Abstract
Cryptococcus neoformans, an encapsulated, pathogenic yeast, is endowed with a variety of virulence factors, including a polysaccharide capsule. During mammalian infection, the outcome of the interaction between C. neoformans and macrophages is central to determining the fate of the host. Previous studies have shown similarities between the interaction of C. neoformans with macrophages and with amoebae, resulting in the proposal that fungal virulence for mammals originated from selection by amoeboid predators. In this study, we investigated the interaction of C. neoformans with the soil amoeba Acanthamoeba castellanii. Comparison of phagocytic efficiency of the wild type, nonencapsulated mutants, and complemented strains showed that the capsule was antiphagocytic for amoebae. Capsular enlargement was associated with a significant reduction in phagocytosis, suggesting that this phenomenon protects against ingestion by phagocytic predators. C. neoformans var. neoformans cells were observed to exit amoebae several hours after ingestion, in a process similar to the recently described nonlytic exocytosis from macrophages. Cryptococcal exocytosis from amoebae was dependent on the strain and on actin and required fungal viability. Additionally, the presence of a capsule was inversely correlated with the likelihood of extrusion in certain strains. In summary, nonlytic exocytosis from amoebae provide another parallel to observations in fungus-macrophage interactions. These results provide additional support for the notion that some mechanisms of virulence observed during mammalian infection originated, and were selected for, by environmental interactions.
The encapsulated yeast Cryptococcus neoformans is an environmental organism that is capable of causing human disease. This fungus is a facultative intracellular pathogen with a unique pathogenic strategy, despite no obvious need for replication in an animal host as part of its life cycle (10). C. neoformans is known to interact with protozoa, some of which have been shown to be effective predators for this fungus (6, 26), and amoebae appear to be important for the control of C. neoformans in the environment (28). Previously, we reported that the interaction of C. neoformans with Acanthamoeba castellanii directly paralleled the interaction with human macrophages (33). Similarities between C. neoformans interactions with amoebae and macrophages included intracellular replication in a phagosome and the release of polysaccharide-containing vesicles into the cytoplasm (33). Furthermore, passage of avirulent C. neoformans and Histoplasma capsulatum through slime mold and amoebae was shown to increase virulence in mice (31, 32). On the basis of these observations, it was proposed that the capacity for mammalian virulence emerged from interactions with phagocytic predators, such as amoebae and slime mold, in the environment (7, 17, 30). Consequently, single-cell protists have emerged as important systems for the study of C. neoformans virulence, and subsequent studies have investigated the interaction of this fungus with slime mold and paramecia (9, 31). Additional evidence for this concept comes from studies of insect fungal pathogens, which suggest that the capacity for insect pathogenicity may follow preadaptation from interactions with amoebae in the environment (4). Understanding the mechanisms by which virulence emerges in environmental microbes is important considering that global warming has been hypothesized to bring about new fungal diseases in the coming century (13).
Recent work in our laboratory and in that of Robin May simultaneously uncovered a novel strategy of avoiding macrophage killing whereby yeast cells were expulsed without lysis of the host cell (2, 19). The process is remarkable in that extrusion of the C. neoformans-filled phagosome is accompanied by the survival of both the host cells and the yeast cells. Phagosome extrusion or fungal exocytosis appears to be a C. neoformans-dictated event that is dependent on both the presence of the polysaccharide capsule and on the depolymerization of actin. A corollary of the hypothesis that C. neoformans virulence emerged from interactions with environmental predators is that phenomena observed with mammalian cells are likely to have a counterpart in free-living phagocytic cells. Consequently, the observation of an apparently unique event such as phagosomal extrusion from mammalian macrophages suggested a need to search for similar events in C. neoformans interactions with environmental phagocytic predators.
In this study, we investigated parallels between the intracellular pathogenic strategy of C. neoformans in both macrophages and A. castellanii, focusing on characterizing the impact of the capsule on protozoan phagocytosis and on ascertaining whether fungal cells could also exit amoebae, including the role of the capsule in that possible mechanism. Using time-lapse microscopy, we observed the exocytosis of C. neoformans from A. castellanii. While there are significant differences in the nonlytic exocytosis process when comparing amoebae and macrophages, the observation of this phenomenon in amoebae provides additional support for the idea that the virulence of C. neoformans was selected for, and is maintained, by interactions in the environment with other soil organisms.
(This research was conducted by Cara Chrisman in partial fulfillment of the requirements for a Ph.D. from the Sue Golding Graduate Division of Medical Science, Albert Einstein College of Medicine, Yeshiva University, Bronx, NY [awarded in 2010].)
MATERIALS AND METHODS
Yeast strains and culture conditions.
