Abstract
Infection with the fish parasite Anisakis following exposure to contaminated fish can lead to allergic reactions in humans. The present study examined the immunological mechanisms underlying the development of allergic airway inflammation in mice after different routes of sensitization to Anisakis. Wild-type and interleukin-4 receptor alpha (IL-4Rα)-deficient BALB/c mice were sensitized intraperitoneally with live or heat-killed Anisakis larvae or by intranasal administration of an Anisakis extract and were subsequently challenged intranasally with an Anisakis extract. Both routes of sensitization induced IL-4Rα-dependent allergic airway responses, whereas allergen-specific antibody responses developed only when mice were sensitized intraperitoneally. Intranasal sensitization induced airway hyperresponsiveness (AHR) in wild-type mice only, showing that AHR was IL-4/IL-13 dependent. Unexpectedly, infection with Anisakis larvae induced AHR in both wild-type and IL-4Rα-deficient mice. IL-4Rα-independent AHR was mediated by gamma interferon (IFN-γ), as evidenced by the fact that in vivo neutralization of IFN-γ abrogated AHR. Together, these results demonstrate that both infection with larvae and inhalational exposure to Anisakis proteins are potent routes of allergic sensitization to Anisakis, explaining food- and work-related allergies in humans. Importantly for diagnosis, allergic airway inflammation can be independent of detectable Anisakis-specific antibodies. Moreover, depending on the route of sensitization, AHR can be induced either by IL-4/IL-13 or by IFN-γ.
While it is believed that many parasitic worms protect against allergy, the fish parasite Anisakis can induce acute gastroallergic or anaphylactic reactions in a subset of infected patients (4). Anisakis spp. are nematode parasites of marine mammals with larval stages that pass through several intermediate hosts. The infectious-stage larvae (L3) are found worldwide in sea fish or cephalopods and can be accidentally ingested by humans (32). If ingested live due to consumption of raw or undercooked fish, Anisakis L3 are able to parasitize humans, causing the zoonotic disease known as anisakiasis. This is usually an acute and transient infection, with the larvae dying within a few weeks, since the host environment does not permit development into adult worms (6). Within hours of being ingested, Anisakis L3 penetrate the mucosal layers of the gastrointestinal tract, causing direct tissue damage that may lead to abdominal pain, nausea, and/or diarrhea. Furthermore, some patients develop an immunoglobulin E (IgE)-mediated “gastroallergic anisakiasis,” which presents with clinical manifestations ranging from urticaria to allergic reactions and life-threatening anaphylactic shock (5, 13, 14). To date, nine allergens from Anisakis simplex, some of which are cross-reactive to other seafood allergens, have been characterized on a molecular level (28). The importance of the problem is demonstrated by studies that have found Anisakis to be a leading cause of food allergy in Spain (2) and have found a higher prevalence of sensitization to Anisakis than to seafood among almost 5 million Japanese (24). Anisakis is also an important cause of occupational allergy in fish-processing workers, as shown in a recent epidemiological study by our group, including 578 workers from fish-processing factories in South Africa, in which Anisakis sensitization was associated with dermatitis and nonspecific bronchial hyperreactivity (37). Because sensitization to Anisakis was associated with increased fish consumption, we investigated the underlying immune mechanisms by infecting mice with Anisakis L3 and subsequently challenging them orally with an Anisakis crude antigen extract. This induced striking allergic reactions, including airway inflammation and lung mucus hypersecretion, which were associated with T-helper 2 (Th2)/type 2 responses (37). Furthermore, mice exposed epicutaneously to Anisakis proteins developed protein contact dermatitis (36). Local skin pathology was interleukin-13 (IL-13) dependent, as evidenced by the fact that it was abolished in IL-13- and IL-4 receptor alpha (IL-4Rα)-deficient mice, whereas IL-4 was important for systemic allergic sensitization (36). Together, these studies show that sensitization by Anisakis infection and subsequent oral challenge with an Anisakis extract can cause allergic airway disease, while epicutaneous exposure to Anisakis proteins can lead to dermatitis, explaining the observations of human prevalence studies.
However, it is still unclear whether live infection is needed for the development of allergic airway reactions or whether exposure to nematode-derived protein is sufficient (4). This is an important clinical question in view of the fact that several case and prevalence studies, including ours, indicate that sensitization by inhalation of Anisakis proteins might be an important cause of work-related allergies (1, 3, 7, 37, 39, 40). Aerosolized food allergens cause as much as 10% of asthma in the occupational environment (41), and Anisakis allergens aerosolized during fish cleaning, cooking, or fish meal production may therefore pose a risk for workers.
In this study we aimed to determine whether sensitization through Anisakis infection is essential for the induction of allergic airway disease or if exposure to larval proteins or heat-killed larvae is sufficient to induce allergic airway reactions. In addition, we investigated the effects of IL-4/IL-13 signaling by using mice deficient in IL-4Rα, an important receptor chain in allergic airway disease through which both IL-4 and IL-13 signal (8). Wild-type and IL-4Rα−/− mice were either sensitized intraperitoneally (i.p.) with live or heat-killed Anisakis L3 or sensitized intranasally to an Anisakis extract and were subsequently challenged by intranasal administration of an Anisakis extract in order to mimic aerosolized exposure. All sensitization protocols induced symptoms of allergic airway disease, but allergen-specific antibody responses were present only when mice were sensitized intraperitoneally by live or heat-killed larvae. Interestingly, while the development of airway hyperresponsiveness (AHR) was dependent on IL-4Rα responsiveness when mice were sensitized intranasally, mice sensitized with live or heat-killed Anisakis L3 developed AHR independently of IL-4Rα responsiveness. This IL-4Rα-independent AHR was shown to be dependent on gamma interferon (IFN-γ).
MATERIALS AND METHODS
Mice.
Experiments were performed with 6- to 8-week-old wild-type BALB/c mice or IL-4Rα−/− mice on a BALB/c genetic background (34). Animals were housed in the University of Cape Town animal unit under specific-pathogen-free conditions. All experiments were approved by the Research Animal Ethics Committee of the University of Cape Town.
Anisakis live infection and intranasal challenge.
