Abstract
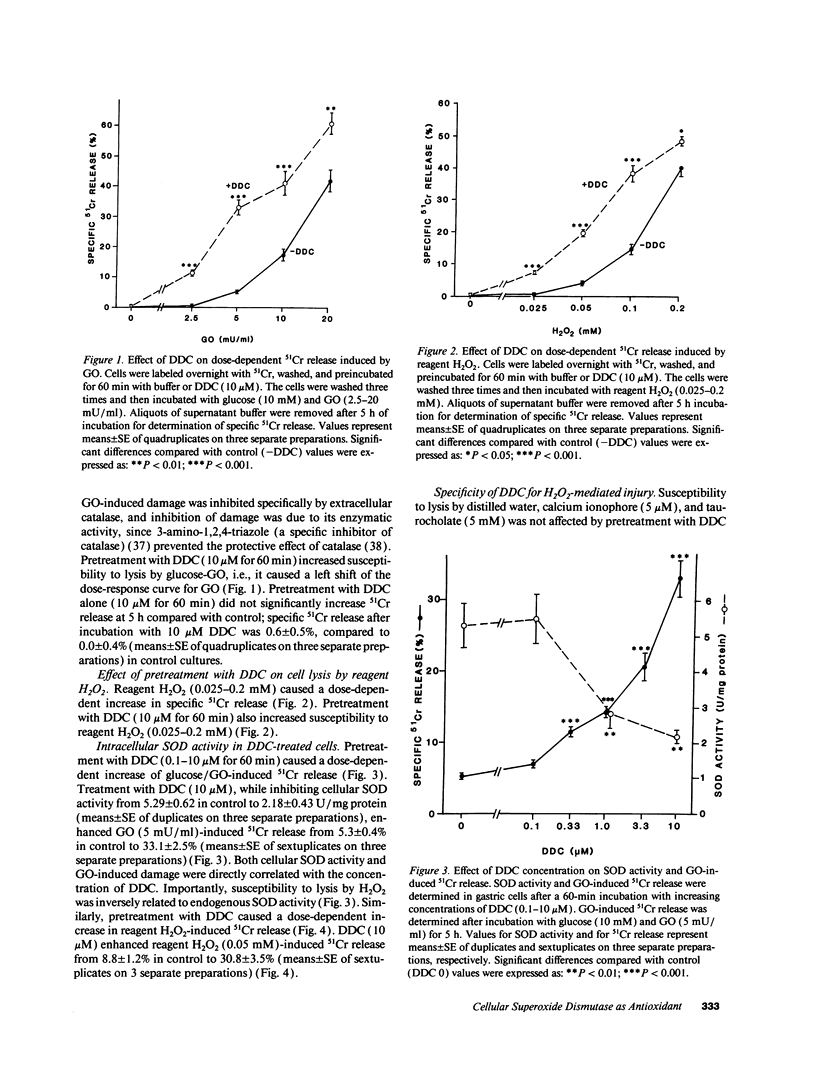
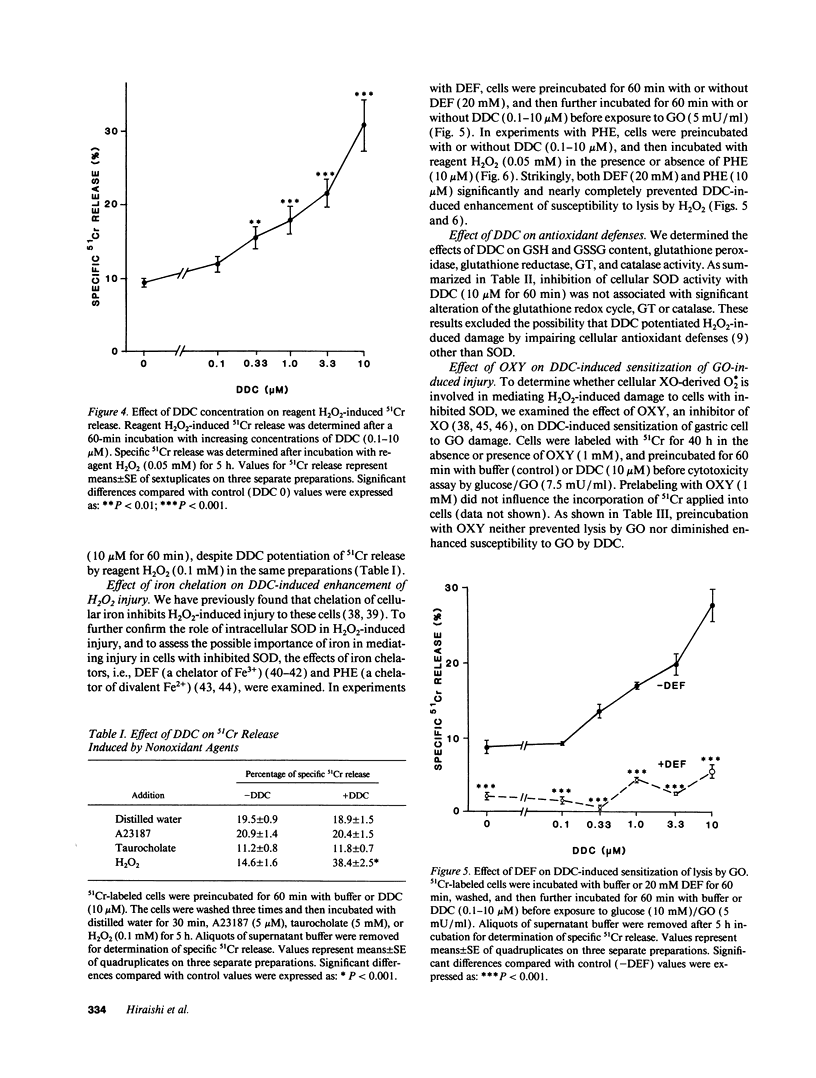
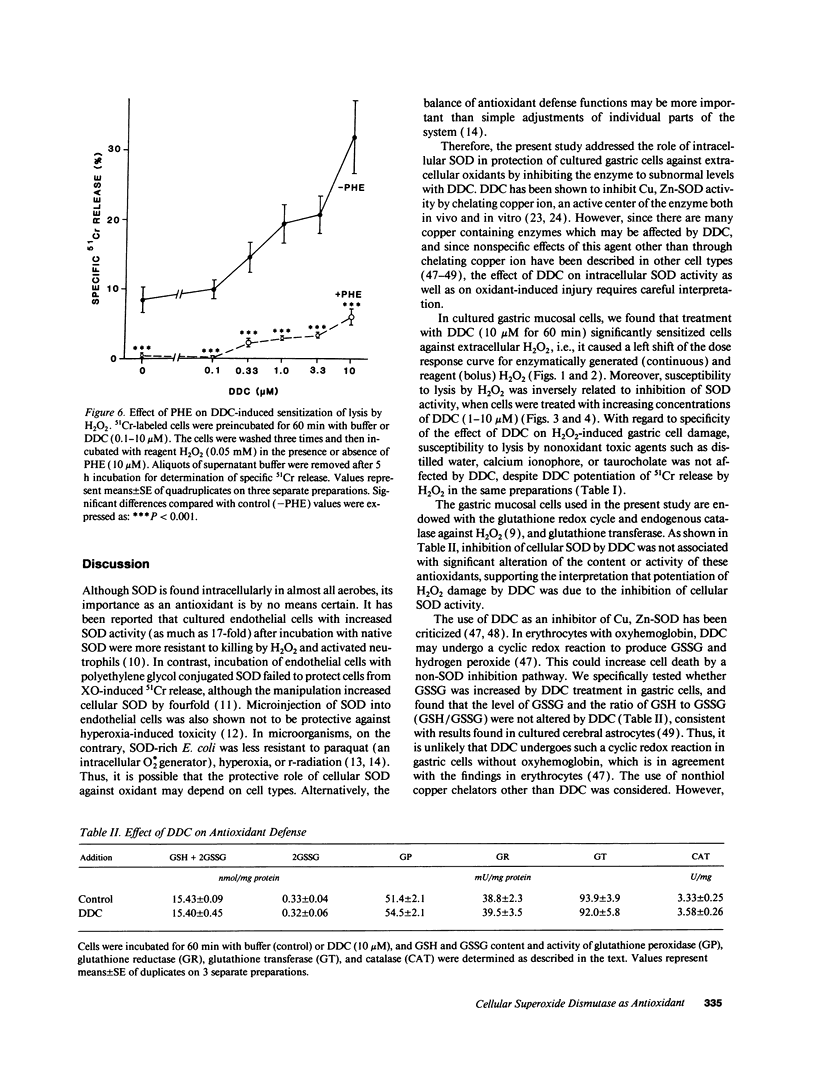
We examined the role of intracellular superoxide dismutase (SOD) as an antioxidant by studying the effect of diethyldithiocarbamate (DDC) on extracellular H2O2-induced damage in cultured rat gastric mucosal cells. 51Cr-labeled monolayers from rat stomachs were exposed to glucose oxidase-generated H2O2 or reagent H2O2, which both caused a dose-dependent increase in 51Cr release. DDC dose-dependently enhanced 51Cr release by hydrogen peroxide, corresponding with inhibition of endogenous SOD activity. This inhibition was not associated either with modulation of other antioxidant defenses, or with potentiation of injury by nonoxidant toxic agents. Enhanced hydrogen peroxide damage by DDC was significantly prevented by chelating cellular iron with deferoxamine or phenanthroline. Inhibition of cellular xanthine oxidase (possible source of superoxide production) by oxypurinol neither prevented lysis by hydrogen peroxide nor diminished DDC-induced sensitization to H2O2. We conclude that (a) extracellular H2O2 induces dose dependent damage to cultured gastric mucosal cells; (b) intracellular SOD plays an important role in preventing H2O2 damage; (c) generation of superoxide seems to occur intracellularly after exposure to H2O2, but independent of cellular xanthine oxidase; and (d) cellular iron mediates the damage by catalyzing the production of more reactive species from superoxide and H2O2, the process which causes ultimate cell injury.