Cryptococcus neoformans strains 24067 (obtained from the American Type Culture Collection [ATCC], Rockville, MD), 11, 13, 14, 16, J4, J8, J10, J11, J20, and J40 (21) were grown from frozen stocks and maintained in Sabouraud dextrose broth. All strains were agitated at 150 to 180 rpm in 30°C incubators. The C. neoformans var. grubii strain, H99, was obtained from John Perfect (Durham, NC). The acapsular strain, CAP67, was obtained from the ATCC. The CAP59 mutant, parent, and complemented strains were obtained from K. J. Kwon-Chung (Bethesda, MD) and grown in yeast nitrogen base (YNB) for 3 days at 30°C. C. neoformans strain 24067 cells were killed by heating at 65°C for 1 h.
Amoebae.
Acanthamoeba castellanii strain 30324 was acquired from the ATCC. This strain was cultured at 28°C in peptone-yeast extract-glucose broth (PYG; ATCC medium 354) as described previously (22). A. castellanii cells were passaged every 7 to 10 days and used when they were confluent on the bottom of the flasks.
Induction of capsule.
C. neoformans capsules were induced through overnight incubation in the presence of 10% heat-inactivated fetal calf serum as described previously (35). India ink staining and light microscopy verified the increase in capsule size as previously described (35). Briefly, cells were stained with India ink and imaged using an Olympus AX70 microscope, and photos were taken with a QImaging Retiga 1300 digital camera using the QCapture suite V2.46 software (QImaging, Burnaby, British Columbia, Canada). Capsule diameters were measured with Adobe Photoshop CS for Windows (San Jose, CA) where the diameter was defined as the difference between the total cell and the cell body.
Phagocytosis assays.
Confluent A. castellanii cells were removed from culture flasks and counted using a hemocytometer. After A. castellanii cells were plated in 96-well plates at 1 × 106 cells/ml, they were allowed to settle for 1 h at 28°C. During this time, C. neoformans cells were also isolated from fungal cultures by centrifugation, washed in phosphate-buffered saline (PBS), counted with a hemocytometer, and resuspended at a density of 1 × 106 cells/ml. C. neoformans cells were then incubated with A. castellanii at 28°C for variable times. Following incubation, the microtiter plates were centrifuged so that A. castellanii would settle to the bottom of the plate, and the supernatant was removed by careful aspiration. A 100-μl volume of ice-cold methanol was added to each well, and the plates were incubated at 4°C for 30 min. The methanol was then removed, and PBS was added 2 times to wash the wells. To visualize A. castellanii cells, a 1:20 dilution of Giemsa stain was added for 30 min. After the plates were rinsed twice with PBS, they were viewed at a magnification of ×40 and counted to determine the phagocytic index. Internalization of C. neoformans was confirmed through experiments with fluorescent C. neoformans. C. neoformans cells were labeled with fluorescent 18B7 and used in phagocytosis assays. With an Olympus AX70 microscope, the internalization observed using bright-field microscopy could be confirmed with observation of internalized C. neoformans visualized with fluorescence. The phagocytic index is defined as the number of A. castellanii with internalized yeast per total number of A. castellanii cells and shown as a percentage.
Imaging of amoebae.
Amoebae were plated at a density of 1 × 105 or 1 × 106 cells/ml on polylysine coverslips at the bottom of wells on MatTek plates (Ashland, MA) in PBS and allowed to settle for 1 h at 28°C. C. neoformans cells of the various strains tested were then added. The cell suspension was then allowed to incubate for an additional hour before centrifuging the plates so that the A. castellanii cells would settle on the coverslip. The plates were then washed with PBS, and the amoebae were resuspended in feeding medium for subsequent imaging.
Reducing amoeba motility.
Protoslo quieting solution (Carolina Biological Supply, Burlington, NC) was used to suspend the organisms for imaging. Additionally, the organisms were suspended in increasing concentrations of soft agar diluted in PBS.
Analysis of exocytosis events.
An Axiovert 200 M inverted microscope was used in conjunction with an AxiocamMR camera controlled by the Axio Vision 4.4 software (Carl Zeiss Micro Imaging, New York). The microscope was encased in a Plexiglas box with conditions as previously described (2). Time-lapse movies were generated by collecting images of amoeba-cryptococcus interactions at magnifications of ×10 and ×20 every 30, 60, 120, or 240 s for 18 to 24 h and then assembling these images into animated movies using ImageJ software (1). For each condition, at least 150 amoebae were observed by eye and their fate was recorded, and each condition was done in triplicate on different days with independent cultures.
Actin depolymerization and polystyrene beads.