Anisakis pegreffii larvae were collected, and an Anisakis extract was prepared, as described previously (37). The endotoxin concentration in Anisakis extract preparations was between 2.5 and 5 endotoxin units (EU)/mg. For Anisakis infection studies, anesthetized mice were injected intraperitoneally with two larvae in 1 ml phosphate-buffered saline (PBS) at day 0 and day 21. Where indicated, Anisakis larvae were heat killed for 20 min at 95°C before injection. On days 42, 43, and 44, anesthetized mice were challenged with 0.1 mg Anisakis protein extract in 50 μl PBS by intranasal administration. For neutralization studies, mice received anti-IFN-γ monoclonal antibodies (rat IgG2a, clone ANI 18KL6; 0.2 mg/mouse; endotoxin level, 10 to 70 EU) intraperitoneally on days 41 and 44. Lung tissue was homogenized in PBS plus protease inhibitors (Roche Diagnostics, Germany) and was then centrifuged for 20 min at 12,000 × g for determination of IFN-γ concentrations after treatment with anti-IFN-γ antibodies. IFN-γ levels in the supernatant were measured by enzyme-linked immunosorbent assays (ELISA). For nasal sensitization studies with an Anisakis extract, anesthetized mice were sensitized on days 0, 7, and 14 with 50 μg Anisakis protein extract-1.3 mg/ml aluminum hydroxide (Sigma, Germany) in 10 μl PBS by intranasal administration. On days 21, 22, and 23, anesthetized mice were challenged with 0.1 mg Anisakis extract in 50 μl PBS by intranasal administration. PBS control groups were treated identically during sensitization or infection, but Anisakis extract was excluded from the solutions during allergen challenge. In separate experiments, naïve mice were challenged intranasally with 1 μg recombinant mouse IFN-γ (endotoxin concentration, <0.1 ng per μg; BD Pharmingen) in 50 μl PBS-bovine serum albumin (BSA) or PBS-BSA alone on 4 consecutive days, and airway hyperresponsiveness was measured 6 h after each challenge. Endotoxin levels were determined by a Limulus amebocyte lysate assay (QCL-1000; Lonza).
Airway hyperresponsiveness.
Responsiveness to β-methacholine (Sigma) by conscious, unrestrained mice was assessed using a whole-body plethysmograph (emka Technologies, France). This system measures pressure changes induced by the breathing of the mice and computes values for enhanced pause (Penh), a dimensionless parameter reflecting airway hyperresponsiveness. It has been shown previously that changes in Penh values correlate with changes in airway resistance in the BALB/c mouse strain under conditions similar to those used here (18). Baseline readings were taken for 5 min before each exposure to increasing doses of aerosolized β-methacholine in PBS. The results are computed as the average for the first 5 min minus the average of the baseline readings.
Serum antibodies.
Blood samples were taken directly from mice after killing, and antibody concentrations were measured in blood serum by ELISA as described previously (37). Ninety-six-well plates were coated with 5 μg/ml or 1 mg/ml Anisakis extract in PBS for the antigen-specific IgG or IgE antibody ELISA, respectively. For the total-IgE ELISA, plates were coated with an anti-mouse IgE antibody (1 μg/ml; clone 84.1C).
Bronchoalveolar lavage (BAL).
Mice were killed by CO2 asphyxiation, and lungs were washed once with 1 ml PBS. A maximum of 2 × 105 cells were centrifuged on a microscope slide and were stained with the Rapi Diff stain set (Clinical Diagnostics CC, South Africa). Differential cell counts were made at a magnification of ×400, and at least 100 cells were counted per slide.
In vitro restimulation of lymphocytes.
Lymphocytes were purified from pooled lung-draining lymph nodes for in vitro restimulation experiments. A total of 2 × 105 lymphocytes were restimulated in 96-well plates (Nunc, Denmark) with 100 μg/ml Anisakis extract for 96 h in Iscove's modified Dulbecco's medium (IMDM)-10% fetal calf serum (FCS) at 37°C. Cytokine concentrations in the supernatant were determined by ELISA using anti-IL-4 (clone 11B11), anti-IL-5 (BD), anti-IL-13 (R&D Systems), or anti-IFN-γ (clone AN18KL6) capture antibodies and biotinylated secondary antibodies.
Lung histology.
Lung tissue was fixed in 4% formaldehyde-PBS, embedded in paraffin, and cut into 5- to 7-μm-thick sections. Sections were stained with periodic acid-Schiff reagent (PAS) or hematoxylin and eosin (H&E). Pictures were taken at magnifications of ×100 for PAS staining and ×800 for H&E staining.
Statistical analysis.
Values are given as means ± standard deviations (SD), and significant differences were determined using analysis of variance (ANOVA) and a Bonferroni posttest (GraphPad Prism software). P values of <0.05 were considered significant.
RESULTS
Antibody responses after infection of mice with Anisakis larvae and nasal exposure to an Anisakis extract.
To investigate the influence that different sensitization routes might have on the development of allergic airway disease in response to inhaled Anisakis allergens, wild-type and IL-4Rα-deficient BALB/c mice were either infected with 2 live Anisakis larvae on day 0 and day 21 postinfection or sensitized by intranasal administration of an Anisakis extract with aluminum hydroxide on days 0, 7, and 14. After injection, Anisakis L3 survive for approximately 14 to 21 days in the peritoneal cavity without penetrating the intestines or causing any obvious intestinal inflammation (reference 23 and our own observations). In both sensitization models, mice were subsequently challenged intranasally with an Anisakis protein extract to induce allergic airway responses to inhaled allergens (Fig. 1A).
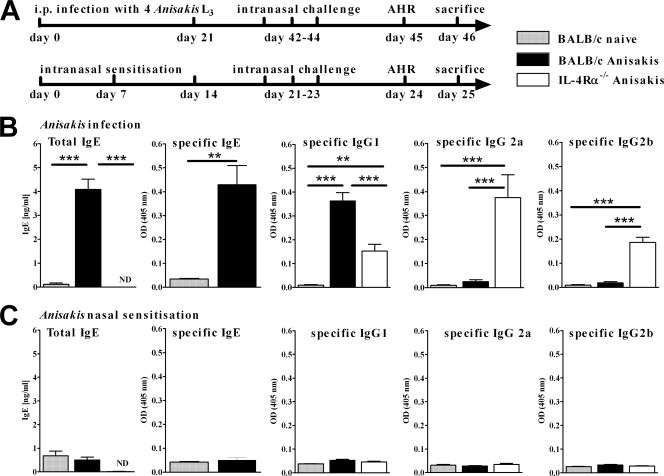
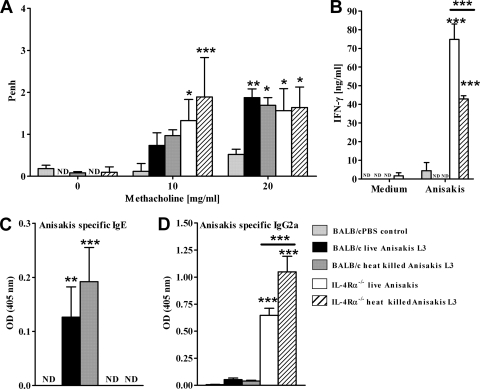
FIG. 1.
