Abstract
Varicella-zoster virus (VZV) is an alphaherpesvirus that is restricted to humans. VZV infection of differentiated cells within the host and establishment of latency likely require evasion of innate immunity and limited secretion of antiviral cytokines. Since interferons (IFNs) severely limit VZV replication, we examined the ability of VZV to modulate the induction of the type I IFN response in primary human embryonic lung fibroblasts (HELF). IFN-β production was not detected, and transcription of two interferon response factor 3 (IRF3)-dependent interferon-stimulated genes (ISGs), ISG54 and ISG56, in response to poly(I:C) stimulation was downregulated in VZV-infected HELF. Inhibition of IRF3 function did not require VZV replication; the viral immediate-early protein 62 (IE62) alone was sufficient to produce this effect. IE62 blocked TBK1-mediated IFN-β secretion and IRF3 function, as shown in an IFN-stimulated response element (ISRE)-luciferase reporter assay. However, IRF3 function was preserved if constitutively active IRF3 (IRF3-5D) was expressed in VZV-infected or IE62-transfected cells, indicating that VZV interferes with IRF3 phosphorylation. IE62-mediated inhibition was mapped to blocking phosphorylation of at least three serine residues on IRF3. However, IE62 binding to TBK1 or IRF3 was not detected and IE62 did not perturb TBK1-IRF3 complex formation. IE62-mediated inhibition of IRF3 function was maintained even if IE62 transactivator activity was disrupted. Thus, IE62 has two critical but discrete roles following VZV entry: to induce expression of VZV genes and to disarm the IFN-dependent antiviral defense through a novel mechanism that prevents IRF3 phosphorylation.
Varicella-zoster virus (VZV) is an alphaherpesvirus with a 125-kb double-stranded DNA (dsDNA) genome encoding approximately 70 unique proteins that are expressed during the virus life cycle. VZV is a pathogen that is restricted to humans and that exhibits tropism for specific differentiated cell types, including epithelial cells, T cells, and neurons (4, 15, 28, 54). Primary VZV infection causes varicella (“chicken pox”), which is manifested by the occurrence of vesicular cutaneous lesions; VZV persists in sensory neurons, and reactivation from latency may cause herpes zoster (“shingles”) (6). Clinical observations and studies of VZV pathogenesis in human tissue xenografts in the severe combined immunodeficiency (SCID) mouse model suggest that primary infection is initiated by transfer of the virus from respiratory mucosal epithelial cells to T cells in tonsils and other local lymphoid tissues. Infected T cells can then transport VZV to skin; access to sensory ganglion neurons where VZV establishes latency may occur by retrograde axonal transport from skin lesions or by direct transfer from T cells (16).
Like other viral pathogens, herpesviruses have evolved mechanisms to subvert the early innate host responses mediated through the activation of interferons (IFNs) and IFN-stimulated genes (ISGs). The type I IFNs, IFN-α and IFN-β, are involved in early pathogen detection (14, 37, 47). In most mammalian cell types, virus infection induces IFN-β transcription and secretion; secreted IFN-β has paracrine and autocrine effects, inducing IFN-α and other IFN-responsive genes and amplifying IFN-β secretion. IFN-β induction is mediated by binding and activation of latent transcription factors, including interferon response factor 3 (IRF3) and NF-κB (37, 42, 45). The recognition of viral pathogen-associated molecular patterns (PAMPs) by various cellular pathogen recognition receptors (PRRs) leads to a signaling cascade that converges on the IRF3 kinases TBK1 and/or IKKi (7, 22, 26). Activation of TBK1/IKKi in turn results in IRF3 phosphorylation at the C-terminal cluster of serine/threonine residues, which triggers IRF3 dimerization, nuclear translocation, and binding to the IFN-β gene promoter in association with other transcription factors (5, 19, 20, 35). Phosphorylation at alternate sites, e.g., serine 339, has also been shown to be important for IRF3 activation and function (5). Activated IRF3 then binds to IFN-stimulated response elements (ISRE) located in the promoter regions of various ISGs, including ISG54, ISG56, ISG15, and others, thereby enabling direct IFN-independent activation of some of these ISGs (41, 46).
Infection of human skin xenografts in vivo in the SCID mouse model of VZV pathogenesis is characterized by gradual cell-cell spread in which VZV-infected cells are surrounded by uninfected epidermal cells that show extensive expression of IFN-α. VZV must overcome this potent IFN barrier to reach the skin surface and cause the vesicular lesions that contain infectious virus for transmission to other susceptible individuals. The biological importance of IFN signaling in modulating VZV infection was confirmed by the substantial increase in the extent of lesions and infectious virus production in skin xenografts when animals were given monoclonal antibody to the IFN-α/β receptor (16). The VZV immediate-early 63 (IE63) protein is known to inhibit downstream events in the IFN pathway (3). VZV also impedes NF-κB activation both in differentiated epidermal cells of the skin and in cultured primary human embryonic lung fibroblasts (HELF). However, while NF-κB is required for optimal induction of IFN-β, IRF3 is essential for the initial phase of IFN-β induction (42).
Given the importance of this pathway, herpesviruses have evolved distinct mechanisms and virus-encoded factors to subvert IRF3-dependent induction of IFN-β. For example, three of six anti-IFN proteins encoded by herpes simplex virus type 1 (HSV-1) regulate IRF3 activation and function, including ICP0, ICP34.5, and ICP27 (21, 23-25, 33, 49), and human cytomegalovirus (HCMV) pp65 and Kaposi's sarcoma herpesvirus (KSHV)-encoded viral IRF1 inhibit IRF3 activity (1, 18).
Herpesvirus IE proteins are of interest for their potential to inhibit IFN responses because viral replication is not required for their expression (39). In VZV, IE62 is a 1,310-amino-acid protein redundantly encoded by open reading frame 62 (ORF62) and ORF71. IE62 is an abundant component of the virion tegument and is the major VZV transactivator, inducing transcription of IE, early, and late viral genes (9, 13, 29, 34). Among the putative IE proteins encoded by ORF4, ORF61, ORF62, and ORF63, we have shown that only IE62 and ORF61 were detectable within 1 h after infection (38).
The purpose of this study was to assess VZV-mediated regulation of type I IFNs and to identify viral factors that modulated innate cell defenses. We show that VZV blocks IRF3 activation in infected cells, thereby evading the signaling cascade that triggers synthesis of antiviral factors. In contrast to HSV-1 (21), VZV interference with IRF3 is a replication-independent process. IE62 alone antagonizes IFN induction by blocking IRF3 activation prior to IRF3 phosphorylation. Using a systematic site-directed mutagenesis approach, we show that IE62 interfered with phosphorylation of IRF3 serine residues within the C-terminal serine/threonine cluster. However, IE62 failed to inhibit TBK1-IRF3 complex formation and did not interact directly with either TBK1 or IRF3. Evaluation of an IE62 construct with a targeted mutation that disrupts IE62-mediated transactivation showed that the capacity of IE62 to block IRF3-dependent IFN-β induction and to initiate VZV gene transcription comprises two discrete functions. IE62 is the first VZV-encoded factor found to prevent type I IFN induction, and it is the first herpesvirus protein that exerts this function by preventing the complete phosphorylation of IRF3, which is necessary for its activation as a transcription factor.
MATERIALS AND METHODS
Cell culture.
Melanoma cells (Mel39) were maintained in Eagle's minimum essential medium (MEM; Mediatech, Manassas, VA) supplemented with 10% fetal calf serum (FCS; Gemini Bio-Products, Woodland, CA), nonessential amino acids (100 μM; Omega Scientific Inc., Tarzana, CA), amphotericin (0.5 mg/ml; Omega Scientific Inc., Tarzana, CA), and an antibiotic cocktail of penicillin G and streptomycin (100 U/ml of each; Omega Scientific Inc., Tarzana, CA). Human embryonic lung fibroblasts (HELF) were propagated in the above-mentioned medium but without the nonessential amino acid supplement. HEK293 cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Mediatech, Manassas, VA) supplemented with 10% FCS and 100 U/ml each of penicillin G and streptomycin.
UV inactivation and virus infection.
UV inactivation of VZV was carried out by exposing infected fibroblasts to UV irradiation for 25 min as described previously (11); inactivation was confirmed by an infectious focus assay of melanoma cells. A duplicate infected plate prepared in parallel was left untreated and used as a control to determine the virus titer. For reverse transcription (RT)-PCR assays, HELF were infected with VZV strain pOka at 0.01 to 0.5 PFU/cell as indicated in the figure legends; in the dose experiments, inocula were 0.14 and 0.014 PFU/cell of pOka and equivalent volumes of UV-inactivated pOka. HELF were infected with HSV (KOS strain) at an MOI of 2.5.
Plasmids and cloning.