The effect of actin polymerization was tested using cytochalasin D. Cytochalasin D (2 μM) was added to the medium containing C. neoformans strain 24067 and A. castellanii after the cells were washed with PBS; this resulted in the same amoeba viability but a decrease in motility. To determine whether inert particles were expulsed from A. castellanii, 5.2-mm polystyrene beads (Spherotech, Lake Forest, IL) were added to a suspension of A. castellanii, and the interaction was recorded as described above.
Statistical analysis.
Statistics, including averages, standard deviations, and correlations, were performed using Microsoft Excel and Graphpad Prism 5 (La Jolla, CA).
RESULTS
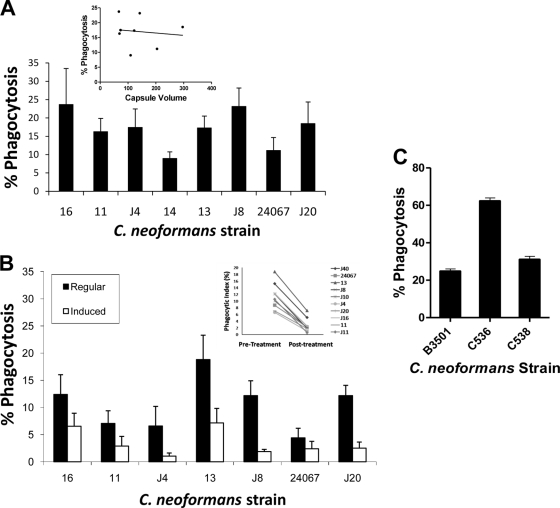
The efficacy of phagocytosis of C. neoformans by A. castellanii is inversely proportional to the diameter of the capsule.
The capsule of C. neoformans has long been known to inhibit the phagocytosis of fungal cells by mammalian macrophages (15). To investigate the role of the capsule in the interaction of C. neoformans with A. castellanii, we measured the phagocytic index for 8 C. neoformans strains (Fig. 1 A). We then correlated capsule size to phagocytic efficacy and found that there was significant strain-to-strain variation in phagocytic efficacy, and no correlation was observed between capsule size and phagocytic efficacy. Given that our prior study suggested a role for the capsule in resisting phagocytosis (33), we inferred that the lack of correlation between strains reflected variation between strains in the contribution of noncapsule variables to the fungus-protist interaction. Consequently, we changed our experimental approach to explore the role of the capsule within strains, and the phagocytic efficacy was determined for each strain before and after capsule growth induction. For all strains, capsule enlargement was associated with a reduction in phagocytic efficacy relative to that measured for the smaller capsule forms (Fig. 1B), and a pair-wise comparison of phagocytic efficacy before and after capsular enlargement showed a statistically significant reduction in phagocytic index in response to capsule growth (Fig. 1B). To further investigate the role of the capsule in amoeba phagocytosis, we compared A. castellanii phagocytic efficacy for an isogenic strain trio, the wild-type strain (B3501), an acapsular mutant resulting from a defined deletion of the CAP59 gene (C536), and the complemented strain (C538). For this strain set, encapsulation was associated with a major reduction in phagocytic efficacy, consistent with an antiphagocytic role for the capsule (Fig. 1C).
FIG. 1.
(A) Percent phagocytosis for 8 different C. neoformans strains from A. castellanii. After incubation at a 1:1 ratio, 96-well plates were washed, cells were stained, and a minimum of 200 cells/well were counted to determine the percent phagocytosis. This value is defined as the number of A. castellanii with internalized yeast per total number of A. castellanii cells counted shown as a percentage. (Inset) Linear regression of capsule volume (μm3) versus percent phagocytosis (R2 = 0.01518). (B) Phagocytosis assays after capsule induction. A. castellanii cells were allowed to phagocytose various strains of C. neoformans before and after the strains had been placed in media to increase the size of the polysaccharide capsule. All strains showed a decrease in the phagocytic index after the capsules had enlarged. (Inset) Comparison of percent phagocytosis before and after capsular enlargement (P < 0.05). The experiments shown in panels A and B were done at different times, and the differences in the values of phagocytosis measured for individual strains are within the experimental error of this assay. (C) Phagocytosis assay with acapsular mutants and capsule complemented strain. A. castellanii phagocytosis assays were performed with the wild type, CAP59 mutant (an acapsular mutant, C536), and complemented strains of C. neoformans (C538).
Yeast phagocytosis and exocytosis.