(A) Mice were either infected intraperitoneally with 4 live Anisakis pegreffii larvae or sensitized intranasally with an Anisakis extract. This was followed by 3 intranasal challenges with an Anisakis extract. (B and C) Infection of mice with Anisakis larvae (B) but not nasal sensitization with an Anisakis extract (C) induces a specific antibody response. Antibody concentrations in serum were determined by ELISA. Data represent pooled results of two independent experiments ± SD (6 to 8 animals per group). ND, not detected. **, P < 0.01; ***, P < 0.001.
After infection with Anisakis larvae, antibody levels in wild-type and IL-4Rα−/− mice were determined on day 46 postinfection and were compared to those of noninfected mice. Levels of total IgE and Anisakis-specific IgG1 and IgE in serum were significantly higher in infected than in unsensitized wild-type mice (Fig. 1B). IL-4Rα−/− mice produced specific IgG2a and IgG2b and, to a lesser extent, IgG1. IgE antibodies were not detected in IL-4Rα−/− mice (Fig. 1B). In contrast, repeated nasal sensitization did not induce a significant specific IgG or IgE antibody response in either wild-type or IL-4Rα−/− mice, and concentrations of total IgE were not affected by intranasal Anisakis sensitization (Fig. 1C). The Anisakis extract was immunogenic, and the failure to induce an antibody response was not due to dose-dependent effects, since sensitization by intraperitoneal injection with an equal antigen dose and an identical time course induced robust antigen-specific IgG1 and IgE responses in wild-type mice and IgG2a and IgG2b responses in IL-4Ra−/− mice in separate experiments (data not shown). Together, these results suggest that sensitization with Anisakis L3, but not intranasal sensitization with an Anisakis extract, induces a predominant type 2 antibody response, which is dependent on IL-4Rα responsiveness.
Allergen-specific cytokine responses after intranasal allergen challenge.
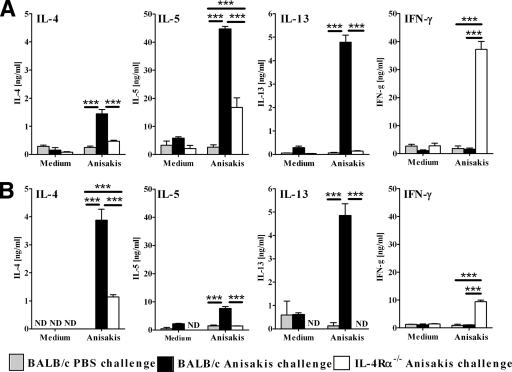
Lymphocytes from lung-draining lymph nodes were restimulated in vitro with an Anisakis extract, and cytokine production was analyzed by ELISA. Wild-type mice sensitized intraperitoneally with live Anisakis larvae (Fig. 2A) or intranasally with an Anisakis extract (Fig. 2B) showed predominantly Th2 type antigen-specific cytokine responses, as demonstrated by higher levels of IL-4, IL-5, and IL-13 than those in PBS-challenged mice but similar levels of IFN-γ (Fig. 2A). In contrast, IL-4Rα−/− mice responded with a predominantly Th1 type cytokine response, characterized by greatly increased IFN-γ production and low IL-4, IL-5, and IL-13 production (Fig. 2A). Of note, sensitization with larvae induced strikingly higher production of IL-5 in wild-type mice, and of IL-5 and IFN-γ in IL-4Rα−/− mice, than in the corresponding mice sensitized intranasally with an Anisakis extract. Together, these results demonstrate that both routes of sensitization induce IL-4Rα-dependent Th2-type cytokine responses in wild-type mice.
FIG. 2.
IL-4Rα-dependent Th2-type cytokine production in lung-draining lymph nodes of mice challenged with Anisakis antigens after sensitization with Anisakis larvae (A) or nasal administration of an Anisakis extract (B). Cytokine secretion by ex vivo-stimulated lymphocytes (4 mice/group) is shown. Data represent one of two independent experiments ± SD. ND, not detected. **, P < 0.01; ***, P < 0.001.
Recruitment of inflammatory cells to the lungs and pulmonary pathology after airway challenge with an Anisakis extract.
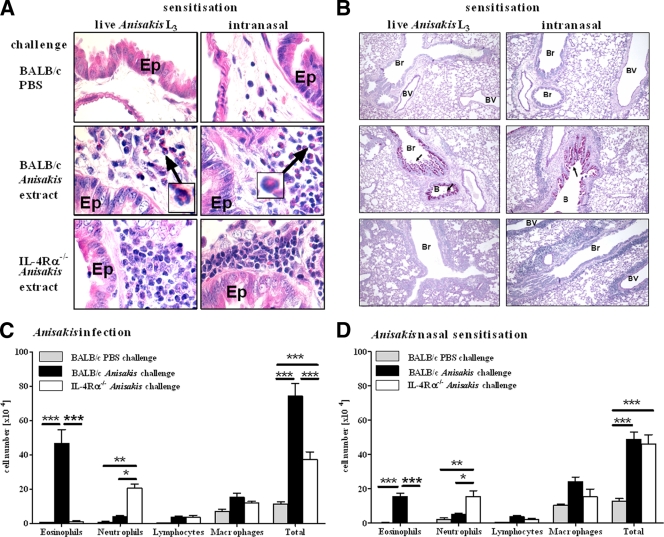
During allergic airway reactions, inflammatory cells infiltrate the lung tissue around blood vessels and airways. Following sensitization of mice by intraperitoneal infection with live larvae or intranasal administration of an Anisakis extract, we challenged mice intranasally with the Anisakis extract for three consecutive days. This resulted in perivascular and peribronchial inflammation in both wild-type and IL-4Rα−/− mice, which was not observed in PBS-challenged controls (Fig. 3A). Many eosinophils, characteristic of allergic asthma, were present in the inflammatory-cell infiltrates in wild-type mice (Fig. 3A, arrows) but not IL-4Rα−/− mice. Hyperplasia of mucus-producing goblet cells in the lung epithelium is also a feature of allergic asthma and similarly occurred only in the lungs of challenged wild-type mice, irrespective of the route of sensitization (Fig. 3B), indicating that goblet cell hyperplasia was IL-4Rα dependent. In allergic asthma, bronchi and alveoli are infiltrated by inflammatory cells such as eosinophils, a hallmark of allergic inflammation, and neutrophils, associated with asthma exacerbations. Differential cell counts revealed that the predominant cell type in the bronchoalveolar lavage (BAL) fluid from wild-type mice was eosinophils, whereas neutrophils were the most common cell type in IL-4Rα−/− mice (Fig. 3C). This is consistent with the higher production of IL-5, a known eosinophilic growth factor, in wild-type mice than in IL4Rα−/− mice (Fig. 2). The total number of cells in the BAL fluid was significantly higher in wild-type than in IL-4Rα−/− mice (7.3 × 105 versus 3.7 × 105) after sensitization with live Anisakis larvae (Fig. 3C) but not after nasal sensitization with an Anisakis extract (4.8 × 105 versus 4.5 × 105) (Fig. 3D). In summary, both routes of sensitization induced infiltration of inflammatory cells into the lungs, and eosinophils were the predominant cell type in wild-type mice.