ORF62 from strain pOka was amplified by PCR using pLitmus 59-65 as the template, AccuPrime Pfx (Invitrogen, Carlsbad, CA), and the following set of primers: NheI-ORF62-F 5′-GCTAGCGCCACCATGGATACGCCGCCGATGCACCGC-3′ and XbaI-ORF62-R-5′-TCTAGATCATCACCCCCGACTCTGCGGGGGGCTCC-3′. The bases in bold indicate restriction enzyme sites added for cloning purposes. The underlined bases indicate the start codon in the forward primer and the tandem stop codons in the reverse primer. The 4-kb amplified product was TOPO cloned (Invitrogen, Carlsbad, CA) and sequenced (Elim Biopharmaceuticals, Hayward, CA). IE62-TOPO clone was subsequently digested with NheI and XbaI and inserted into the NheI/XbaI sites of pcDNA3.1 mammalian expression vector (Invitrogen, Carlsbad, CA). Point mutations were introduced in IE62-pcDNA3.1 to disrupt the TRAF binding motifs (Traf-M1 and Traf-M2) by using the following common primer set: sense, 5′-GTGTGTTGGAGGTCGCTGAGTAGTG-3′ (anneals to the pcDNA3.1 vector); antisense. 5′-ACAGGTTGGCAAACGCAGTCTCGAT-3′ (anneals in ORF62). The following motif-specific internal primers were also used: Traf-M1-sense, GATACGCCGgCGATGgcGCGCgCTACACCCCAACG; Traf-M1-antisense, CGTTGGGGTGTAGcGCGCgcCATCGcCGGCGTATC; Traf-M2-sense, GGTCT ATAgCCCGAgcAACGgCGAGAGGAAATTCA; Traf-M2-antisense, TGAATTTCCTCTCGcC GTTgcTCGGGgTATAGACC. The lowercase bases indicate the changes incorporated in the internal primer sets to convert the conserved residues within the two motifs—amino acids P5, Q7, and S9 (Traf-M1) and P188, Q190, and T192 (Traf-M2) in wild-type IE62—to alanine. The IE62 Traf-M1/M2 mutant, in which both the motifs were mutated, was generated by digesting the IE62 Traf-M2 mutant with HindIII/BstEII and cloning the fragment containing the mutation into the HindIII/BstEII-digested IE62 Traf-M1. Each clone was verified by sequencing (Elim Biopharmaceuticals). IE62-K548E and IE62-L446P point mutations were introduced in the IE62-pcDNA3.1 expression plasmid by amplification using Accuprime Pfx and the following set of common primers: HindIII sense, GTATGCGAGGACGGTCAGGA; and HindIII antisense, TCTGTCGCGAGGGTGCTCTC. Internal primers for the specific mutations used were as follows: K548E sense, gAGCTGACCGGTGTCAACTC; K548E antisense, GGGGTTCTGGAGC CATGCTA; L446p sense, cGCCGCGATCCAGAACCCCG; and L446P antisense, GGGAAAATGATTTCTGTCTC. The lowercase bases in the forward internal primers indicate the specific base changes. The amplified products and wild-type IE62-pcDNA3.1 were digested with HindIII and cloned by triple ligation. The clones were screened for orientation and sequenced to verify the presence of the desired mutation. Plasmids encoding the human IRF3-5D and green fluorescent protein (GFP)-IRF3, TBK1, and HA-TRAF3 were kindly provided by Adolfo Garcia-Sastre (Mount Sinai School of Medicine), David Baltimore (California Institute of Technology), and Michael Karin (University of California, San Diego). Flag-TBK1 expression plasmid was a kind gift from Harry Greenberg (Stanford University), while the human IRF3 plasmid was purchased from Invivogen (San Diego, CA). IRF3-serine/threonine mutants and the TBK1 kinase dead mutant were generated by site-directed mutagenesis using the Quickchange site-directed mutagenesis kit (Stratagene). Primers for site-directed mutagenesis were designed using the PrimerX software (http://www.bioinformatics.org/primerx/). The ISRE-luciferase plasmid was kindly provided by Dingxiang Liu, Institute of Molecular and Cell Biology, Singapore.
Reagents and antibodies.
Poly(I:C) was obtained from Invivogen (San Diego, CA) and used at the concentrations indicated in the figure legends. Immunoblotting was done using primary antibodies to IRF3 (sc9082) and p65 (sc8008) (Santa Cruz Biotechnology Inc., Santa Cruz, CA); pIRF3-S396 (4D4G; Cell Signaling Technology, Boston, MA); p56 (Thermo Scientific, Rockford, IL); Flag-M2 monoclonal antibody, β-actin, and α-tubulin (Sigma, Saint Louis, MO); and IE62 (52). Horseradish peroxidase-conjugated anti-rabbit and anti-mouse secondary antibodies were purchased from Amersham Biosciences. The restriction enzymes used for cloning were all purchased from New England Biolabs (Ipswich, MA).
RNA isolation and RT-PCR assay.
RNA was isolated from infected and uninfected HELF using TRIzol reagent (Invitrogen) per the manufacturer's instructions at the times postinfection indicated in the figures. RNA concentration was measured by spectrophotometry, and the quality of purified RNA was determined on a 1% glyoxal-agarose gel by using a ratio of 28S:18S rRNA. Total RNA (1 μg) was used for DNase digestion and RT with the First Strand cDNA synthesis kit (Invitrogen, Carlsbad, CA). Five percent of the first-strand reaction was used for PCR analysis to detect ISG56 (sense, CCTGGAGTACTATGAGCGGGC; antisense, TGGGTGCCTAAGGACCTTGTC; 30 cycles), ISG54 (sense, AGAAATCAAGGGAGAAAGAA; antisense, AAGGTGACTAAGCAAATGGT; 30 cycles), and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (sense, CACCACACTGCTTAGCAC; antisense, CCCTGTTGCTGTAGCCAAAT; 25 cycles) transcripts. Control reactions were performed in parallel in the absence of reverse transcriptase.
Cell sorting.
HELF were infected with VZV-GFP (55) 24 h before sorting and trypinized (TrypLE Express; Invitrogen) rapidly, and a single-cell suspension was made in phosphate-buffered saline (PBS; Mediatech Inc., Manassas, VA) containing 10% fetal bovine serum and passed through a 70-μm nylon cell strainer (BD Biosciences, San Jose, CA). The cells were sorted using a Vantoo sorter and resuspended to 5,000 cells per μl; 1 × 104 cells from GFP+ and GFP− populations were used for cDNA synthesis (SuperscriptIII cell direct cDNA synthesis system; Invitrogen, Carlsbad, CA).
ELISA.
Cell culture supernatants were collected at the times indicated in the figure legends and assayed for IFN-β secretion using the Verikine human IFN-β enzyme-linked immunosorbent assay (ELISA) kit (PBL Interferon Source Inc.).
Transfection, Western blotting, and coimmunoprecipitation.
HEK293 cells that had been plated onto 10-cm culture dishes 24 h earlier were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) and the amounts of the expression plasmids indicated in the figure legends. Whole-cell lysates were prepared by lysing cells in 2× Laemmli sample buffer (Bio-Rad, Hercules, CA) containing 10% β-mercaptoethanol (GIBCO, Invitrogen Inc., Grand Island, NY). The lysed samples were boiled for 5 min and separated on a 10% SDS-PAGE gel, followed by immunoblotting using the antibodies indicated in the figures. For coimmunoprecipitation (coIP), cells were lysed at 48 h posttransfection in 1% NP-40 buffer supplemented with protease inhibitor (Sigma, St. Louis, MO) and Halt phosphatase inhibitor cocktails (Thermo Scientific). Cell lysates were analyzed for coIP using the ExactaCruz kits (Santa Cruz Biotechnology, Santa Cruz, CA). Briefly, lysates were incubated with the preclearing matrix for at least 1 h at 4°C while being rotated, and beads were conjugated to anti-Flag antibody for 4 h at 4°C. The antibody-bound beads were washed with PBS and incubated with precleared lysates overnight at 4°C and then washed and resuspended in electrophoresis buffer. Samples were loaded onto a 10% SDS-PAGE gel and analyzed by immunoblotting using the antibodies indicated in the figures.
Transfection and luciferase reporter assay.
HEK293 cells (1.7 × 106) were seeded on a 24-well plate 18 to 24 h before transfection. Cells in antibiotic-free medium were transfected with Fugene6 (Roche Diagnostics, Indianapolis, IN) per the manufacturer's instructions using a DNA-to-Fugene6 ratio of 1:3. Cells were transfected in duplicate with 50 ng of ISRE firefly luciferase reporter, 0.1 ng of Renilla luciferase reporter, and 50 or 100 ng of the IRF3 pathway inducers. Plasmids expressing VZV proteins were cotransfected with plasmids encoding specific IRF3 inducers at a (VZV protein-to-inducer) ratio of 1:1, 2:1, or 4:1. Transfected cells were lysed at 24 h using 1× passive lysis buffer (Promega, Madison, WI). For transfection-infection luciferase assays, melanoma cells were seeded on a 24-well plate 24 h before transfection. Cells were transfected with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) and infected with strain pOka at 24 h.
Image quantification.
Densitometric analysis was performed on scanned immunoblots using the ImageJ software (2). The gel analysis tool was used to obtain the absolute intensity for each experimental band and the corresponding control actin band. Relative densitometric value (RDV) for each experimental band was calculated by normalizing the absolute intensity of the experimental band with respect to that of the control (actin or GAPDH) bands.
RESULTS
VZV infection blocks the transcription of IRF3-responsive genes and the secretion of IFN-β.
Primary fibroblasts are IFN-competent cells that respond to virus infection by triggering IFN-β induction mediated by cellular transcription factors, including IRF3 (32, 37). ISG56 mRNA is upregulated in response to IRF3 activation, an essential step in early IFN induction in fibroblasts, and is a marker for this response. In order to examine how VZV affects the early innate immune response, RT-PCR analysis of ISG56 transcripts in HELF infected with pOka or UV-inactivated pOka (UV-pOka) was done at sequential time points over 12 h postinfection (Fig. 1A). HELF exhibited significant upregulation of ISG56 transcription in response to the control, poly(I:C), a synthetic dsRNA/single-stranded RNA (ssRNA) analog that triggers IFN-β induction via PRRs like TLR3 and RIG-I. When ISG56 transcripts were measured by RT-PCR at 24 h after inoculation of HELF with two concentrations of VZV pOka or UV-inactivated pOka, none induced ISG56 mRNA transcripts whereas IFN-α induced robust ISG56 transcription. As we have reported previously, newly synthesized IE62 and ORF61 proteins can be detected in HELF within 1 h postinfection (38). However, ISG56 transcripts were not detected in infected cells between 4 and 24 h postinfection (Fig. 1A and B). UV-inactivated VZV also failed to induce any detectable ISG56 transcripts between 2 and 24 h postinfection. These results indicate that VZV did not induce early ISG56 transcription, which requires IRF3, and suggested that VZV encoded replication-independent mechanisms which might suppress induction of IFN-β, which also depends upon IRF3 activation.