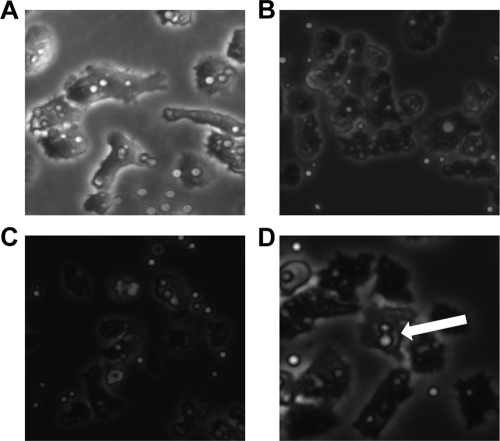
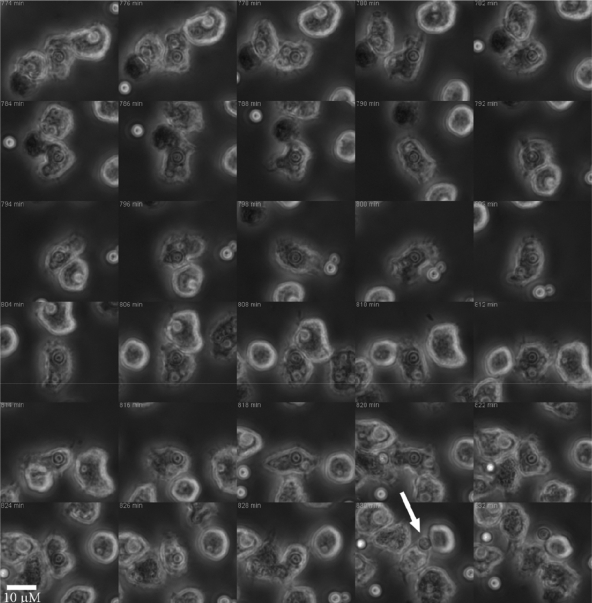
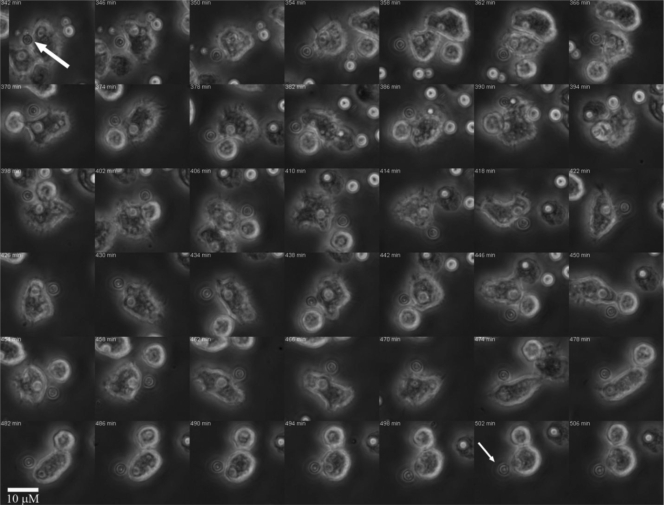
Incubation of A. castellanii with C. neoformans strains H99 and 24067 resulted in the ingestion of both cryptococcal strains (Fig. 2 B and C). However, after a certain time, yeast cells of C. neoformans strain 24067 were occasionally expulsed from amoebae (Fig. 3; see Video S1 in the supplemental material), whereas no expulsion events were observed with strain H99 within 24 h in the course of analyzing the results of three separate experiments that included at least 500 A. castellanii cells. The earliest expulsion with strain 24067 cells was observed 4 h after the experiment commenced. The exocytosis phenomenon always involved single yeast cells and occurred in up to 17% of amoebae with internalized C. neoformans when observed over a period of 18 to 24 h. However, this number may be an underestimate given the high mobility of amoebae. Amoebae moved rapidly in the field and often in the direction away from the light beam. This limited our ability to track all individual host cells during the course of time-lapse microscopy, as many migrated outside the field (approximately 45% of amoebae left the field in the course of each experiment). Attempts to slow the amoeba, including the use of soft agar and Protoslo, were unsuccessful. If the medium was too solid, the amoebae encysted. On the other hand, if the medium was diluted to allow mobility, the protists retained their high motility and exited the microscope viewing field. Extruded yeast cells consistently remained attached to the amoeba surface as the cell moved about the field (Fig. 4; see Video S2 in the supplemental material). Attachment was broken by contact with other amoebae. After exocytosis events, amoebae and their attached fungal cells continued to move about the field, and host cells with their attached fungal cells moved sufficiently to leave the field of vision (see Videos S1 and S2 in the supplemental material).
FIG. 2.