FIG. 3.
(A and B) Airway inflammation after intranasal challenge with an Anisakis extract. Lung sections were stained with H&E (A) to visualize eosinophils (insets) or with PAS (B) to visualize mucus production (arrows). Pictures were taken at magnifications of ×800 for H&E staining and ×100 for PAS staining. Ep, epithelial cells; Br, bronchiole; BV, blood vessel. (C and D) Cellular infiltrates in the BAL fluid of mice sensitized by infection with Anisakis larvae (C) or nasal administration of an Anisakis extract (D). Pooled data from two independent experiments ± SD (6 to 8 mice/group) are shown. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Airway hyperresponsiveness after intranasal allergen challenge.
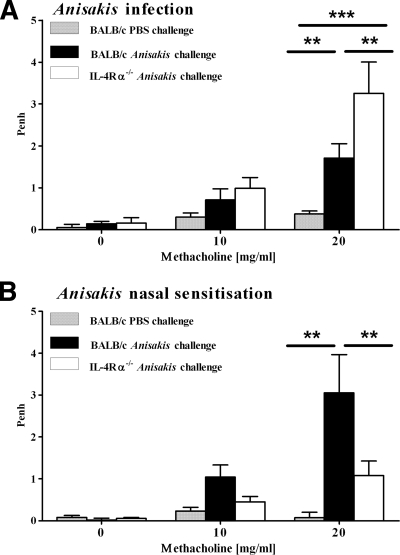
Airway hyperresponsiveness (AHR) to nonspecific bronchoconstrictors is characteristic of allergic asthma. Intranasal challenge with an Anisakis extract induced airway hyperresponsiveness in wild-type mice irrespective of the route of sensitization, as reflected by significantly increased Penh values after exposure to 20 mg/ml aerosolized β-methacholine (Fig. 4A and B). Interestingly, AHR was present in IL-4Rα−/− mice sensitized with Anisakis larvae (Fig. 4A) but not in mice sensitized intranasally with an Anisakis extract (Fig. 4B). To test whether live larvae were necessary for the induction of IL-4Rα-independent AHR, we sensitized mice with heat-killed Anisakis L3. Subsequent intranasal challenge induced AHR in both wild-type and IL-4Rα−/− mice, as seen when live larvae were used (Fig. 5A). Moreover, sensitization with live or heat-killed Anisakis L3 induced a strong antigen-specific IFN-γ response in lung-draining lymph nodes in IL-4Rα−/− mice compared to control mice (Fig. 5B). Heat-treated Anisakis L3 induced a specific IgE response in wild-type mice, indicating allergic sensitization (Fig. 5C), whereas IL-4Rα−/− mice responded with a Th1-type antibody response reflected by specific IgG2a (Fig. 5D). Together, these data demonstrate that intranasal challenge with an Anisakis extract after intraperitoneal sensitization with larvae or sensitization with an Anisakis extract is able to induce airway hyperresponsiveness. Furthermore, AHR can occur independently of IL-4Rα responsiveness after intraperitoneal sensitization with larvae but was strictly dependent on IL-4Rα responsiveness after intranasal sensitization.
FIG. 4.
Airway hyperresponsiveness after intranasal challenge with Anisakis antigens is IL-4Rα independent after Anisakis infection (A) but not after nasal sensitization (B). The airway response to increasing concentrations of β-methacholine was measured by whole-body plethysmography 1 day after the final allergen challenge. Pooled data from two independent experiments ± SD (6 to 8 mice/group) are shown. **, P < 0.01; ***, P < 0.001.
FIG. 5.
(A) Airway hyperresponsiveness is induced in BALB/c and IL-4Rα−/− mice after injection of live or heat-killed Anisakis L3 on days 0 and 21 and intranasal challenge with Anisakis antigens. (B) Injection of live or heat-killed Anisakis L3 induced a strong antigen-specific IFN-γ response in lung-draining lymph nodes in IL-4Rα−/− mice compared to control mice. (C and D) Injection of live or heat-killed Anisakis L3 induced specific IgE responses in BALB/c mice (C) and IgG2a responses in IL-4Rα−/− mice (D). Data are means ± SD for 6 mice per group. Asterisks indicate significant differences (*, P < 0.05; **, P < 0.01; ***, P < 0.001) from PBS control groups. No significant differences between injection of live or heat-killed parasites were found.
Airway hyperresponsiveness in IL-4Rα−/− mice is dependent on IFN-γ.