FIG. 1.
VZV infection blocks transcription of IRF3-responsive genes and IFN-β secretion. (A) HELF inoculated with UV-pOka (lanes 1 to 5) or with pOka at an MOI of 0.01 (lanes 6 and 7) were harvested for cDNA synthesis at the indicated hours postinfection (hpi) and analyzed by RT-PCR for ISG56 transcript levels at each time point. Cells treated with 10 μg/ml of poly(I:C) for 12 h or left untreated were used as positive (lane 8) and negative (lane 9) controls, respectively. GAPDH was used as an internal control for the assay. One microgram of total RNA from each sample was analyzed on a 1% glyoxal-agarose gel. (B) HELF inoculated with UV-pOka (lanes 1 and 2) or pOka (lanes 3 and 4) at 0.014 and 0.14 MOI were harvested at 24 hpi for RT-PCR analysis of ISG56 transcripts compared to mock-inoculated (control [C]) cells (lane 5). Cells treated with 104 U/ml of IFN-α for 6 h served as a positive control. *, RT-negative control using IFN-α-treated HELF. (C) Secretion of IFN-β into the culture supernatant of pOka-infected and UV-pOka-inoculated HELF was measured by ELISA at 18 hpi and compared with IFN-β secreted by 2.5 and 10 μg/ml poly(I:C)-treated HELF that served as a control for the assay. **, P values are 0.0025 for pOka-infected samples and 0.0010 for UV-Oka-inoculated samples. Error bars indicate the standard errors of the mean. hpt, hours posttreatment.
To assess IFN-β induction, supernatants were collected from Oka-infected and UV-pOka-inoculated HELF at 18 h postinfection and tested for IFN-β release by ELISA. As a control, HELF were treated for the same period of time with poly(I:C). As shown in Fig. 1C, while poly(I:C) induces IFN-β secretion, IFN-β concentrations in supernatants from pOka-infected and UV-pOka-inoculated HELF were similar to the basal levels observed in supernatants from uninfected/untreated HELF. These results demonstrate that IFN-β was not secreted by VZV-infected HELF, further indicating that VZV encodes antagonists of IFN induction.
VZV infection blocks transcription of IRF3-responsive genes and IFN-β secretion in response to poly(I:C).
Since upregulation of ISG56 transcription was not detectable in VZV-infected cells, we next determined whether VZV infection actively inhibits IRF3-mediated ISG56 induction in response to poly(I:C); transcription of ISG54, another IRF3-responsive gene, was also measured. As shown in Fig. 2A, treatment of HELF with 10 μg/ml poly(I:C) for 18 h increased ISG56 mRNA synthesis to 2.7-fold above that of the control. However, this induction was reduced to 1.3-fold in HELF infected with pOka 6 h before poly(I:C) treatment. VZV also downregulated ISG54 transcription in response to poly(I:C). HSV-1, which was used as a control, abolished ISG56 induction by poly(I:C). This difference between VZV and HSV-1 is explained by the fact that inoculum titers achievable with VZV do not permit synchronous infection of all cells in the monolayer; at this time point, only about 15 to 20% of HELF are infected. A reduction of ISG56 transcription from 3.4-fold to 1.5-fold was also seen in poly(I:C)-induced cells that were inoculated with UV-inactivated VZV (Fig. 2B). To further verify that VZV interfered with ISG56 mRNA upregulation, HELF were infected with a recombinant pOka strain that expresses GFP and, at 20 h postinfection, were treated with 5 μg/ml of poly(I:C) for 4 h. The poly(I:C)-treated and VZV-infected cells were then sorted for GFP+ and GFP− populations by fluorescence-activated cell sorting (FACS) and tested for ISG56 or internal control GAPDH mRNA by RT-PCR. When the fraction of VZV-infected cells was enriched using the GFP marker, ISG56 mRNA levels were 2-fold lower than those of the GFP-negative cell population.
FIG. 2.
VZV infection blocks transcription of IRF3-responsive genes and IFN-β secretion in response to exogenous poly(I:C). (A) HELF were mock infected (control [C]; lanes 1 and 2), infected with HSV at an MOI of 2.5 (lanes 3 and 4) or with VZV at an MOI of 0.5 (lanes 5 and 6) and treated (+) with 10 μg/ml of poly(I:C) or left untreated (−). RNA was isolated at 24 hpi and analyzed for ISG54 and ISG56 transcripts by RT-PCR. GAPDH mRNA was used as an internal control for the assay. The relative densitometric value (RDV) of each band was calculated with respect to GAPDH and is represented on the bar graph. (B) HELF infected with pOka at an MOI of 0.04 (lanes 3 and 4) or inoculated with UV-pOka (lanes 5 and 6) and treated with (+) or without (−) poly (I:C) were analyzed for ISG56 transcripts. The RDV of each ISG56 PCR product was calculated with respect to GAPDH and is represented on the bar graph. (C) GFP-VZV-infected and poly(I:C)-treated HELF were sorted by FACS at 24 hpi and analyzed by RT-PCR for ISG56 and GAPDH transcripts. The intensities of the PCR products were densitometrically analyzed and are represented on the bar graph. (D) pOka (lane 3)- and UV-pOka (lane 4)-inoculated HELF were harvested in 2× sample lysis buffer at 24 hpi and analyzed for p56 expression by SDS-PAGE, followed by Western blotting with anti-p56 antibody. β-Actin served as the loading control. α, anti-. (E) Uninfected HELF (lanes 1 and 2), mock-infected HELF (lanes 3 and 4), and those inoculated with increasing amounts of pOka (lanes 5 and 6) or UV-pOka (lanes 7 and 8) were treated with (+) or without (−) poly (I:C). Cells were harvested in 2× sample lysis buffer at 24 hpi and analyzed for p56 expression. The band intensity of p56 for each sample was normalized to the respective β-actin levels and is represented in the bar graph. (F) VZV- or UV-VZV-inoculated HELF were treated with 2.5 μg/ml poly(I:C) at 24 hpi, and samples were assayed by ELISA for IFN-β secretion 18 h posttreatment. Error bars indicate the standard errors of the mean.
Based on these analyses of ISG56 transcription, we evaluated ISG56 protein (p56) expression in HELF that were inoculated with pOka or UV-inactivated pOka and either treated with poly(I:C) or the dimethyl sulfoxide (DMSO) control. As shown in Fig. 2D, the 56-kDa p56 was not detected in cells inoculated with infectious or inactivated VZV (lanes 3 and 4), although poly(I:C) alone strongly induced p56 (lane 2). In addition, both pOka and inactivated pOka efficiently downregulated p56 expression in response to stimulation with poly(I:C), as shown in Fig. 2E (lanes 5 to 8). The VZV-mediated downregulation of p56 production that was elicited by poly(I:C) occurred in a dose-dependent manner. At a low inoculum, the downregulation was approximately 35% in cells inoculated with pOka and 50% with UV-inactivated pOka (lanes 5 and 7). At a higher inoculum, inhibition was nearly complete with both (lanes 6 and 8). Thus, both infectious and replication-deficient VZV interfered with IRF3-dependent ISG56 induction and blocked p56 expression.
We next determined whether the ability to block poly(I:C)-dependent upregulation of mRNA transcripts correlated with the ability of the virus to abrogate poly(I:C)-dependent IFN-β secretion. HELF were inoculated with two concentrations of pOka or UV-inactivated pOka for 24 h prior to treatment with 2.5 μg/ml of poly(I:C) for an additional 18 h. Uninfected HELF treated with poly(I:C) or mock treated were used as controls. Supernatant from each sample was analyzed by IFN-β ELISA (Fig. 2F). A dose-dependent decrease in IFN-β secretion triggered by poly(I:C) treatment was observed in the presence of both pOka and UV-inactivated pOka.
VZV infection blocks TBK1-mediated ISRE activation.
Since IRF3 activation requires the cellular kinase TBK1, we used the well-established ISRE-luciferase reporter assay to determine if TBK1-mediated activation of this reporter was modulated during VZV infection. Melanoma cells were transfected with the ISRE-firefly luciferase and Renilla luciferase (internal control) reporter constructs in the presence and absence of TBK1. Six hours posttransfection, cells were infected with VZV or mock infected and the luciferase assay was done at 24 h postinfection. TBK1 activated the ISRE-luciferase reporter to a level about 180-fold above the basal level at 24 h (Fig. 3A), whereas TBK1-mediated activation of the ISRE-luciferase reporter was reduced by 50% in VZV-infected cells. This preliminary observation indicated that VZV may have mechanisms to evade the TBK1-IRF3 arm of ISG induction and IFN-β production. As observed in the ISG transcription experiments, the incomplete inhibition of TBK1-mediated activation is attributable to the presence of residual uninfected cells. In order to determine whether VZV blocked IRF3 phosphorylation, which is necessary to activate IRF3, these experiments were repeated with a construct that expresses IRF3-5D. IRF3-5D, a potent inducer of the ISRE-luciferase reporter, is a constitutively active form of IRF3 in which five Ser/Thr residues at the C terminus of IRF3 are replaced with the phosphomimetic aspartic acid residue. IRF3-5D activated the ISRE-luciferase reporter about 200-fold at 24 h (Fig. 3A), and VZV infection failed to inhibit IRF3-5D-mediated ISRE activation. Thus, the specificity of viral interference with TBK1-mediated IRF3 phosphorylation was shown by the failure of VZV to inhibit activation of ISRE-luciferase reporter when it was stimulated by IRF3-5D.
