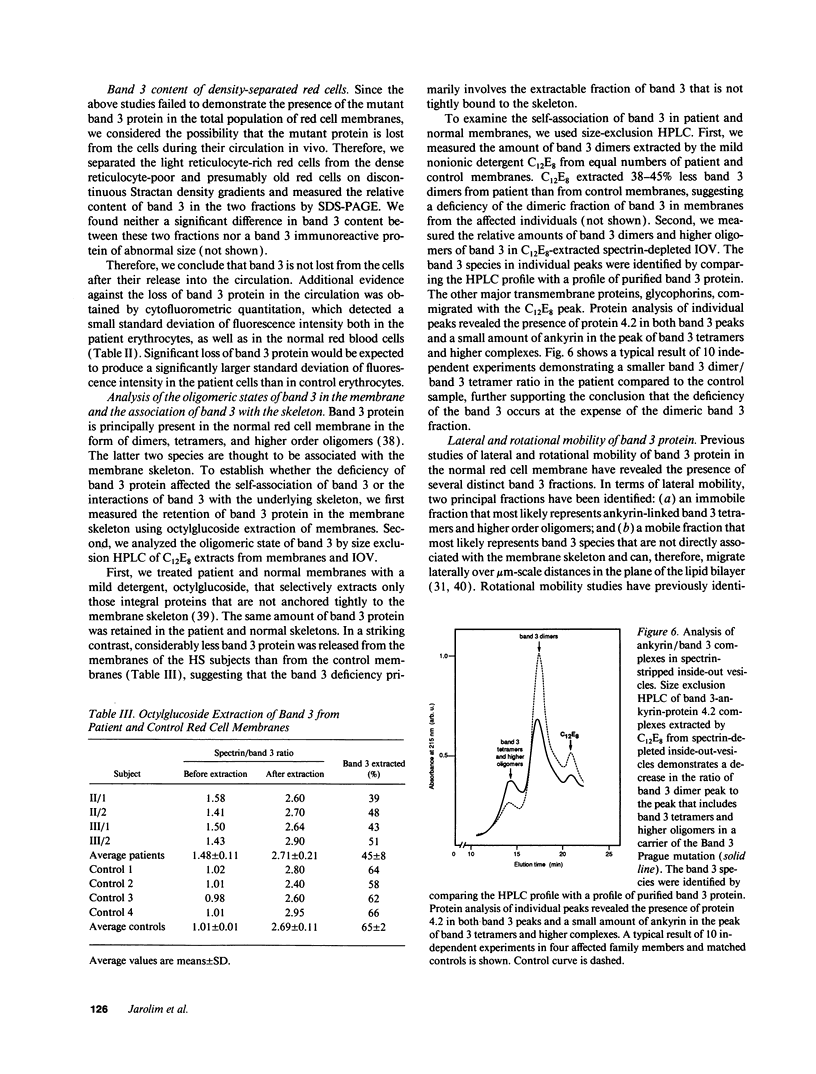
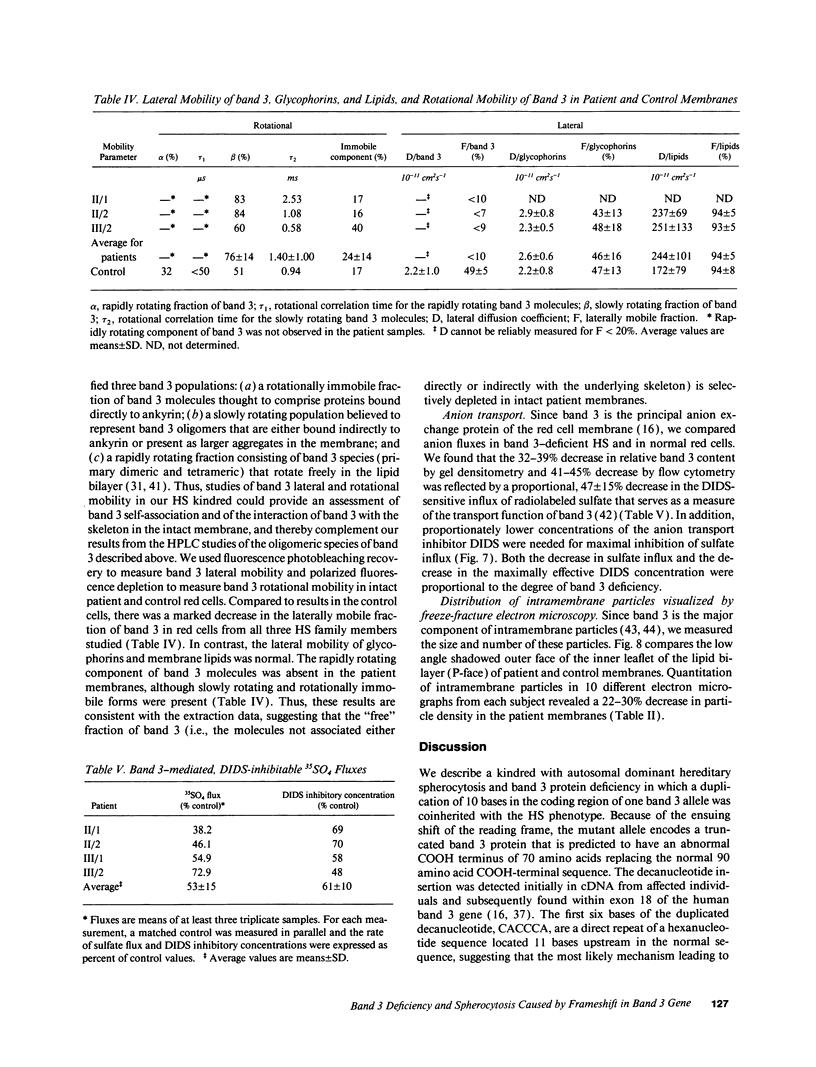
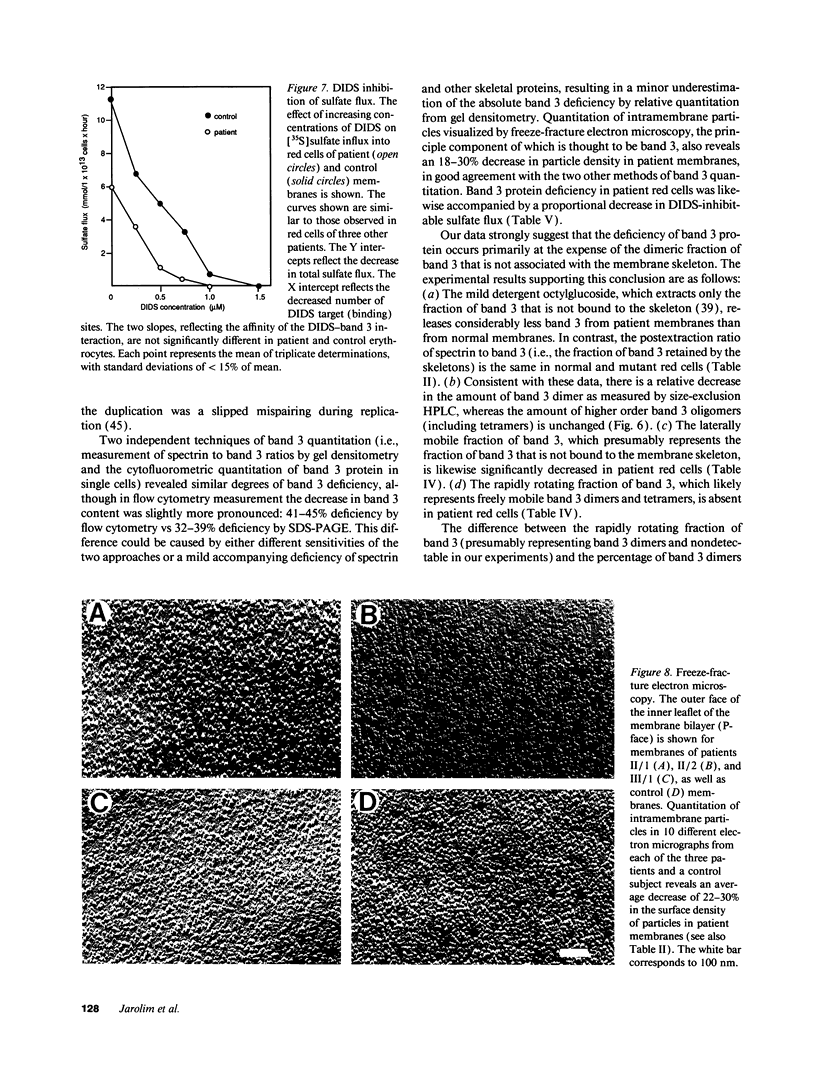
We describe a duplication of 10 nucleotides (2,455-2,464) in the band 3 gene in a kindred with autosomal dominant hereditary spherocytosis and a partial deficiency of the band 3 protein that is reflected by decreased rate of transmembrane sulfate flux and decreased density of intramembrane particles. The mutant allele potentially encodes an abnormal band 3 protein with a 3.5-kD COOH-terminal truncation; however, we did not detect the mutant protein in the membrane of mature red blood cells. Since the mRNA levels for the mutant and normal alleles are similar and since the band 3 content is the same in the light and dense red cell fractions, we conclude that the mutant band 3 is either not inserted into the plasma membrane or lost from the membrane prior to the release of red blood cells into circulation. We further show that the decrease in band 3 content principally involves the dimeric laterally and rotationally mobile fraction of the band 3 protein, while the laterally immobile and rotationally restricted band 3 fraction is left essentially intact. We propose that the decreased density of intramembrane particles decreases the stability of the membrane lipid bilayer and causes release of lipid microvesicles that leads to surface area deficiency and spherocytosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Casella J. F., Zinkham W. H., McMillan C., Bennett V. Partial deficiency of erythrocyte spectrin in hereditary spherocytosis. 1985 Mar 28-Apr 3Nature. 314(6009):380–383. doi: 10.1038/314380a0. [DOI] [PubMed] [Google Scholar]
- Casey J. R., Reithmeier R. A. Analysis of the oligomeric state of Band 3, the anion transport protein of the human erythrocyte membrane, by size exclusion high performance liquid chromatography. Oligomeric stability and origin of heterogeneity. J Biol Chem. 1991 Aug 25;266(24):15726–15737. [PubMed] [Google Scholar]
- Corash L. M., Piomelli S., Chen H. C., Seaman C., Gross E. Separation of erythrocytes according to age on a simplified density gradient. J Lab Clin Med. 1974 Jul;84(1):147–151. [PubMed] [Google Scholar]