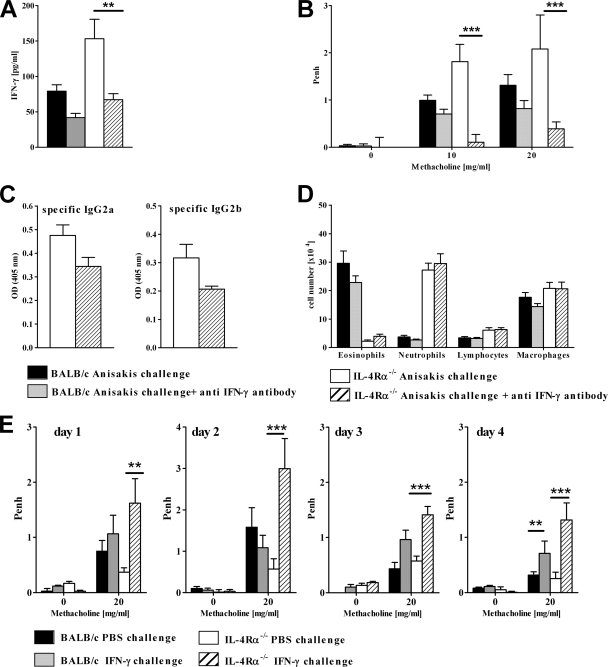
Several studies have demonstrated IL-4Rα-independent AHR in murine models of ovalbumin-induced allergic airway hyperresponsiveness, but the factors involved remain elusive (31, 38, 46). IFN-γ-associated AHR has been described previously in a mouse model of chronic asthma, where administration of anti-IFN-γ antibodies reduced AHR (26). Since AHR was associated with strikingly increased IFN-γ levels in IL-4Rα−/− mice sensitized intraperitoneally with live or heat-killed larvae, we hypothesized that IFN-γ might play a role in IL-4Rα-independent AHR. In order to test this, we sensitized mice with live larvae and injected neutralizing anti-IFN-γ antibodies prior to allergen challenge, resulting in significantly reduced levels of IFN-γ in the lungs (Fig. 6A). Of importance, in vivo IFN-γ depletion had no significant effect on AHR in wild-type mice but abrogated AHR in IL-4Rα−/− mice (Fig. 6B), providing evidence that IFN-γ was responsible for IL-4Rα-independent AHR. Impairment of AHR following IFN-γ depletion was not due to altered Th1-type antibody responses; levels of Anisakis-specific IgG2a and IgG2b were not significantly different from those for untreated controls (Fig. 6C). Depletion of IFN-γ also had no significant effect on airway inflammation or the cellular composition of the BAL fluid (Fig. 6D). Together these data indicated that IFN-γ might directly induce AHR similarly to IL-13 (17). To investigate this possibility, we challenged naïve wild-type and IL-4Rα−/− mice intranasally with recombinant IFN-γ or saline on 4 consecutive days, measuring AHR after each challenge (Fig. 6E). AHR increased significantly on days 1 to 4 in IL-4Rα−/− mice administered IFN-γ, compared to that for PBS controls. In wild-type mice, a significant effect on AHR was evident at day 4 only. These results strongly suggest that IFN-γ is able to induce AHR in mice, particularly in the absence of IL-4Rα responsiveness.
FIG. 6.
IL-4Rα-independent airway hyperresponsiveness is dependent on IFN-γ. (A) Concentrations of IFN-γ in lung tissue homogenates of mice treated with anti-IFN-γ antibodies were determined by ELISA. (B) Airway response to β-methacholine after Anisakis infection and nasal challenge with an Anisakis extract in mice treated with anti-IFN-γ antibodies prior to nasal allergen challenge. Statistically significant differences between anti-IFN-γ antibody-treated and untreated groups are indicated by asterisks (***, P < 0.001). (C and D) Antibody response (C) and airway inflammation (D) were not affected by treatment with an antibody against IFN-γ. An IgG2 antibody isotype had no effect on AHR in an independent experiment. OD, optical density. For panels A through D, pooled data from two independent experiments ± SD (8 to 11 mice/group) are shown. (E) Naïve BALB/c and IL-4Rα−/− mice were challenged intranasally on days 1 to 4 with 1 μg recombinant IFN-γ, and AHR was measured 6 h after each challenge. Data represent results of one experiment ± SD (3 to 5 mice per group). Asterisks indicate significant differences from PBS control groups (**, P < 0.01; ***, P < 0.001).
DISCUSSION
Accidental infection of humans with Anisakis L3 after ingestion of fish can cause disease ranging from acute gastrointestinal reactions to anaphylactic shock (6). In this study, we investigated the influence of different routes of sensitization to Anisakis pegreffii on the outcome of experimental allergic airway inflammation. Sensitization by infection with live or heat-killed Anisakis larvae was compared with nasal sensitization of mice with an Anisakis extract. Both routes of sensitization primed mice to exhibit allergic airway inflammation after subsequent intranasal challenge with an Anisakis extract. This finding is important, because it strongly suggests that repeated inhalation of aerosolized Anisakis proteins is able to cause respiratory reactions, as suggested by previous studies of humans (1, 3, 37). This is particularly relevant for food- and work-related allergies in exposed individuals. Both live and heat-killed Anisakis larvae induced specific IgE in wild-type mice. This outcome suggests the presence of heat-stable somatic allergens and confirms evidence that cooking of contaminated fish may not be sufficient to prevent allergic reactions to Anisakis (10, 35). Furthermore, our additional finding that intranasal sensitization/challenge with an Anisakis extract induced allergic airway inflammation and AHR in the absence of a detectable antibody response may have consequences for the diagnosis of Anisakis allergies, which currently relies on the detection of specific IgE antibodies (29).
As expected, AHR was dependent on the Th2-type cytokines IL-4 and IL-13 in wild-type mice. However, following i.p. sensitization with live or heat-killed Anisakis L3, AHR was dependent on IFN-γ in the absence of IL-4Rα responsiveness. Indeed, recombinant IFN-γ was sufficient to induce AHR in the absence of allergic sensitization of IL-4Rα−/− mice. These results support a very recent allergic airway inflammation model, which demonstrated that cooperative signals of IFN-γ and Toll-like receptor 4 (TLR4)/MyD88 are able to induce profound AHR (47).
Intranasal challenge with an Anisakis extract after nasal or intraperitoneal sensitization resulted in profound allergic airway inflammation in wild-type mice, characterized by AHR, eosinophilic airway inflammation, Th2-type cytokine responses, and goblet cell hyperplasia. In contrast, IL-4Rα−/− mice developed mixed Th1/Th2 cytokine responses and neutrophilic airway inflammation but no goblet cell hyperplasia. Importantly, AHR was dependent on IL-4Rα responsiveness only after nasal sensitization, not after injection with live or heat-killed Anisakis larvae. This was somewhat unexpected, since IL-4Rα expression is generally considered critical for the development of AHR (17). Previous studies have implicated IL-5 and IL-13 in IL-4Rα-independent AHR in murine models of ovalbumin-induced allergic AHR, but possible mechanisms have remained elusive (31, 38, 46). Our data showed that IL-4Rα-independent AHR after intraperitoneal sensitization with larvae was associated with elevated IFN-γ production. Consequently, we performed in vivo neutralization studies and found that IFN-γ was critical for the development of IL-4Rα-independent AHR. This result supports the findings of a recent study that used a mouse model of ovalbumin-induced allergic airway disease to demonstrate that glucocorticoid-resistant AHR was dependent on cooperative signals from IFN-γ and TLR4/MyD88 and that it could be abolished by treatment with anti-IFN-γ antibodies without affecting the numbers of neutrophils in the BAL fluid (47). We did not investigate the Toll receptor pathway in our model, but in vivo neutralization of IFN-γ abrogated AHR without affecting neutrophil numbers in the BAL fluid. Furthermore, IFN-γ-induced AHR was not affected by neutrophil depletion (data not shown).