FIG. 3.
VZV infection and IE62 overexpression interferes with activation of the IRF3 pathway. (A) Melanoma cells were transfected with TBK1 or IRF3-5D together with ISRE-firefly luciferase and Renilla luciferase reporter plasmids. Six hours posttransfection, the cells were either mock infected (−) or infected with pOka (+). Cells were lysed in 1× passive lysis buffer and assayed for luciferase activity at 24 hpi. The graph represents ISRE-luciferase activity normalized to the Renilla luciferase activity. (B) HEK293 cells were transfected with TBK1 together with ISRE-firefly luciferase and Renilla luciferase reporter plasmids and increasing concentrations of either IE62, (C) IE63, or (D) ORF9 and ORF11 expression plasmids. Cells were lysed at 24 hpt and assayed for luciferase activation. Data are presented as a bar graph of ISRE-luciferase activities normalized to the Renilla luciferase activity, and the P values are indicated. (E) HEK293 cells were cotransfected with IRF3, the luciferase reporters, and increasing concentrations of IE62 expression plasmids. Luciferase activity was measured at 24 hpt, and the normalized responses are presented in a bar graph. (F) IFN-β secretion in culture supernatant of HEK293 cells transfected with TBK1 in the presence and absence of increasing amounts of IE62 and IE63 was measured by ELISA at 48 hpt. **, P value = 0.0011 (<0.05). Error bars indicate the standard errors of the mean.
Effects of VZV gene products on activation of the IRF3 pathway.
Given this evidence that VZV encodes one or more viral proteins that function as IFN antagonists and that viral replication was not necessary for the effect, plasmids bearing several individual VZV ORFs were analyzed in the ISRE-luciferase reporter assay. The VZV proteins tested were the ORF9, ORF11, and ORF63 products, which, like IE62, are present in the virion tegument and are delivered into the target cell upon viral entry. Plasmids bearing VZV ORFs were cotransfected with TBK1, ISRE-luciferase, and Renilla luciferase reporter plasmids. Of the VZV proteins tested, IE62 resulted in significant inhibition of TBK1-mediated activation of the ISRE-luciferase reporter. The ISRE-luciferase reporter was activated approximately 550-fold in HEK293 cells in response to TBK1, but activation was reduced by more than 90% in the presence of the highest concentration of the IE62 plasmid (Fig. 3B). IE63 also had some modulatory effect on TBK1 activation of the ISRE-luciferase reporter, but this downregulation was about 50%, which was significantly less than that observed with IE62 (Fig. 3C); ORF9 and ORF11 did not downregulate the ISRE-luciferase reporter activation by TBK1 (Fig. 3D). The expression of each of the viral proteins from their respective plasmids was confirmed by immunoblotting (data not shown).
IE62-mediated interference with activation of the IRF3 pathway.
To determine whether IE62 could also inhibit ISRE-luciferase activity in response to the overexpression of IRF3 in a dose-dependent manner, HEK293 cells were cotransfected with IRF3, the ISRE-luciferase reporter, the Renilla luciferase control, and increasing concentrations of the IE62 plasmid. Overexpression of IRF3 resulted in ∼200-fold activation of the ISRE-luciferase reporter (Fig. 3E). As observed with TBK1, coexpression of IE62 in IRF3-transfected cells resulted in an 80 to 90% reduction in IRF3-mediated activation of the ISRE-luciferase reporter.
To confirm these observations at the level of protein synthesis, we investigated whether IE62 could block IFN-β secretion induced by TBK1. HEK293 cells were transfected with the TBK1 plasmid in the presence and absence of the IE62 plasmid, and IFN-β concentrations were measured in supernatants collected at 48 h. These experiments showed that IE62 efficiently blocked IFN-β secretion in response to TBK1 at both doses examined (Fig. 3F), whereas IE63 failed to block IFN-β secretion. Together, these results indicated that IE62 mediates the inhibition of IRF3 activation and therefore also prevents IRF3-dependent IFN-β secretion in response to TBK-1. Despite a 50% decrease in the TBK1-mediated induction of the ISRE-luciferase reporter, IE63 did not inhibit IFN-β secretion.
Given the inhibitory effects of IE62 on the IRF3 pathway, a bioinformatics analysis of the IE62 amino acid sequence was done to identify possible functional motifs which revealed that IE62 contains two putative TRAF-binding PXQX(T/S) (amino acids [aa] 5 to 9, PMQRS; and aa 188 to 192, PRQTT) motifs within its N-terminal region (Fig. 4 A). TRAF3 is an adaptor protein that links antiviral signals originating at the pathogen recognition receptors to TBK1 and IRF3 in the IFN pathway (30). The PXQX(T/S) motif is found in several TRAF3 binding proteins, including the cellular CD40 protein and Epstein-Barr virus (EBV) LMP1 (50, 53). Since a TRAF3-TBK1 interaction is required for TBK1 to phosphorylate IRF3 (8, 30), we investigated whether TRAF3 is the molecular target for IE62 in blocking the IRF3 pathway. HEK293 cells were cotransfected with constant amounts of TBK1 and IE62 plasmids and increasing concentrations of a TRAF3 plasmid. Since TRAF3 alone is unable to activate the ISRE-luciferase reporter as has been reported earlier (30), we investigated whether exogenous TRAF3 could overcome the inhibition mediated by IE62. As shown in Fig. 4B, IE62-mediated inhibition was not reversed by expression of increasing amounts of TRAF3. In addition, we made IE62 plasmids in which the two putative TRAF-binding motifs in IE62 were mutated. The IE62-TRAF domain mutants were tested for protein expression and localization by immunofluorescence (data not shown). Mutations performed within the TRAF-binding motifs either singly (Traf-M1 and Traf-M2) or together (Traf-M1/M2) did not alter the ability of IE62 to block TBK1-mediated ISRE-luciferase reporter activation (Fig. 4C and D), and no effect was observed when higher concentrations of the construct with the double mutation were used (Fig. 4D), indicating that the putative TRAF-binding motifs in IE62 do not contribute to IE62-dependent inhibition of TBK1-mediated IRF3 activation.
FIG. 4.
Mutation of the two TRAF-binding motifs in IE62 did not abolish its inhibitory effect on IRF3 activation. (A) The two TRAF-binding PXQX(T/S) motifs on IE62 (M1, aa 5 to 9; M2, aa 188 to 192) are indicated in underlined bold type. (B) HEK293 cells were cotransfected with TBK1, ISRE-luciferase, and Renilla luciferase reporter plasmids in the presence of increasing amounts of TRAF3 expression plasmid. Cells were lysed at 24 hpt and assayed for luciferase activity, and data are presented as a bar graph of ISRE-luciferase activity normalized to the Renilla luciferase activity. (C) Increasing concentrations of the IE62 mutants Traf-M1 and Traf-M2, in which TRAF-binding motifs were mutated singly, were cotransfected with TBK1 and the luciferase reporter plasmids. Cell lysates were harvested at 24 hpt, and ISRE-luciferase activity was measured and is represented on a bar graph normalized to the Renilla luciferase internal control. (D) Increasing concentrations of the IE62 mutant Traf-M1/M2, in which both TRAF-binding motifs were mutated, were cotransfected with TBK1 and the luciferase reporter plasmids. Luciferase activity was measured at 24 hpt, and the normalized ISRE-luciferase response was charted on a bar graph. Error bars indicate the standard errors of the mean.
IE62 blocks endogenous IRF3 phosphorylation.
Because infection with pOka did not inhibit the function of fully phosphorylated IRF3, we investigated the effects of IE62 when IRF3-5D was used to activate the ISRE-luciferase reporter. HEK293 cells were cotransfected with the luciferase reporters, IRF3-5D, and increasing concentrations of the IE62 plasmid. IE62 did not cause a dose-dependent inhibition of IRF3-5D-mediated activation of the ISRE-luciferase reporter, in contrast to its effect when endogenous IRF3 was activated by TBK1 (Fig. 5A). In fact, we observed a slight increase in activation by IRF3-5D in the presence of IE62. Thus, once IRF3 is phosphorylated at the C-terminal serine/threonine cluster, IE62 cannot block activation of the ISRE-luciferase reporter. Therefore, it is likely that IE62 interferes with IRF3 phosphorylation either by blocking TBK1 function or by making IRF3 unavailable as a substrate for phosphorylation by TBK1.
FIG. 5.
IE62 blocks endogenous IRF3 phosphorylation. (A) HEK293 cells were cotransfected with IRF3-5D and the luciferase reporter plasmids in the presence or absence of increasing amounts of IE62 expression plasmid. Cells were harvested in 1× passive lysis buffer, and luciferase activity was measured at 24 hpt. ISRE-luciferase activity normalized to Renilla luciferase activity was plotted and is represented on a bar graph. Error bars indicate the standard errors of the mean. (B) HEK293 cells were transfected with TBK1 alone (lanes 1 and 2), with the indicated amounts of IE62 (lanes 3 to 6) or IE63 (lanes 7 to 10), or with 2 μg of pCDNA3.1 empty vector (lanes 11 and 12). Transfected cells were lysed to extract the nuclear and cytoplasmic fractions at 48 hpt. Equivalent amounts of protein from each fraction were electrophoresed on a 10% SDS-PAGE gel and immunoblotted (WB) for endogenous IRF3 levels. A black arrow indicates the phosphorylated forms of IRF3. An asterisk indicates a nonspecific band detected in the cytoplasmic lysates with the IRF3 antibody. Fractionation was confirmed by immunoblotting for α-tubulin. (C) Immunoblot analysis of endogenous p65 in mock- and TBK1-transfected cytoplasmic (lanes C) and nuclear (lanes N) fractions. (D) Immunoblot detection of phosphorylated IRF3 in the cytoplasmic and nuclear fractions of TBK1- and TBK1-plus-IE62-transfected HEK293 cells using phosphospecific IRF3 (S396) antibody. (E) HEK293 cells transfected with 0.5 μg of Flag-TBK1 with or without increasing amounts of IE62 as indicated were lysed and immunoblotted for total endogenous IRF3 and TBK1 levels. The RDV for TBK1 expression in each lane normalized to actin is indicated below the TBK1 blot. Actin served as the loading control, while immunoblotting for IE62 showed increasing expression levels of the protein with increasing amounts of plasmid transfection. α, anti-.