- Corbett J. D., Golan D. E. Band 3 and glycophorin are progressively aggregated in density-fractionated sickle and normal red blood cells. Evidence from rotational and lateral mobility studies. J Clin Invest. 1993 Jan;91(1):208–217. doi: 10.1172/JCI116172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Davis L., Lux S. E., Bennett V. Mapping the ankyrin-binding site of the human erythrocyte anion exchanger. J Biol Chem. 1989 Jun 5;264(16):9665–9672. [PubMed] [Google Scholar]
- Elgsaeter A., Shotton D. M., Branton D. Intramembrane particle aggregation in erythrocyte ghosts. II. The influence of spectrin aggregation. Biochim Biophys Acta. 1976 Feb 19;426(1):101–122. doi: 10.1016/0005-2736(76)90433-8. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gilman J. G., Johnson M. E., Mishima N. Four base-pair DNA deletion in human A gamma globin-gene promoter associated with low A gamma expression in adults. Br J Haematol. 1988 Apr;68(4):455–458. doi: 10.1111/j.1365-2141.1988.tb04235.x. [DOI] [PubMed] [Google Scholar]
- Goossens M., Kan Y. Y. DNA analysis in the diagnosis of hemoglobin disorders. Methods Enzymol. 1981;76:805–817. doi: 10.1016/0076-6879(81)76159-7. [DOI] [PubMed] [Google Scholar]
- Groves J. D., Tanner M. J. Glycophorin A facilitates the expression of human band 3-mediated anion transport in Xenopus oocytes. J Biol Chem. 1992 Nov 5;267(31):22163–22170. [PubMed] [Google Scholar]
- Hanspal M., Palek J. Biogenesis of normal and abnormal red blood cell membrane skeleton. Semin Hematol. 1992 Oct;29(4):305–319. [PubMed] [Google Scholar]
- Hanspal M., Yoon S. H., Yu H., Hanspal J. S., Lambert S., Palek J., Prchal J. T. Molecular basis of spectrin and ankyrin deficiencies in severe hereditary spherocytosis: evidence implicating a primary defect of ankyrin. Blood. 1991 Jan 1;77(1):165–173. [PubMed] [Google Scholar]
- Jarolim P., Palek J., Rubin H. L., Prchal J. T., Korsgren C., Cohen C. M. Band 3 Tuscaloosa: Pro327----Arg327 substitution in the cytoplasmic domain of erythrocyte band 3 protein associated with spherocytic hemolytic anemia and partial deficiency of protein 4.2. Blood. 1992 Jul 15;80(2):523–529. [PubMed] [Google Scholar]
- Jay D., Cantley L. Structural aspects of the red cell anion exchange protein. Annu Rev Biochem. 1986;55:511–538. doi: 10.1146/annurev.bi.55.070186.002455. [DOI] [PubMed] [Google Scholar]
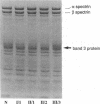