In order to investigate whether IFN-γ could directly induce AHR, we challenged naïve wild-type and IL-4Rα−/− mice intranasally with recombinant IFN-γ for 4 consecutive days. This treatment induced AHR from day 1 onward in IL-4Rα−/− mice and from day 4 in wild-type mice, demonstrating that recombinant IFN-γ was sufficient to induce AHR, particularly in IL-4Rα−/− mice. Since the endotoxin level of the IFN-γ administered was less than 0.1 ng/μg, it is unlikely that TLR induction took place due to endotoxin contamination. We found low concentrations of endotoxin in the Anisakis extract used for intranasal sensitization (2.5 to 5 EU/mg), which are below the LPS concentrations used previously to determine the influence of endotoxin on AHR in mice (15, 44, 47). Since Anisakis L3 remain in the peritoneal cavity after injection into mice for approximately 14 to 21 days without penetrating the intestine (reference 23 and our own observations), it is unlikely that leakage of intestinal contents into the peritoneum was a source of endotoxin. Furthermore, IL-4Rα-independent AHR also occurred in mice injected with heat-killed larvae. This may indicate that IFN-γ-dependent AHR is also possible via TLR4-independent mechanisms. The involvement of IFN-γ in the pathology of asthma may extend to humans, since a positive correlation between serum IFN-γ concentrations and the severity of asthma pathogenesis has been described for asthmatic patients, and higher numbers of IFN-γ-producing T cells were found in the BAL fluid and blood of asthmatics than in those of healthy control subjects (9, 30, 45).
In wild-type mice, AHR was accompanied by goblet cell hyperplasia and eosinophilic lung inflammation. This pathology is in agreement with the strong Th2 cytokine response induced by Anisakis infection (37), which could drive goblet cell hyperplasia through increased IL-4Rα signaling in airway epithelial cells (27) and eosinophilia through IL-5 (25). Eosinophilia may also be promoted by the chemotactic properties of the Anisakis extract (16). Intranasal sensitization resulted in lower numbers of eosinophils in the BAL fluid and lower levels of IL-5 production than those obtained with live infection. Together, these results demonstrate that Anisakis infection and intraperitoneal sensitization are more potent inducers of Th2-type immune responses than nasal sensitization with an Anisakis extract. In IL-4Rα−/− mice, neutrophils but not eosinophils were recruited to the airways after allergen challenge. Augmented neutrophil recruitment and impaired eosinophil recruitment in IL-4Rα−/− mice also occurred in our previous studies and were shown to be IL-17 dependent (42). The production of IL-17 in response to allergens is increased in the absence of IL-4/IL-13 responsiveness (19, 42), and conversely, IL-17 downregulates the production of Th2 cytokines (42). This may play an important role during sensitization to Anisakis by promoting a strong IFN-γ response and the development of IFN-γ-dependent AHR.
An important finding of this study was that, unlike mice sensitized intraperitoneally with Anisakis larvae, nasally sensitized mice failed to launch Anisakis-specific IgE and IgG antibody responses, despite a normal Th2-type allergic inflammatory disease phenotype. Allergic airway symptoms in the absence of IgE have been described in models using aerosolized ovalbumin or Aspergillus allergens in either IL-4-, B-cell-, or IgE-deficient mice (12, 20, 33). Interestingly, failure to induce Anisakis-specific IgE and IgG antibody responses was dependent on the intranasal sensitization route, since mice sensitized intraperitoneally with an Anisakis extract did produce specific antibodies (our unpublished data). Non-IgE-mediated asthma has been described in human studies, and respiratory symptoms have been ascribed to the presence of IL-4, IL-5, and IL-13 in the lung (11, 21, 22). The outcome of our studies could be important for improving the diagnosis of Anisakis-related allergic reactions and explaining airway hyperreactivity in the absence of specific IgE antibodies.
Acknowledgments
We thank Hiram Arendse, Lizette Fick, Marilyn Tyler, Wendy Green, and Reagon Petersen for assistance.
This work is based on research supported by the Medical Research Council (MRC), the National Research Foundation (NRF), and the South African Research Chair initiative of the Department of Science and Technology (DST).
We declare no financial or commercial conflict of interest.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 6 July 2010.