To test whether IE62 might interfere with IRF3 phosphorylation, HEK293 cells were cotransfected with TBK1 and IE62. The transfected cells were processed at 48 h to prepare cytoplasmic and nuclear fractions; equivalent amounts of the cytoplasmic and nuclear fractions were separated on a 10% SDS-PAGE gel and probed using total IRF3 (Fig. 5B) and phosphospecific IRF3 (S396) (Fig. 5D) antibodies. The 52-kDa latent form of IRF3 (Fig. 5B, lanes 11 and 12), but not phosphorylated IRF3 (Fig. 3D, lanes 1 and 2), was detected both in the nucleus and in the cytoplasm in mock-transfected cells, consistent with earlier reports of IRF3 shuttling between the nucleus and cytoplasm, with nuclear export being predominant in resting cells (17). Biochemical fractionation of the cell lysates was confirmed by probing for tubulin (Fig. 5B, lower panel) and also for NF-κB-p65, a 65-kDa protein which is known to be exclusively cytoplasmic until it translocates to the nucleus upon activation (Fig. 5C), in this case with TBK1 transfection. Endogenous phosphorylated IRF3 was detected in the cytoplasmic and nuclear fractions in cells transfected with the TBK1 plasmid, as expected (Fig. 5B, lanes 1 and 2, and Fig. 5D, lanes 3 and 4). However, very low levels of phosphorylated IRF3 were detected in either the cytoplasmic or nuclear fractions in the presence of IE62 (Fig. 5B, lanes 3 to 6, and Fig. 5D, lanes 5 to 8). In contrast, IE63 coexpression did not perturb phosphorylation of IRF3 by TBK1 (Fig. 5B, lanes 7 to 10). Since IRF3 is phosphorylated in the cytoplasm before its transport into the nucleus, these experiments indicate that IE62 blocks IRF3 phosphorylation in the cytoplasm, which is known to be mediated by TBK1.
An assessment of the total IRF3 levels in the presence of IE62 at increasing concentrations showed no change with or without the addition of the proteasome inhibitor MG132 (Fig. 5E, upper panel). These data indicated that the decrease in phosphorylated IRF3 associated with IE62 expression is not due to alterations in the production or degradation of IRF3 in the presence of IE62. To determine if TBK1 expression was modulated by IE62, the TBK1 expression plasmid was cotransfected with increasing amounts of the IE62 plasmid; the transfected cells were lysed at 48 h posttransfection and immunoblotted for TBK1 (84 kDa) detection using anti-Flag antibody (Fig. 5E, second panel). A 30% decrease in total TBK1 levels was observed between the lowest and higher doses of IE62 (Fig. 5E, compare lane 2 with lanes 3 and 4), although this decrease was not overcome by the addition of MG132 (Fig. 5E, lane 5), indicating that IE62 was not likely targeting TBK1 for proteasomal degradation. At a TBK1/IE62 DNA ratio of 1:1, there was no decrease in total TBK1 levels compared to TBK1 alone (Fig. 5E, compare lane 2 with lane 1), whereas under similar conditions, IE62 was able to dramatically reduce both TBK1-mediated ISRE luciferase activation and IFN-β secretion (Fig. 3B and F). Therefore, the decrease in IRF3 phosphorylation associated with IE62 expression is not likely due to reduced expression or degradation of TBK1.
IE62 blocks sequential phosphorylation of IRF3 by TBK1.
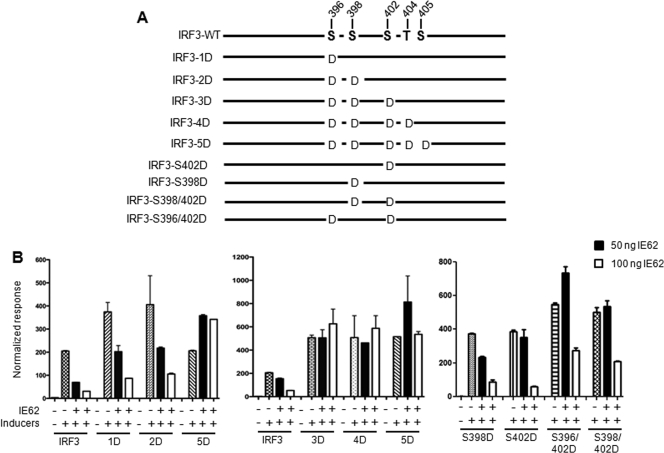
IRF3 phosphorylation has been shown to occur in incremental steps, where serine 402 (in cluster III) and serine 396 (in cluster II) are predicted to be the sites for initial phosphorylation, which is followed by phosphorylation at the remaining Ser/Thr residues (5). In order to determine the stage of phosphorylation that is blocked by IE62, we made a series of four IRF3 serine/threonine mutants (Fig. 6 A). Beginning with serine 396 within cluster II, we sequentially converted the phosphoacceptor sites to phosphomimetic aspartic acid. Some of the mutations have been characterized earlier and are known to activate the ISRE (5). Each of the IRF3 serine mutants was cotransfected with the ISRE-firefly luciferase and control Renilla luciferase reporters together with increasing concentrations of IE62 plasmid and tested at 24 h. IE62 showed a dose-dependent downregulation of the ISRE-luciferase reporter activation mediated by the IRF3-1D (S396D) and IRF3-2D (S396D and S398D) mutants, as was observed with intact IRF3 (Fig. 6B). In contrast, IE62 was unable to exert any inhibitory effect on ISRE-luciferase activation mediated by IRF3-3D (S396, S398, and S402) and IRF3-4D (S396, S398, S402, and T404) (Fig. 6B). Thus, in addition to IRF3-5D, IRF3 mutants, IRF3-3D, and IRF3-4D exhibited resistance to regulation by IE62.
FIG. 6.
IE62 blocks phosphorylation of IRF3 at serine residues at positions 396, 398, and 402. (A) Schematic representation of the IRF3 Ser/Thr mutants. (B) IRF3 and IRF3-Ser/Thr mutants (Inducers) were cotransfected with increasing amounts of IE62 plasmid in HEK293 cells together with the ISRE-firefly luciferase and Renilla luciferase reporter plasmids. Cell lysates were analyzed for luciferase activity at 24 hpt. ISRE-luciferase activity normalized to Renilla luciferase activity was plotted and is represented on a bar graph. Error bars indicate the standard errors of the mean.
Since serine 402 was the only additional mutation in IRF3-3D that was not present in IRF3-2D, we reasoned that serine 402 may be critical for the IE62-mediated block of the TBK1-IRF3 pathway. To evaluate this possibility, we made another IRF3 mutant, IRF3-S402D, in which only S402 was mutated to aspartic acid, and tested it in the ISRE-luciferase reporter assay with IE62. As shown in Fig. 6B, the mutation at serine 402 alone was insufficient to block the ability of IE62 to regulate the IRF3-mediated activation of the ISRE reporter. We next made two additional double mutants (S396D S402D [S396/402D] and S398D S402D [S398/402D]) and tested the inhibitory activity of IE62 using these partially phosphorylated IRF3 proteins. None of the mutations that altered two of the three important IRF3 serine residues to aspartic acid was able to completely reverse IE62 inhibition. These data indicated that IE62 interferes with phosphorylation of serine residues at positions 396, 398, and 402, resulting in inhibition of downstream signaling mediated by phosphorylated IRF3. Once these three serine residues are phosphorylated together, IE62 loses the ability to inhibit IRF3 function.
IE62 does not block the interaction of TBK-1 and IRF3.
A point mutation changing the TBK1 lysine residue at position 38 to alanine (TBK1-K38A) renders the protein kinase inactive; this TBK1 kinase dead (TBK1-KD) mutant does not activate the ISRE-luciferase reporter. We hypothesized that if IE62 binds TBK1 and blocks the downstream pathway, the addition of mutant TBK1-KD would function to sequester IE62 and induce partial, if not complete, reversal of IE62-mediated inhibition. We therefore cotransfected HEK293 cells with increasing concentrations of the TBK1-KD mutant with TBK1, IE62, and the luciferase reporters. ISRE-luciferase activity measured at 24 h posttransfection showed that the presence of increasing amounts of the TBK1-KD mutant had no effect on IE62-mediated inhibition of the IRF3 pathway (Fig. 7A). Although this result suggests that IE62 may not bind TBK1, it does not rule out the possibility of an IE62-TBK1 interaction that is dependent on TBK1 kinase activity. Therefore, to examine the possibility of a direct interaction between the TBK1-IRF3 complex and IE62, we cotransfected plasmids expressing the three proteins in HEK293 cells; 48 h posttransfection, cell lysates were analyzed by coIP and Western blotting. Despite using various combinations of antibodies, we were unable to detect any significant interaction between IE62 and TBK1 or between IE62 and IRF3 (data not shown).
FIG. 7.