A. castellanii phagocytoses C. neoformans strains 24067, H99, and C536 and polystyrene beads. For imaging of extrusion, movies were recorded following incubation of amoeba and C. neoformans for 1 h and subsequent washing of extracellular C. neoformans. Recording then commenced, and the images represent still photos taken from movies at a magnification of ×10. (A) A. castellanii with ingested 5.2-mm polystyrene beads. (B) A. castellanii with ingested C. neoformans strain 24067. (C) A. castellanii with ingested C. neoformans strain H99. (D) A. castellanii with ingested C. neoformans strain C536. The large white arrow shows the internal replication.
FIG. 3.
Exocytosis of C. neoformans strain 24067 from A. castellanii. Montage taken from a video of interactions between the two organisms. Video was started 1 h after the commencement of phagocytosis, and the frames are labeled accordingly. The large white arrow indicates the expulsion event, which was followed by both organisms continuing to move about the field. Magnification, ×10.
FIG. 4.
Persistence of C. neoformans-A. castellanii attachment following exocytosis of yeast cells from the host phagocytic cell. The large white arrow indicates the expulsion of a C. neoformans cell from inside A. castellanii. Following the expulsion, the amoeba actively moves about the field while the yeast cell remains attached, indicated by the small white arrow. Magnification, ×10.
Actin polymerization.
To investigate the effect of actin polymerization, the organisms were observed after the addition of cytochalasin D. Cytochalasin D did not affect the viability of the amoebae; however, the motility was decreased. In the presence of cytochalasin D, no exocytosis events were observed compared to extrusion rates without the addition of cytochalasin D (0% versus 17.4%; P = 0.0082, Fisher exact test).
Interaction with polystyrene beads, acapsular mutants, and heat-killed C. neoformans.
Incubation of A. castellanii with polystyrene beads resulted in highly efficient phagocytosis of the beads (Fig. 2A), but no exocytosis events were observed in the course of analysis (data not shown). Additionally, no exocytosis events were observed after A. castellanii organisms were coincubated with heat-killed C. neoformans strain 24067 (0% versus 17.4%; P = 0.0064, Fisher exact test), implying that fungal viability was required to exit the host cell. When A. castellanii was incubated with the acapsular C. neoformans strains, CAP67 and CAP59, high levels of exocytosis resulted, averaging 17% of the cells with internalized C. neoformans. For strain CAP67, the extrusion rate was significantly higher than for the parental wild-type strain (B3501), which exhibited no extrusion events in the course of analysis. As in the case of the wild-type cells, the extruded acapsular cells were observed to replicate following exocytosis. The exocytosis of the acapsular strains may represent an early burst of exocytosis, as they were observed to extrude within 30 min of the start of the movies, as opposed to 4 h for other wild-type strains.
DISCUSSION
Nonvertebrate hosts have emerged as powerful tools for the study of microbial virulence (25). In recent years, amoebae, slime mold, worms, and insects have become useful models for evaluating the virulence characteristics of fungi (3, 23, 24, 31, 32). This study represented a further investigation of the interaction between two soil-dwelling organisms, C. neoformans and A. castellanii, that were previously shown to interact in the laboratory (6, 26, 33) and may do so in the environment. Amoebae are associated with biological control of C. neoformans in the environment (28). Prior studies had established that many aspects of the intracellular pathogenic strategy of C. neoformans for macrophages could be reproduced in amoebae (33). Here, we considered two phenomena associated with C. neoformans and macrophage interactions, namely, that the capsule inhibited phagocytosis and that C. neoformans was capable of nonlytic exocytosis, and investigated whether these also applied to C. neoformans-A. castellanii interactions.
The capsule is known to have an antiphagocytic function in C. neoformans-macrophage interactions such that phagocytosis is extremely inefficient unless antibody and/or complement opsonins are present (14). In contrast, amoebae are able to ingest encapsulated cryptococci without opsonins, and the role of the capsule is uncertain in this fungus-protozoan interaction (33). A prior study involving a limited number of strains had suggested a role for the capsule in protecting cryptococci against phagocytosis (33). Two lines of evidence in this study indicate that the capsule has an antiphagocytic role in C. neoformans interactions with A. castellanii. First, capsular enlargement was associated with major reductions in phagocytic efficacy for all 7 strains tested. Hence, capsular enlargement, a phenomenon associated with evasion of phagocytosis by macrophages and virulence in mice, also has a corresponding protective function in interactions with amoebae (34, 36). Second, comparison of phagocytic efficacy of three isogenic strains differing in capsular phenotype revealed that, in the absence of capsule, C. neoformans was much more easily ingested by A. castellanii. However, when we compared the relationship between uninduced capsule size and phagocytic efficacy across diverse strains, we observed considerable interstrain variability, and no correlation was apparent between capsule size and phagocytic efficacy. These experimental results suggest the existence of variables other than capsular diameter that contribute to amoeba phagocytic efficacy. In this regard, we note that C. neoformans strains manifest differences in polysaccharide capsule structure which could affect the interaction of the capsule with A. castellanii receptors as was observed with C. neoformans var. gattii (20), and that C. neoformans makes antiphagocytic proteins (18). Overall, these results confirm that the C. neoformans capsule is antiphagocytic for environmental amoeboid predators, implying a major defensive role for this distinctive fungal structure and establishing another correlate for cryptococcal interactions between amoebae and macrophages.