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerboom T. P., Sies H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981;77:373–382. doi: 10.1016/s0076-6879(81)77050-2. [DOI] [PubMed] [Google Scholar]
- Andreoli S. P., Liechty E. A., Mallett C. Exogenous adenine nucleotides replete endothelial cell adenosine triphosphate after oxidant injury by adenosine uptake. J Lab Clin Med. 1990 Mar;115(3):304–313. [PubMed] [Google Scholar]
- Bacon B. R., Tavill A. S. Role of the liver in normal iron metabolism. Semin Liver Dis. 1984 Aug;4(3):181–192. doi: 10.1055/s-2008-1041769. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Minor R. L., Jr, White C. W., Repine J. E., Rosen G. M., Freeman B. A. Superoxide dismutase and catalase conjugated to polyethylene glycol increases endothelial enzyme activity and oxidant resistance. J Biol Chem. 1988 May 15;263(14):6884–6892. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Iwami Y., Yamada T. Hydrogen peroxide excretion by oral streptococci and effect of lactoperoxidase-thiocyanate-hydrogen peroxide. Infect Immun. 1983 Apr;40(1):70–80. doi: 10.1128/iai.40.1.70-80.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers D. E., Parks D. A., Patterson G., Roy R., McCord J. M., Yoshida S., Parmley L. F., Downey J. M. Xanthine oxidase as a source of free radical damage in myocardial ischemia. J Mol Cell Cardiol. 1985 Feb;17(2):145–152. doi: 10.1016/s0022-2828(85)80017-1. [DOI] [PubMed] [Google Scholar]