IE62 does not block the interaction of TBK-1 and IRF3. (A) HEK293 cells were cotransfected with TBK1 and the luciferase reporters with and without increasing amounts of the TBK1-KD mutant, and luciferase activity was measured at 24 hpt. Error bars indicate the standard errors of the mean. (B) Flag-TBK1 and GFP-IRF3 expression plasmids were cotransfected in the presence or absence of IE62 expression plasmid. Transfected HEK293 cells were harvested at 48 hpt in 1× radioimmunoprecipitation assay (RIPA) lysis buffer and coimmunoprecipitated using anti-Flag antibody (Ab) (+) or no antibody (−). The coIP samples were electrophoresed on a 10% SDS-PAGE gel and immunoblotted using anti-IRF3 antibody. Preimmunoprecipation samples were immunoblotted using the indicated antibodies to determine expression levels from each expression plasmid. α, anti-.
We next investigated whether the presence of IE62 hindered the formation of the TBK1-IRF3 complex. HEK293 cells were cotransfected with Flag-TBK1 and GFP-IRF3 in the presence and absence of IE62. Cell lysates collected at 48 h posttransfection were analyzed by coIP using anti-Flag antibody and Western blotting using anti-IRF3 antibody. As shown in Fig. 7B, IE62 failed to block TBK1-IRF3 complex formation (lane 4). Furthermore, the presence of IE62 seemed to enhance the stability of the TBK1-IRF3 complex (lane 5). These results indicated that IE62 did not hinder the formation of the TBK1-IRF3 complex, but we were unable to detect the presence of IE62 in this complex. Expression levels of the each of the proteins were verified by immunoblotting with the cell lysates prior to coIP, as shown in the right panel of Fig. 7B.
IE62 interference with IRF3 phosphorylation is independent of its transactivating function.
To determine if the ability of IE62 to block IRF3 phosphorylation is dependent on its ability to transactivate VZV genes, the effects of IE62 were compared with those of IE62-K548E. The IE62 K548E mutation has been shown to inhibit activation of heterologous HSV-1 and simian virus (SV40) promoters (48) but has not been tested previously in the context of native VZV promoters. A reporter plasmid containing the VZV gE promoter driving expression of firefly luciferase was cotransfected with IE62 and IE62-K548E and tested for luciferase activity at 24 h. As shown in Fig. 8B, IE62-K548E was a poor inducer of the gE-luciferase promoter; luciferase activity was reduced by >80% compared to that of intact IE62. Nevertheless, the IE62-K548E mutant efficiently blocked TBK1-mediated ISRE-luciferase activity in a dose-dependent manner (Fig. 8C). Interestingly, IE62-K548E seemed to have a greater inhibitory effect on the TBK1-IRF3 pathway than IE62 did. The ability of IE62-K548E to downregulate ISRE activation dependent on TBK1 phosphorylation of IRF3 was confirmed by IFN-β ELISA (Fig. 8D).
FIG. 8.
IE62 interference with IRF3 phosphorylation is independent of its transactivating function. (A) Schematic representation of the point mutations introduced into full-length IE62. (B) gE-luciferase activity was measured in HEK293 cells cotransfected with gE-luciferase reporter construct, IE62, and its mutants at 24 hpt, and the normalized activity was plotted on the graph as shown. *, P value = 0.0197 (<0.05). (C) HEK293 cells were cotransfected with TBK1, ISRE-firefly luciferase, and Renilla luciferase reporters together with increasing concentrations of plasmids expressing IE62 and the IE62-K548E and -L446P point mutants. Cells were lysed in 1× passive lysis buffer, and luciferase activity was determined at 24 hpt. ISRE-luciferase activity normalized to Renilla luciferase activity was plotted and is represented in a bar graph as shown. ***, P = 0.0003 (<0.05). The efficiency of inhibition of IE62-K548E in increasing doses compared to that of IE62 and IE62-L446P is represented in the bar graph depicting fold inhibition. (D) IFN-β secretion in HEK293 cells transfected with TBK1 in the presence and absence of increasing amounts of IE62 and IE62 point mutants was measured by ELISA at 48 hpt. ***, P = 0.0003 (<0.05). Error bars indicate the standard errors of the mean.
Another IE62 mutant, IE62-L446P, was also tested for effects on the TBK1/IRF3 pathway. Reversion of this mutation has been described to occur in viruses isolated from skin rashes following immunization with Oka-derived varicella vaccines (36). We observed no effect of IE62-L446P on VZV transactivation (Fig. 8B), and an ISRE-luciferase assay with TBK1 revealed that a mutation at position 446 has no effect on IE62 inhibition of the TBK1-IRF3 pathway (Fig. 8C and D).
DISCUSSION
The recognition of molecular patterns (nucleic acids or proteins) presented by incoming pathogens triggers activation of the cytoplasmic latent transcription factors, IRF3, NF-κB, and others, which in turn induce the synthesis and secretion of many cytokines, including IFNs. Secreted IFNs establish an antiviral state not only within the infected cells but also in neighboring uninfected cells through the induction of numerous ISGs that inhibit virus replication. However, most successful viral pathogens encode anti-IFN factors that dysregulate the IFN pathway in order for the virus to replicate and spread to neighboring cells. We found that VZV, through its IE62 protein, repressed IFN-β secretion from infected fibroblasts, which is consistent with this paradigm. Inhibition of IFN-β secretion and IRF3 transcriptional activity was mediated as efficiently by inactivated VZV, demonstrating the replication-independent nature of the block. Interference was due to the ability of IE62 to intervene at the stage of sequential phosphorylation-mediated activation of IRF3 at the three discrete C-terminal serine residues of IRF3, at positions 396, 398, and 402. Once the three serine residues were phosphorylated, IE62 lost its ability to block the pathway, although IRF3-TBK1 complex formation remained unperturbed in the presence of IE62. The ability of IE62 to inhibit the IRF3-IFN-β signaling axis was independent of its function as a viral gene transactivator, indicating that these two critical functions of IE62 are distinct and mediated by different functional domains of the protein.
IE62 is an abundant virus tegument protein and is thus introduced into the host cell upon virus entry. IE62 is localized predominantly to the cell nucleus at early times postinfection and during transient transfections; IE62 export to the cytoplasm occurs later in infection as a result of ORF66-directed phosphorylation of IE62 (12). Like IE62, the HCMV major tegument protein, pp65, exhibits similar distributions to both the nucleus and the cytoplasm and pp65 alters IRF3 phosphorylation (1, 27). The precise mechanism by which pp65 blocks IRF3 phosphorylation is not known but is hypothesized to be either through IRF3 dephosphorylation within the nucleus or through blocking TBK1 in the cell cytoplasm. A direct interaction between pp65 and IRF3 was not detected. We found that IE62 inhibited TBK1-mediated accumulation of phospho-IRF3 both in the nucleus and in the cytoplasm. This observation suggested that IE62 most likely does not function by dephosphorylating IRF3 in the nucleus, as suggested for HCMV pp65, but rather through the blocking of cytoplasmic phosphorylation of IRF3 by TBK1. Therefore, it is likely that cytoplasmic IE62 is responsible for the anti-IFN function of the protein. Although other VZV proteins may modulate the induction of IFN-β by other mechanisms, the functional role of IE62 in IRF3 inhibition is likely to be independent of other viral proteins and occurs at the earliest step in the IFN pathway. In this study, we found that IE62 binding partners like IE63 and ORF9, which are also part of the virus tegument, had no significant effect on the TBK1-IFN-β axis. Interestingly, IE63 has been shown to play a role in antagonism of downstream IFN signaling by inhibiting eIF2α phosphorylation in response to IFN-α treatment (3).
The ability of UV-inactivated VZV to block ISG54 and ISG56 transcription and IFN-β secretion indicated that IE62 present as part of the “incoming” viral tegument is sufficient for exerting the IFN inhibitory property. This observation further substantiates the importance of cytoplasmic IE62 in preventing IFN-β induction in VZV-infected cells. UV-inactivated adenoviruses have similarly been shown to be poor inducers of ISG56 transcription while, in contrast, UV-inactivated HSV-1 induces ISG56 at early times postinoculation. Therefore, unlike HSV-1, the ability of VZV to disarm the IRF3-IFN-β axis does not appear to depend on de novo viral protein synthesis, although further studies are required to conclusively determine the role of tegument-associated and newly synthesized IE62 in IFN regulation. An earlier study by Jones and Arvin demonstrated that NF-κB activation cannot be repressed by UV-inactivated VZV (11) and therefore the observed block in IFN-β is likely due specifically to the nonavailability of fully phosphorylated, transcriptionally active IRF3.
As exemplified by the influenza A virus NS1 protein, viral IFN antagonists have often been associated with multiple functions. NS1 is a potent inhibitor of the RIG-I pathway of IFN induction and also of the export and processing of mRNAs of cellular genes from the nucleus (31, 43). Similarly, in addition to its IFN inhibitory function, IE62 has long been established to be a potent transactivator of VZV genes. We found that IE62-mediated transactivation and IFN inhibition were independent functions. Thus, it may be hypothesized that the nuclear and cytoplasmic IE62 proteins execute two distinct but crucial functions, namely, viral gene transcription and IFN inhibition, respectively.
Since IRF3 is a critical transcription factor in early IFN-β induction that functions to integrate signals converging from various pathogen recognition receptors, IRF3 is very commonly targeted by several viral proteins as a strategy to abrogate IFN induction and signaling. HSV-1 encodes three proteins—ICP0 (VZV ORF61), ICP34.5, and ICP27 (VZV ORF4)—that inhibit IRF3 through various mechanisms. However, ICP4, which is the IE62 protein homolog in HSV-1, has not been found to play a role in IFN inhibition. VZV ORF4 did not inhibit TBK1-mediated ISRE-luciferase activation (data not shown), but a role for the protein in events upstream of TBK1 cannot be ruled out. HSV-1 ICP0 protein impedes phospho-IRF3 function by sequestration of IRF3 and CBP/p300 to nuclear structures away from the chromatin within the nucleus, while ICP34.5 restricts IRF3 phosphorylation by directly binding to TBK1, thereby limiting IRF3 binding to its kinase (25, 49). The bovine herpesvirus ICP0 protein directs IRF3 proteasomal degradation, whereas the human herpesvirus 6 (HHV-6) immediate early protein IE1 interferes with IRF3 dimerization and binding to the IFN-β promoter sequence (10, 40). More recently, a direct and interesting correlation between IRF3 regulation and host range restriction has been described for rotaviruses (44). Since VZV is essentially a pathogen restricted to humans, it would be interesting to elucidate whether the host restriction is mediated in part by the inability of the virus and/or IE62 to block IRF3 activation in other species.