REFERENCES
- 1.Añibarro, B., and F. J. Seoane. 1998. Occupational conjunctivitis caused by sensitization to Anisakis simplex. J. Allergy Clin. Immunol. 102:331-332. [DOI] [PubMed] [Google Scholar]
- 2.Añibarro, B., F. J. Seoane, and M. V. Mugica. 2007. Involvement of hidden allergens in food allergic reactions. J. Investig. Allergol. Clin. Immunol. 17:168-172. [PubMed] [Google Scholar]
- 3.Armentia, A., M. Lombardero, A. Callejo, J. M. Martin Santos, F. J. Gil, J. Vega, M. L. Arranz, and C. Martinez. 1998. Occupational asthma by Anisakis simplex. J. Allergy Clin. Immunol. 102:831-834. [DOI] [PubMed] [Google Scholar]
- 4.Audicana, M. T., I. J. Ansotegui, L. F. de Corres, and M. W. Kennedy. 2002. Anisakis simplex: dangerous—dead and alive? Trends Parasitol. 18:20-25. [DOI] [PubMed] [Google Scholar]
- 5.Audicana, M. T., L. Fernandez de Corres, D. Munoz, E. Fernandez, J. A. Navarro, and M. D. del Pozo. 1995. Recurrent anaphylaxis caused by Anisakis simplex parasitizing fish. J. Allergy Clin. Immunol. 96:558-560. [DOI] [PubMed] [Google Scholar]
- 6.Audicana, M. T., and M. W. Kennedy. 2008. Anisakis simplex: from obscure infectious worm to inducer of immune hypersensitivity. Clin. Microbiol. Rev. 21:360-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbuzza, O., F. Guarneri, G. Galtieri, S. Gangemi, and M. Vaccaro. 2009. Protein contact dermatitis and allergic asthma caused by Anisakis simplex. Contact Dermatitis 60:239-240. [DOI] [PubMed] [Google Scholar]
- 8.Brombacher, F. 2000. The role of interleukin-13 in infectious diseases and allergy. Bioessays 22:646-656. [DOI] [PubMed] [Google Scholar]
- 9.Brown, V., T. J. Warke, M. D. Shields, and M. Ennis. 2003. T cell cytokine profiles in childhood asthma. Thorax 58:311-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caballero, M. L., and I. Moneo. 2004. Several allergens from Anisakis simplex are highly resistant to heat and pepsin treatments. Parasitol. Res. 93:248-251. [DOI] [PubMed] [Google Scholar]
- 11.Corrigan, C. J. 2004. Mechanisms of intrinsic asthma. Curr. Opin. Allergy Clin. Immunol. 4:53-56. [DOI] [PubMed] [Google Scholar]
- 12.Corry, D. B., G. Grunig, H. Hadeiba, V. P. Kurup, M. L. Warnock, D. Sheppard, D. M. Rennick, and R. M. Locksley. 1998. Requirements for allergen-induced airway hyperreactivity in T and B cell-deficient mice. Mol. Med. 4:344-355. [PMC free article] [PubMed] [Google Scholar]
- 13.Daschner, A., A. Alonso-Gomez, R. Cabanas, J. M. Suarez-de-Parga, and M. C. Lopez-Serrano. 2000. Gastroallergic anisakiasis: borderline between food allergy and parasitic disease—clinical and allergologic evaluation of 20 patients with confirmed acute parasitism by Anisakis simplex. J. Allergy Clin. Immunol. 105:176-181. [DOI] [PubMed] [Google Scholar]
- 14.Domínguez-Ortega, J., A. Alonso-Llamazares, L. Rodriguez, M. Chamorro, T. Robledo, J. M. Bartolome, and C. Martinez-Cocera. 2001. Anaphylaxis due to hypersensitivity to Anisakis simplex. Int. Arch. Allergy Immunol. 125:86-88. [DOI] [PubMed] [Google Scholar]
- 15.Eisenbarth, S. C., D. A. Piggott, J. W. Huleatt, I. Visintin, C. A. Herrick, and K. Bottomly. 2002. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J. Exp. Med. 196:1645-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gómez, B., A. I. Tabar, T. Tunon, B. Larrinaga, M. J. Alvarez, B. E. Garcia, and J. M. Olaguibel. 1998. Eosinophilic gastroenteritis and Anisakis. Allergy 53:1148-1154. [DOI] [PubMed] [Google Scholar]
- 17.Grünig, G., M. Warnock, A. E. Wakil, R. Venkayya, F. Brombacher, D. M. Rennick, D. Sheppard, M. Mohrs, D. D. Donaldson, R. M. Locksley, and D. B. Corry. 1998. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 282:2261-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamelmann, E., J. Schwarze, K. Takeda, A. Oshiba, G. L. Larsen, C. G. Irvin, and E. W. Gelfand. 1997. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am. J. Respir. Crit. Care Med. 156:766-775. [DOI] [PubMed] [Google Scholar]
- 19.He, R., H. Y. Kim, J. Yoon, M. K. Oyoshi, A. MacGinnitie, S. Goya, E.-J. Freyschmidt, P. Bryce, A. N. J. McKenzie, D. T. Umetsu, H. C. Oettgen, and R. S. Geha. 2009. Exaggerated IL-17 response to epicutaneous sensitization mediates airway inflammation in the absence of IL-4 and IL-13. J. Allergy Clin. Immunol. 124:761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hogan, S. P., A. Mould, H. Kikutani, A. J. Ramsay, and P. S. Foster. 1997. Aeroallergen-induced eosinophilic inflammation, lung damage, and airways hyperreactivity in mice can occur independently of IL-4 and allergen-specific immunoglobulins. J. Clin. Invest. 99:1329-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Humbert, M., S. Durham, S. Ying, P. Kimmitt, J. Barkans, B. Assoufi, R. Pfister, G. Menz, D. Robinson, A. Kay, and C. Corrigan. 1996. IL-4 and IL-5 mRNA and protein in bronchial biopsies from patients with atopic and nonatopic asthma: evidence against “intrinsic” asthma being a distinct immunopathologic entity. Am. J. Respir. Crit. Care Med. 154:1497-1504. [DOI] [PubMed] [Google Scholar]
- 22.Humbert, M., S. R. Durham, P. Kimmitt, N. Powell, B. Assoufi, R. Pfister, G. Menz, A. B. Kay, and C. J. Corrigan. 1997. Elevated expression of messenger ribonucleic acid encoding IL-13 in the bronchial mucosa of atopic and nonatopic subjects with asthma. J. Allergy Clin. Immunol. 99:657-665. [DOI] [PubMed] [Google Scholar]
- 23.Jones, R. E., T. L. Deardorff, and S. G. Kayes. 1990. Anisakis simplex: histopathological changes in experimentally infected CBA/J mice. Exp. Parasitol. 70:305-313. [DOI] [PubMed] [Google Scholar]
- 24.Kimura, S. 2001. Positive ratio of allergen specific IgE antibodies in serum, from a large scale study. Rinsho Byori 49:376-380. [PubMed] [Google Scholar]