- Jennings L. K., Brown L. K., Dockter M. E. Quantitation of protein 3 content of circulating erythrocytes at the single-cell level. Blood. 1985 May;65(5):1256–1262. [PubMed] [Google Scholar]
- Kopito R. R., Andersson M., Lodish H. F. Structure and organization of the murine band 3 gene. J Biol Chem. 1987 Jun 15;262(17):8035–8040. [PubMed] [Google Scholar]
- Kunimoto M., Shibata K., Miura T. Comparison of the cytoskeleton fractions of rat red blood cells prepared with non-ionic detergents. J Biochem. 1989 Feb;105(2):190–195. doi: 10.1093/oxfordjournals.jbchem.a122638. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Low P. S. Structure and function of the cytoplasmic domain of band 3: center of erythrocyte membrane-peripheral protein interactions. Biochim Biophys Acta. 1986 Sep 22;864(2):145–167. doi: 10.1016/0304-4157(86)90009-2. [DOI] [PubMed] [Google Scholar]
- Lux S. E., John K. M., Kopito R. R., Lodish H. F. Cloning and characterization of band 3, the human erythrocyte anion-exchange protein (AE1). Proc Natl Acad Sci U S A. 1989 Dec;86(23):9089–9093. doi: 10.1073/pnas.86.23.9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux S. E., Tse W. T., Menninger J. C., John K. M., Harris P., Shalev O., Chilcote R. R., Marchesi S. L., Watkins P. C., Bennett V. Hereditary spherocytosis associated with deletion of human erythrocyte ankyrin gene on chromosome 8. Nature. 1990 Jun 21;345(6277):736–739. doi: 10.1038/345736a0. [DOI] [PubMed] [Google Scholar]
- Matayoshi E. D., Jovin T. M. Rotational diffusion of band 3 in erythrocyte membranes. 1. Comparison of ghosts and intact cells. Biochemistry. 1991 Apr 9;30(14):3527–3538. doi: 10.1021/bi00228a025. [DOI] [PubMed] [Google Scholar]
- Morrison M., Grant W., Smith H. T., Mueller T. J., Hsu L. Catabolism of the anion transport protein in human erythrocytes. Biochemistry. 1985 Oct 22;24(22):6311–6315. doi: 10.1021/bi00343a041. [DOI] [PubMed] [Google Scholar]
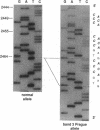
- Orita M., Iwahana H., Kanazawa H., Hayashi K., Sekiya T. Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2766–2770. doi: 10.1073/pnas.86.8.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Palek J., Jarolim P. Clinical expression and laboratory detection of red blood cell membrane protein mutations. Semin Hematol. 1993 Oct;30(4):249–283. [PubMed] [Google Scholar]