IRF3 phosphorylation at the C-terminal Ser/Thr cluster occurs in a sequential fashion with either S396, S402, or both serving as the initial target sites of TBK1 (5). Phosphorylation at these two Ser residues attributes a conformational change that leads to phosphorylation of the remaining three residues (S398, T404, and S405). We used a systematic approach to mutate each of the five phosphoacceptor sites to the phosphomimetic residue Asp either singly or in combination and found that each of the IRF3 mutants generated retained their transcriptional activity, as determined by the ISRE-luciferase reporter assay, which was consistent with earlier reports. Interestingly, mutation of IRF3 sites S396, S398, and S402 to Asp was required to overcome IE62-mediated IRF3 inhibition, but none of the IRF3 constructions with single or double mutations at these sites was as effective at downmodulating the IE62 effect. Therefore, the IE62-mediated inhibition of IRF3 transcriptional function likely involves a mechanism that is critically dependent on preserving IRF3 residues 396, 398, and 402 in a nonphosphorylated state. IE62 thereby prevents the sequential and complete TBK1-mediated phosphorylation of the IRF3 C-terminal cluster, leading to functional inhibition of IRF3. Since we could not document a direct interaction between IE62 and either TBK1 or IRF3, we determined the status of the TBK1-IRF3 complex in the presence of IE62. In contrast to our expectation, IE62 appeared to enhance the stability of the TBK1-IRF3 complex. Normally, the TBK1-IRF3 complex exists in a dynamic equilibrium wherein IRF3 molecules are recruited to activated TBK1 and turned over following pathway stimulation. Once IRF3 is completely phosphorylated, it undergoes a conformational switch and dissociates from the complex, as demonstrated by a lack of interaction between IRF3-5D and TBK1 (51), allowing other IRF3 molecules to be similarly phosphorylated by TBK1.
Based on our observations that the TBK1-IRF3 complex is preserved in the presence of IE62, we hypothesize that IRF3 molecules that are bound to activated TBK1 fail to dissociate because the process of sequential phosphorylation at the critical IRF3 residues 396, 398, and/or 402 is incomplete. Without full IRF3 phosphorylation, the conformational switch for dissociation of IRF3 from TBK1 is not triggered. This leads to intermediate pIRF3 isoforms that are transcriptionally nonfunctional and also renders activated TBK1 unavailable for phosphorylating other IRF3 molecules. This explanation is supported by the observation that despite its inability to disrupt IRF3-TBK1 complexes, expression of IE62 resulted in significantly reduced phosphorylation of IRF3 in response to TBK-1 overexpression. Thus, IE62 may create a “sink” for both IRF3 and its kinase TBK1 by blocking their dissociation, which avoids IRF3-mediated transactivation of ISGs and subsequently abrogates early type I IFN secretion in response to VZV infection (Fig. 9). The precise mechanism by which IE62 blocks IRF3 phosphorylation without disrupting bimolecular IRF3-TBK1 complexes remains to be elucidated. It is possible that IE62 interaction with either TBK1 or IRF3 or both is transient and therefore undetectable under the conditions used for coIP. Alternatively, IE62 may be acting indirectly via recruitment of other cellular proteins to such complexes.
FIG. 9.
Proposed “sink” model of IE-62-mediated IRF3-IFN-β inhibition. (Step 1) IE62, present on the viral tegument, upon entering the cell inhibits the ability of TBK1 to phosphorylate IRF3 at the C terminus. (Step 2) This leads to incomplete phosphorylation or complete lack of phosphorylation of IRF3 that is required for binding to promoter regions of IFN-β and IRF3-dependent ISGs. (Step 3) The incompletely phosphorylated IRF3 probably remains bound to TBK1, thereby making the kinase inaccessible to other IRF3 substrate molecules.
While the fact that VZV IE62 is essential has been attributed to its central role as a potent viral gene transactivator, our observations indicate that the novel and potent mechanism by which IE62 blocks IFN induction is likely to be equally important for the pathogenesis of VZV infection.
Acknowledgments
This work was supported by NIH grants AI053846 and AI20459 and National Cancer Institute grant CA49605.
We thank Adrish Sen, Department of Gastroenterology, Stanford University, for his helpful discussions and critical review of the manuscript. We also thank Tim Knaak at the Stanford Shared FACs Facility for his technical assistance.
Footnotes
Published ahead of print on 14 July 2010.
REFERENCES
- 1.Abate, D. A., S. Watanabe, and E. S. Mocarski. 2004. Major human cytomegalovirus structural protein pp65 (ppUL83) prevents interferon response factor 3 activation in the interferon response. J. Virol. 78:10995-11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abramoff, M. D., P. J. Magalhaes, and S. J. Ram. 2004. Image processing with Image J. Biophotonics International 11:36-42. [Google Scholar]
- 3.Ambagala, A. P., and J. I. Cohen. 2007. Varicella-zoster virus IE63, a major viral latency protein, is required to inhibit the alpha interferon-induced antiviral response. J. Virol. 81:7844-7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Besser, J., M. Ikoma, K. Fabel, M. H. Sommer, L. Zerboni, C. Grose, and A. M. Arvin. 2004. Differential requirement for cell fusion and virion formation in the pathogenesis of varicella-zoster virus infection in skin and T cells. J. Virol. 78:13293-13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clément, J. F., A. Bibeau-Poirier, S. P. Gravel, N. Grandvaux, E. Bonneil, P. Thibault, S. Meloche, and M. J. Servant. 2008. Phosphorylation of IRF-3 on Ser 339 generates a hyperactive form of IRF-3 through regulation of dimerization and CBP association. J. Virol. 82:3984-3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen, J. I., S. E. Straus, and A. M. Arvin. 2007. Varicella-zoster virus replication, pathogenesis, and management, p. 2773-2818. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Strause (ed.), Fields virology, 5th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 7.Fitzgerald, K. A., S. M. McWhirter, K. L. Faia, D. C. Rowe, E. Latz, D. T. Golenbock, A. J. Coyle, S. M. Liao, and T. Maniatis. 2003. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4:491-496. [DOI] [PubMed] [Google Scholar]
- 8.Häcker, H., V. Redecke, B. Blagoev, I. Kratchmarova, L. C. Hsu, G. G. Wang, M. P. Kamps, E. Raz, H. Wagner, G. Hacker, M. Mann, and M. Karin. 2006. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature 439:204-207. [DOI] [PubMed] [Google Scholar]
- 9.Inchauspe, G., S. Nagpal, and J. M. Ostrove. 1989. Mapping of two varicella-zoster virus-encoded genes that activate the expression of viral early and late genes. Virology 173:700-709. [DOI] [PubMed] [Google Scholar]
- 10.Jaworska, J., A. Gravel, K. Fink, N. Grandvaux, and L. Flamand. 2007. Inhibition of transcription of the beta interferon gene by the human herpesvirus 6 immediate-early 1 protein. J. Virol. 81:5737-5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones, J. O., and A. M. Arvin. 2006. Inhibition of the NF-kappaB pathway by varicella-zoster virus in vitro and in human epidermal cells in vivo. J. Virol. 80:5113-5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinchington, P. R., K. Fite, and S. E. Turse. 2000. Nuclear accumulation of IE62, the varicella-zoster virus (VZV) major transcriptional regulatory protein, is inhibited by phosphorylation mediated by the VZV open reading frame 66 protein kinase. J. Virol. 74:2265-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinchington, P. R., J. K. Hougland, A. M. Arvin, W. T. Ruyechan, and J. Hay. 1992. The varicella-zoster virus immediate-early protein IE62 is a major component of virus particles. J. Virol. 66:359-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koyama, S., K. J. Ishii, C. Coban, and S. Akira. 2008. Innate immune response to viral infection. Cytokine 43:336-341. [DOI] [PubMed] [Google Scholar]
- 15.Ku, C. C., J. A. Padilla, C. Grose, E. C. Butcher, and A. M. Arvin. 2002. Tropism of varicella-zoster virus for human tonsillar CD4+ T lymphocytes that express activation, memory, and skin homing markers. J. Virol. 76:11425-11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ku, C. C., L. Zerboni, H. Ito, B. S. Graham, M. Wallace, and A. M. Arvin. 2004. Varicella-zoster virus transfer to skin by T cells and modulation of viral replication by epidermal cell interferon-alpha. J. Exp. Med. 200:917-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar, K. P., K. M. McBride, B. K. Weaver, C. Dingwall, and N. C. Reich. 2000. Regulated nuclear-cytoplasmic localization of interferon regulatory factor 3, a subunit of double-stranded RNA-activated factor 1. Mol. Cell. Biol. 20:4159-4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin, R., P. Genin, Y. Mamane, M. Sgarbanti, A. Battistini, W. J. Harrington, Jr., G. N. Barber, and J. Hiscott. 2001. HHV-8 encoded vIRF-1 represses the interferon antiviral response by blocking IRF-3 recruitment of the CBP/p300 coactivators. Oncogene 20:800-811. [DOI] [PubMed] [Google Scholar]