- 25.Kopf, M., F. Brombacher, P. D. Hodgkin, A. J. Ramsay, E. A. Milbourne, W. J. Dai, K. S. Ovington, C. A. Behm, G. Kohler, I. G. Young, and K. I. Matthaei. 1996. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity 4:15-24. [DOI] [PubMed] [Google Scholar]
- 26.Kumar, R. K., C. Herbert, D. C. Webb, L. Li, and P. S. Foster. 2004. Effects of anticytokine therapy in a mouse model of chronic asthma. Am. J. Respir. Crit. Care Med. 170:1043-1048. [DOI] [PubMed] [Google Scholar]
- 27.Kuperman, D. A., X. Huang, L. Nguyenvu, C. Holscher, F. Brombacher, and D. J. Erle. 2005. IL-4 receptor signaling in Clara cells is required for allergen-induced mucus production. J. Immunol. 175:3746-3752. [DOI] [PubMed] [Google Scholar]
- 28.Lopata, A. L., and S. B. Lehrer. 2009. New insights into seafood allergy. Curr. Opin. Allergy Clin. Immunol. 9:270-277. [DOI] [PubMed] [Google Scholar]
- 29.Lorenzo, S., R. Iglesias, J. Leiro, F. M. Ubeira, I. Ansotegui, M. Garcia, and L. Fernandez de Corres. 2000. Usefulness of currently available methods for the diagnosis of Anisakis simplex allergy. Allergy 55:627-633. [DOI] [PubMed] [Google Scholar]
- 30.Magnan, A. O., L. G. Mely, C. A. Camilla, M. M. Badier, F. A. Montero-Julian, C. M. Guillot, B. B. Casano, S. J. Prato, V. Fert, P. Bongrand, and D. Vervloet. 2000. Assessment of the Th1/Th2 paradigm in whole blood in atopy and asthma. Increased IFN-γ-producing CD8+ T cells in asthma. Am. J. Respir. Crit. Care Med. 161:1790-1796. [DOI] [PubMed] [Google Scholar]
- 31.Mattes, J., M. Yang, A. Siqueira, K. Clark, J. MacKenzie, A. N. J. McKenzie, D. C. Webb, K. I. Matthaei, and P. S. Foster. 2001. IL-13 induces airways hyperreactivity independently of the IL-4Rα chain in the allergic lung. J. Immunol. 167:1683-1692. [DOI] [PubMed] [Google Scholar]
- 32.Mattiucci, S., and G. Nascetti. 2007. Genetic diversity and infection levels of anisakid nematodes parasitic in fish and marine mammals from Boreal and Austral hemispheres. Vet. Parasitol. 148:43-57. [DOI] [PubMed] [Google Scholar]
- 33.Mehlhop, P. D., M. van de Rijn, A. B. Goldberg, J. P. Brewer, V. P. Kurup, T. R. Martin, and H. C. Oettgen. 1997. Allergen-induced bronchial hyperreactivity and eosinophilic inflammation occur in the absence of IgE in a mouse model of asthma. Proc. Natl. Acad. Sci. U. S. A. 94:1344-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohrs, M., B. Ledermann, G. Kohler, A. Dorfmuller, A. Gessner, and F. Brombacher. 1999. Differences between IL-4- and IL-4 receptor alpha-deficient mice in chronic leishmaniasis reveal a protective role for IL-13 receptor signaling. J. Immunol. 162:7302-7308. [PubMed] [Google Scholar]
- 35.Moneo, I., M. L. Caballero, M. Gonzalez-Munoz, A. I. Rodriguez-Mahillo, R. Rodriguez-Perez, and A. Silva. 2005. Isolation of a heat-resistant allergen from the fish parasite Anisakis simplex. Parasitol. Res. 96:285-289. [DOI] [PubMed] [Google Scholar]
- 36.Nieuwenhuizen, N., D. R. Herbert, F. Brombacher, and A. L. Lopata. 2009. Differential requirements for interleukin (IL)-4 and IL-13 in protein contact dermatitis induced by Anisakis. Allergy 64:1309-1318. [DOI] [PubMed] [Google Scholar]
- 37.Nieuwenhuizen, N., A. L. Lopata, M. F. Jeebhay, D. R. Herbert, T. G. Robins, and F. Brombacher. 2006. Exposure to the fish parasite Anisakis causes allergic airway hyperreactivity and dermatitis. J. Allergy Clin. Immunol. 117:1098-1105. [DOI] [PubMed] [Google Scholar]
- 38.Proust, B., M. A. Nahori, C. Ruffie, J. Lefort, and B. B. Vargaftig. 2003. Persistence of bronchopulmonary hyper-reactivity and eosinophilic lung inflammation after anti-IL-5 or -IL-13 treatment in allergic BALB/c and IL-4Rα knockout mice. Clin. Exp. Allergy 33:119-131. [DOI] [PubMed] [Google Scholar]
- 39.Pulido-Marrero, Z., E. Gonzalez-Mancebo, T. Alfaya-Arias, B. de la Hoz-Caballer, and M. Cuevas-Agustin. 2000. Unusual sensitization to Anisakis simplex. Allergy 55:586-587. [DOI] [PubMed] [Google Scholar]
- 40.Purello-D'Ambrosio, F., E. Pastorello, S. Gangemi, G. Lombardo, L. Ricciardi, O. Fogliani, and R. A. Merendino. 2000. Incidence of sensitivity to Anisakis simplex in a risk population of fishermen/fishmongers. Ann. Allergy Asthma Immunol. 84:439-444. [DOI] [PubMed] [Google Scholar]
- 41.Roberts, G., and G. Lack. 2003. Relevance of inhalational exposure to food allergens. Curr. Opin. Allergy Clin. Immunol. 3:211-215. [DOI] [PubMed] [Google Scholar]
- 42.Schnyder-Candrian, S., D. Togbe, I. Couillin, I. Mercier, F. Brombacher, V. Quesniaux, F. Fossiez, B. Ryffel, and B. Schnyder. 2006. Interleukin-17 is a negative regulator of established allergic asthma. J. Exp. Med. 203:2715-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reference deleted.
- 44.Tan, A. M., H. C. Chen, P. Pochard, S. C. Eisenbarth, C. A. Herrick, and H. K. Bottomly. 2010. TLR4 signaling in stromal cells is critical for the initiation of allergic Th2 responses to inhaled antigen. J. Immunol. 184:3535-3544. [DOI] [PubMed] [Google Scholar]
- 45.ten Hacken, N. H., Y. Oosterhoff, H. F. Kauffman, L. Guevarra, T. Satoh, D. J. Tollerud, and D. S. Postma. 1998. Elevated serum interferon-gamma in atopic asthma correlates with increased airways responsiveness and circadian peak expiratory flow variation. Eur. Respir. J. 11:312-316. [DOI] [PubMed] [Google Scholar]
- 46.Webb, D. C., S. Mahalingam, Y. Cai, K. I. Matthaei, D. D. Donaldson, and P. S. Foster. 2003. Antigen-specific production of interleukin (IL)-13 and IL-5 cooperate to mediate IL-4Rα-independent airway hyperreactivity. Eur. J. Immunol. 33:3377-3385. [DOI] [PubMed] [Google Scholar]
- 47.Yang, M., R. K. Kumar, and P. S. Foster. 2009. Pathogenesis of steroid-resistant airway hyperresponsiveness: interaction between IFN-γ and TLR4/MyD88 pathways. J. Immunol. 182:5107-5115. [DOI] [PubMed] [Google Scholar]