Given the recent observation that C. neoformans is capable of nonlytic exocytosis from mammalian phagocytic cells (2, 19), we investigated whether similar phenomena occurred following ingestion of yeast cells by A. castellanii. Cells of C. neoformans strain 24067 (serotype D) were observed to exit A. castellanii after phagocytosis. Hence, we now report the phenomenon of cryptococcal nonlytic exocytosis from protozoan phagocytic cells. This observation echoes similar findings with mammalian macrophages with the caveat that there were significant differences between exit from mammalian macrophages and from protozoan phagocytic cells. First, the exocytosis events in amoebae involved single yeast cells whereas macrophage exocytosis events often involve numerous yeast cells exiting a giant phagosome. In this regard, the exit of C. neoformans from amoebae is similar to that described by Ma et al. who reported exocytosis of single yeast cells from mammalian macrophages (19). Second, we observed strain-related differences in the ability of C. neoformans to exit amoebae, as no exit events were observed with yeast cells of C. neoformans var. grubii strain H99 (serotype A). In contrast, all cryptococcal strains studied in macrophages were found capable of nonlytic exocytosis from those cells. Furthermore, in macrophages, the efficiency of exocytosis was higher for strain H99 than for strain 24067 (2), whereas the former was never observed to exit A. castellanii. Given the limitations encountered in observing amoeba-cryptococcus interactions over a prolonged time, relating to their extreme mobility and microscope light aversion, we cannot rule out a false-negative result in the absence of observable exocytosis events with strain H99. Nevertheless, if these exocytosis events occur, exocytosis appears to be a much more frequent event with strain 24067 than with strain H99. Third, exocytosis from amoebae occurred significantly later than macrophage exocytosis, which can occur as early as 2 h after ingestion. Fourth, the addition of the actin inhibitor cytochalasin D abrogated yeast expulsion in amoebae, while the same drug hastens it in macrophages. Comparison of CAP59 and CAP67 mutants to encapsulated strains suggested a role for the capsule in extrusion. The lack of capsule greatly increased the rate of expulsion, something not observed with the macrophage experiments.
C. neoformans exocytosis from amoebae was a microbe-mediated process, as no exit events were observed with heat-killed yeast or polystyrene beads. Yeast exocytosis from amoebae appeared to be a different phenomenon than the cytoplasmic expulsion reported when amoebae are injected with foreign cytoplasm, since yeast exocytosis followed active phagocytosis and occurred much later than cytoplasmic expulsion (11). The capacity of yeast cells to exit amoebae would be an effective survival strategy for escaping predatory protozoa. We note that bacteria have recently been shown to exit species of the protozoan Tetrahymena in a process that can alter bacterial virulence properties (5). Alternatively, it is possible that the amoeba cell expels ingested yeast with a pathogenic potential for that host. In this regard, it is known that Dictyostelium discoideum expulses zymosan particles after ingestion (27). Since fungal passage in amoebae can alter virulence properties, one can imagine how cycles of ingestion and exocytosis could affect the virulence of C. neoformans (31, 32).
In summary, we report that C. neoformans has the capacity for nonlytic exocytosis from amoebae, thus echoing similar observations with macrophages, but there appears to be significant differences in the mechanisms involved (2, 19). The observation that amoebae ingest and occasionally extrude C. neoformans is consistent with the proposal that fungus-protozoan interactions are both common and ancient and represents a mechanism for the selection of fungal traits that confer virulence in the setting of appropriate fungus-animal interactions (7, 30, 33). In considering the implications of these findings, we caution that there are innumerable species of protozoa capable of yeast predation in the environment and that the findings observed here were limited to one amoeba species that has been adapted to laboratory conditions. In fact, the strain of A. castellanii used in this study is unusual among amoeba strains in its capacity to grow in axenic media as a result of laboratory adaptations. Consistent with the notion that each fungus-protozoan interaction may be unique, we recently described the interaction of C. neoformans with Paramecium spp. and noted that it was very different from that described for amoebae (12). Nevertheless, the occurrence of C. neoformans nonlytic exocytosis from amoebae establishes another important global correlate between fungus-protozoan interactions and fungus-macrophage interactions, despite significant differences in the details of this process. An enhanced understanding of the interactions of fungi with pathogenic potential with environmental protists is important in light of the hypothesis that continued climate warming will be associated with new and more frequent fungal diseases (13). Considering the enormous evolutionary time that separates protozoa and mammals, the global similarities between C. neoformans interactions with amoebae and macrophages is consistent and supportive of the proposal that many virulence-associated characteristics emerged from environmental selection pressures (8).