- 19.Lin, R., C. Heylbroeck, P. M. Pitha, and J. Hiscott. 1998. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol. Cell. Biol. 18:2986-2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin, R., Y. Mamane, and J. Hiscott. 1999. Structural and functional analysis of interferon regulatory factor 3: localization of the transactivation and autoinhibitory domains. Mol. Cell. Biol. 19:2465-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin, R., R. S. Noyce, S. E. Collins, R. D. Everett, and K. L. Mossman. 2004. The herpes simplex virus ICP0 RING finger domain inhibits IRF3- and IRF7-mediated activation of interferon-stimulated genes. J. Virol. 78:1675-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McWhirter, S. M., K. A. Fitzgerald, J. Rosains, D. C. Rowe, D. T. Golenbock, and T. Maniatis. 2004. IFN-regulatory factor 3-dependent gene expression is defective in Tbk1-deficient mouse embryonic fibroblasts. Proc. Natl. Acad. Sci. U. S. A. 101:233-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melchjorsen, J., J. Siren, I. Julkunen, S. R. Paludan, and S. Matikainen. 2006. Induction of cytokine expression by herpes simplex virus in human monocyte-derived macrophages and dendritic cells is dependent on virus replication and is counteracted by ICP27 targeting NF-kappaB and IRF-3. J. Gen. Virol. 87:1099-1108. [DOI] [PubMed] [Google Scholar]
- 24.Melroe, G. T., N. A. DeLuca, and D. M. Knipe. 2004. Herpes simplex virus 1 has multiple mechanisms for blocking virus-induced interferon production. J. Virol. 78:8411-8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melroe, G. T., L. Silva, P. A. Schaffer, and D. M. Knipe. 2007. Recruitment of activated IRF-3 and CBP/p300 to herpes simplex virus ICP0 nuclear foci: potential role in blocking IFN-beta induction. Virology 360:305-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyahira, A. K., A. Shahangian, S. Hwang, R. Sun, and G. Cheng. 2009. TANK-binding kinase-1 plays an important role during in vitro and in vivo type I IFN responses to DNA virus infections. J. Immunol. 182:2248-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mocarski, E. S. J., and C. T. Courcelle. 2001. Cytomegaloviruses and their replication. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Strause (ed.), Fields virology, vol. 2, p. 2629-2673. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 28.Moffat, J. F., M. D. Stein, H. Kaneshima, and A. M. Arvin. 1995. Tropism of varicella-zoster virus for human CD4+ and CD8+ T lymphocytes and epidermal cells in SCID-hu mice. J. Virol. 69:5236-5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moriuchi, M., H. Moriuchi, S. E. Straus, and J. I. Cohen. 1994. Varicella-zoster virus (VZV) virion-associated transactivator open reading frame 62 protein enhances the infectivity of VZV DNA. Virology 200:297-300. [DOI] [PubMed] [Google Scholar]
- 30.Oganesyan, G., S. K. Saha, B. Guo, J. Q. He, A. Shahangian, B. Zarnegar, A. Perry, and G. Cheng. 2006. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature 439:208-211. [DOI] [PubMed] [Google Scholar]
- 31.Opitz, B., A. Rejaibi, B. Dauber, J. Eckhard, M. Vinzing, B. Schmeck, S. Hippenstiel, N. Suttorp, and T. Wolff. 2007. IFNbeta induction by influenza A virus is mediated by RIG-I which is regulated by the viral NS1 protein. Cell Microbiol. 9:930-938. [DOI] [PubMed] [Google Scholar]
- 32.Paladino, P., D. T. Cummings, R. S. Noyce, and K. L. Mossman. 2006. The IFN-independent response to virus particle entry provides a first line of antiviral defense that is independent of TLRs and retinoic acid-inducible gene I. J. Immunol. 177:8008-8016. [DOI] [PubMed] [Google Scholar]
- 33.Paladino, P., and K. L. Mossman. 2009. Mechanisms employed by herpes simplex virus 1 to inhibit the interferon response. J. Interferon Cytokine Res. 29:599-607. [DOI] [PubMed] [Google Scholar]
- 34.Perera, L. P., J. D. Mosca, W. T. Ruyechan, G. S. Hayward, S. E. Straus, and J. Hay. 1993. A major transactivator of varicella-zoster virus, the immediate-early protein IE62, contains a potent N-terminal activation domain. J. Virol. 67:4474-4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qin, B. Y., C. Liu, S. S. Lam, H. Srinath, R. Delston, J. J. Correia, R. Derynck, and K. Lin. 2003. Crystal structure of IRF-3 reveals mechanism of autoinhibition and virus-induced phosphoactivation. Nat. Struct. Biol. 10:913-921. [DOI] [PubMed] [Google Scholar]
- 36.Quinlivan, M. L., A. A. Gershon, M. M. Al Bassam, S. P. Steinberg, P. LaRussa, R. A. Nichols, and J. Breuer. 2007. Natural selection for rash-forming genotypes of the varicella-zoster vaccine virus detected within immunized human hosts. Proc. Natl. Acad. Sci. U. S. A. 104:208-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Randall, R. E., and S. Goodbourn. 2008. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 89:1-47. [DOI] [PubMed] [Google Scholar]
- 38.Reichelt, M., J. Brady, and A. M. Arvin. 2009. The replication cycle of varicella-zoster virus: analysis of the kinetics of viral protein expression, genome synthesis, and virion assembly at the single-cell level. J. Virol. 83:3904-3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruyechan, W., P. Ling, P. Kinchington, and J. Hay. 1991. The correlation between varicella zoster virus transcription and the sequence of the viral genome, p. 301-318. In E. K. Wagner (ed.), Herpesvirus transcription and its regulation. CRC Press, Boca Raton, FL.
- 40.Saira, K., Y. Zhou, and C. Jones. 2007. The infected cell protein 0 encoded by bovine herpesvirus 1 (bICP0) induces degradation of interferon response factor 3 and, consequently, inhibits beta interferon promoter activity. J. Virol. 81:3077-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarkar, S. N., and G. C. Sen. 2004. Novel functions of proteins encoded by viral stress-inducible genes. Pharmacol. Ther. 103:245-259. [DOI] [PubMed] [Google Scholar]
- 42.Sato, M., H. Suemori, N. Hata, M. Asagiri, K. Ogasawara, K. Nakao, T. Nakaya, M. Katsuki, S. Noguchi, N. Tanaka, and T. Taniguchi. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 13:539-548. [DOI] [PubMed] [Google Scholar]
- 43.Satterly, N., P. L. Tsai, J. van Deursen, D. R. Nussenzveig, Y. Wang, P. A. Faria, A. Levay, D. E. Levy, and B. M. Fontoura. 2007. Influenza virus targets the mRNA export machinery and the nuclear pore complex. Proc. Natl. Acad. Sci. U. S. A. 104:1853-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sen, A., N. Feng, K. Ettayebi, M. E. Hardy, and H. B. Greenberg. 2009. IRF3 inhibition by rotavirus NSP1 is host cell and virus strain dependent but independent of NSP1 proteasomal degradation. J. Virol. 83:10322-10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sen, R., and D. Baltimore. 1986. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell 47:921-928. [DOI] [PubMed] [Google Scholar]
- 46.Sharma, S., B. R. tenOever, N. Grandvaux, G. P. Zhou, R. Lin, and J. Hiscott. 2003. Triggering the interferon antiviral response through an IKK-related pathway. Science 300:1148-1151. [DOI] [PubMed] [Google Scholar]
- 47.Stetson, D. B., and R. Medzhitov. 2006. Type I interferons in host defense. Immunity 25:373-381. [DOI] [PubMed] [Google Scholar]
- 48.Tyler, J. K., and R. D. Everett. 1994. The DNA binding domains of the varicella-zoster virus gene 62 and herpes simplex virus type 1 ICP4 transactivator proteins heterodimerize and bind to DNA. Nucleic Acids Res. 22:711-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verpooten, D., Y. Ma, S. Hou, Z. Yan, and B. He. 2009. Control of TANK-binding kinase 1-mediated signaling by the gamma(1)34.5 protein of herpes simplex virus 1. J. Biol. Chem. 284:1097-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu, S., P. Xie, K. Welsh, C. Li, C. Z. Ni, X. Zhu, J. C. Reed, A. C. Satterthwait, G. A. Bishop, and K. R. Ely. 2005. LMP1 protein from the Epstein-Barr virus is a structural CD40 decoy in B lymphocytes for binding to TRAF3. J. Biol. Chem. 280:33620-33626. [DOI] [PubMed] [Google Scholar]
- 51.Yang, K., H. Shi, R. Qi, S. Sun, Y. Tang, B. Zhang, and C. Wang. 2006. Hsp90 regulates activation of interferon regulatory factor 3 and TBK-1 stabilization in Sendai virus-infected cells. Mol. Biol. Cell 17:1461-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang, M., H. Peng, J. Hay, and W. T. Ruyechan. 2006. Promoter activation by the varicella-zoster virus major transactivator IE62 and the cellular transcription factor USF. J. Virol. 80:7339-7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Young, L. S., A. G. Eliopoulos, N. J. Gallagher, and C. W. Dawson. 1998. CD40 and epithelial cells: across the great divide. Immunol. Today 19:502-506. [DOI] [PubMed] [Google Scholar]
- 54.Zerboni, L., C. C. Ku, C. D. Jones, J. L. Zehnder, and A. M. Arvin. 2005. Varicella-zoster virus infection of human dorsal root ganglia in vivo. Proc. Natl. Acad. Sci. U. S. A. 102:6490-6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zerboni, L., M. Sommer, C. F. Ware, and A. M. Arvin. 2000. Varicella-zoster virus infection of a human CD4-positive T-cell line. Virology 270:278-285. [DOI] [PubMed] [Google Scholar]