- Pasternack G. R., Anderson R. A., Leto T. L., Marchesi V. T. Interactions between protein 4.1 and band 3. An alternative binding site for an element of the membrane skeleton. J Biol Chem. 1985 Mar 25;260(6):3676–3683. [PubMed] [Google Scholar]
- Romans A. Y., Yeagle P. L., O'Connor S. E., Grisham C. M. Interaction between glycophorin and phospholipids in recombined systems. J Supramol Struct. 1979;10(2):241–251. doi: 10.1002/jss.400100213. [DOI] [PubMed] [Google Scholar]
- Schofield A. E., Reardon D. M., Tanner M. J. Defective anion transport activity of the abnormal band 3 in hereditary ovalocytic red blood cells. Nature. 1992 Feb 27;355(6363):836–838. doi: 10.1038/355836a0. [DOI] [PubMed] [Google Scholar]
- Tanner M. J., Martin P. G., High S. The complete amino acid sequence of the human erythrocyte membrane anion-transport protein deduced from the cDNA sequence. Biochem J. 1988 Dec 15;256(3):703–712. doi: 10.1042/bj2560703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner M. J. Molecular and cellular biology of the erythrocyte anion exchanger (AE1). Semin Hematol. 1993 Jan;30(1):34–57. [PubMed] [Google Scholar]
- Walder J. A., Chatterjee R., Steck T. L., Low P. S., Musso G. F., Kaiser E. T., Rogers P. H., Arnone A. The interaction of hemoglobin with the cytoplasmic domain of band 3 of the human erythrocyte membrane. J Biol Chem. 1984 Aug 25;259(16):10238–10246. [PubMed] [Google Scholar]
- Wickner W. T., Lodish H. F. Multiple mechanisms of protein insertion into and across membranes. Science. 1985 Oct 25;230(4724):400–407. doi: 10.1126/science.4048938. [DOI] [PubMed] [Google Scholar]
- Wolfe L. C., John K. M., Falcone J. C., Byrne A. M., Lux S. E. A genetic defect in the binding of protein 4.1 to spectrin in a kindred with hereditary spherocytosis. N Engl J Med. 1982 Nov 25;307(22):1367–1374. doi: 10.1056/NEJM198211253072203. [DOI] [PubMed] [Google Scholar]
- Yoshida T. M., Barisas B. G. Protein rotational motion in solution measured by polarized fluorescence depletion. Biophys J. 1986 Jul;50(1):41–53. doi: 10.1016/S0006-3495(86)83437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Branton D. Reconstitution of intramembrane particles in recombinants of erythrocyte protein band 3 and lipid: effects of spectrin-actin association. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3891–3895. doi: 10.1073/pnas.73.11.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]