Supplementary Material
Acknowledgments
C.J.C. is supported by the Training Program in Cellular and Molecular Biology and Genetics, T32 GM007491. A.C. is supported by National Institutes of Health grants AI33774, AI33142, AI51519, and HL59842-01.
Footnotes
Published ahead of print on 30 July 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Abramoff, M. D., P. J. Magelhaes, and S. J. Ram. 2004. Image processing with ImageJ. Biophotonics Int. 11:36-42. [Google Scholar]
- 2.Alvarez, M., and A. Casadevall. 2006. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr. Biol. 16:2161-2165. [DOI] [PubMed] [Google Scholar]
- 3.Apidianakis, Y., L. G. Rahme, J. Heitman, F. M. Ausubel, S. B. Calderwood, and E. Mylonakis. 2004. Challenge of Drosophila melanogaster with Cryptococcus neoformans and role of the innate immune response. Eukaryot. Cell 3:413-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bidochka, M. J., D. C. Clark, M. Lewis, and N. O. Keyhani. 2010. Could insect phagocytic avoidance by entomogenous fungi have evolved via selection against soil amoeboid predators? Microbiology 156:2164-2171. [DOI] [PubMed] [Google Scholar]
- 5.Brandl, M. T., B. M. Rosenthal, A. F. Haxo, and S. G. Berk. 2005. Enhanced survival of Salmonella enterica in vesicles released by a soilborne Tetrahymena species. Appl. Environ. Microbiol. 71:1562-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunting, L. A., J. B. Neilson, and G. S. Bulmer. 1979. Cryptococcus neoformans: gastronomic delight of a soil ameba. Sabouraudia 17:225-232. [DOI] [PubMed] [Google Scholar]
- 7.Casadevall, A., and L. A. Pirofski. 2007. Accidental virulence, cryptic pathogenesis, Martians, lost hosts, and the pathogenicity of environmental microbes. Eukaryot. Cell 6:2169-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casadevall, A., J. N. Steenbergen, and J. D. Nosanchuk. 2003. ‘Ready made’ virulence and ‘dual use’ virulence factors in pathogenic environmental fungi — the Cryptococcus neoformans paradigm. Curr. Opin. Microbiol. 6:332-337. [DOI] [PubMed] [Google Scholar]
- 9.Fan, W., A. Idnurm, J. Breger, E. Mylonakis, and J. Heitman. 2007. EcaI, a sarcoplasmic/endoplasmic reticulum Ca2+-ATPase, is involved in stress tolerance and virulence in Cryptococcus neoformans. Infect. Immun. 75:3394-3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldmesser, M., Y. Kress, P. Novikoff, and A. Casadevall. 2000. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect. Immun. 68:4225-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flickinger, C. J., G. A. Read, and E. M. Kabana. 1980. Structural responses of amoebae to the injection of heterologous cytoplasm. J. Cell Sci. 45:1-14. [DOI] [PubMed] [Google Scholar]
- 12.Frager, S. Z., C. J. Chrisman, R. Shakked, and A. Casadevall. 2010. Paramecium species ingest and kill the cells of the human pathogenic fungus Cryptococcus neoformans. Med. Mycol. 48:775-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Solache, M., and A. Casadevall. 2010. Global warming will bring new fungal diseases for mammals. mBio 1:e00061-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozel, T. R. 1993. Opsonization and phagocytosis of Cryptococcus neoformans. Arch. Med. Res. 24:211-218. [PubMed] [Google Scholar]
- 15.Kozel, T. R., and E. C. Gotschlich. 1982. The capsule of Cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J. Immunol. 129:1675-1680. [PubMed] [Google Scholar]
- 16.Kozel, T. R., G. S. Pfrommer, A. S. Guerlain, B. A. Highison, and G. J. Highison. 1988. Strain variation in phagocytosis of Cryptococcus neoformans: dissociation of susceptibility to phagocytosis from activation and binding of opsonic fragments of C3. Infect. Immun. 56:2794-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levitz, S. M. 2001. Does amoeboid reasoning explain the evolution and maintenance of virulence factors in Cryptococcus neoformans? Proc. Natl. Acad. Sci. U. S. A. 98:14760-14762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luberto, C., B. Martinez-Marino, D. Taraskiewicz, B. Bolanos, P. Chitano, D. L. Toffaletti, G. M. Cox, J. R. Perfect, Y. A. Hannun, E. Balish, and M. Del Poeta. 2003. Identification of App1 as a regulator of phagocytosis and virulence of Cryptococcus neoformans. J. Clin. Invest. 112:1080-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma, H., J. E. Croudace, D. A. Lammas, and R. C. May. 2006. Expulsion of live pathogenic yeast by macrophages. Curr. Biol. 16:2156-2160. [DOI] [PubMed] [Google Scholar]
- 20.Malliaris, S. D., J. N. Steenbergen, and A. Casadevall. 2004. Cryptococcus neoformans var. gattii can exploit Acanthamoeba castellanii for growth. Med. Mycol. 42:149-158. [DOI] [PubMed] [Google Scholar]
- 21.Martinez, L. R., J. Garcia-Rivera, and A. Casadevall. 2001. Cryptococcus neoformans var. neoformans (serotype D) strains are more susceptible to heat than C. neoformans var. grubii (serotype A) strains. J. Clin. Microbiol. 39:3365-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moffat, J. F., and L. S. Tompkins. 1992. A quantitative model of intracellular growth of Legionella pneumophila in Acanthamoeba castellanii. Infect. Immun. 60:296-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mylonakis, E. 2008. Galleria mellonella and the study of fungal pathogenesis: making the case for another genetically tractable model host. Mycopathologia 165:1-3. [DOI] [PubMed] [Google Scholar]
- 24.Mylonakis, E., F. M. Ausubel, J. R. Perfect, J. Heitman, and S. B. Calderwood. 2002. Killing of Caenorhabditis elegans by Cryptococcus neoformans as a model of yeast pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 99:15675-15680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mylonakis, E., A. Casadevall, and F. M. Ausubel. 2007. Exploiting amoeboid and non-vertebrate animal model systems to study the virulence of human pathogenic fungi. PLoS Pathog. 3:e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neilson, J. B., M. H. Ivey, and G. S. Bulmer. 1978. Cryptococcus neoformans: pseudohyphal forms surviving culture with Acanthamoeba polyphaga. Infect. Immun. 20:262-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rauchenberger, R., U. Hacker, J. Murphy, J. Niewohner, and M. Maniak. 1997. Coronin and vacuolin identify consecutive stages of a late, actin-coated endocytic compartment in Dictyostelium. Curr. Biol. 7:215-218. [DOI] [PubMed] [Google Scholar]
- 28.Ruiz, A., J. B. Neilson, and G. S. Bulmer. 1982. Control of Cryptococcus neoformans in nature by biotic factors. Sabouraudia 20:21-29. [PubMed] [Google Scholar]
- 29.Small, J. M., and T. G. Mitchell. 1989. Strain variation in antiphagocytic activity of capsular polysaccharides from Cryptococcus neoformans serotype A. Infect. Immun. 57:3751-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steenbergen, J. N., and A. Casadevall. 2003. The origin and maintenance of virulence for the human pathogenic fungus Cryptococcus neoformans. Microbes Infect. 5:667-675. [DOI] [PubMed] [Google Scholar]
- 31.Steenbergen, J. N., J. D. Nosanchuk, S. D. Malliaris, and A. Casadevall. 2003. Cryptococcus neoformans virulence is enhanced after growth in the genetically malleable host Dictyostelium discoideum. Infect. Immun. 71:4862-4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steenbergen, J. N., J. D. Nosanchuk, S. D. Malliaris, and A. Casadevall. 2004. Interaction of Blastomyces dermatitidis, Sporothrix schenckii, and Histoplasma capsulatum with Acanthamoeba castellanii. Infect. Immun. 72:3478-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steenbergen, J. N., H. A. Shuman, and A. Casadevall. 2001. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc. Natl. Acad. Sci. U. S. A. 98:15245-15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaragoza, O., C. J. Chrisman, M. V. Castelli, S. Frases, M. Cuenca-Estrella, J. L. Rodriguez-Tudela, and A. Casadevall. 2008. Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell. Microbiol. 10:2043-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaragoza, O., B. C. Fries, and A. Casadevall. 2003. Induction of capsule growth in Cryptococcus neoformans by mammalian serum and CO2. Infect. Immun. 71:6155-6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaragoza, O., C. P. Taborda, and A. Casadevall. 2003. The efficacy of complement-mediated phagocytosis of Cryptococcus neoformans is dependent on the location of C3 in the polysaccharide capsule and involves both direct and indirect C3-mediated interactions. Eur. J. Immunol. 33:1957-1967. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.