- Cross C. E., Halliwell B., Allen A. Antioxidant protection: a function of tracheobronchial and gastrointestinal mucus. Lancet. 1984 Jun 16;1(8390):1328–1330. doi: 10.1016/s0140-6736(84)91822-1. [DOI] [PubMed] [Google Scholar]
- Flohé L., Otting F. Superoxide dismutase assays. Methods Enzymol. 1984;105:93–104. doi: 10.1016/s0076-6879(84)05013-8. [DOI] [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Biology of disease: free radicals and tissue injury. Lab Invest. 1982 Nov;47(5):412–426. [PubMed] [Google Scholar]
- Freeman B. A., Young S. L., Crapo J. D. Liposome-mediated augmentation of superoxide dismutase in endothelial cells prevents oxygen injury. J Biol Chem. 1983 Oct 25;258(20):12534–12542. [PubMed] [Google Scholar]
- Fuchs H. J., Borders C. L., Jr Affinity inactivation of bovine Cu,Zn superoxide dismutase by hydroperoxide anion, HO2-. Biochem Biophys Res Commun. 1983 Nov 15;116(3):1107–1113. doi: 10.1016/s0006-291x(83)80256-3. [DOI] [PubMed] [Google Scholar]
- Gong D. H., Turner B., Bhaskar K. R., Lamont J. T. Lipid binding to gastric mucin: protective effect against oxygen radicals. Am J Physiol. 1990 Oct;259(4 Pt 1):G681–G686. doi: 10.1152/ajpgi.1990.259.4.G681. [DOI] [PubMed] [Google Scholar]
- Granger D. N., Rutili G., McCord J. M. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981 Jul;81(1):22–29. [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Grisham M. B., Hernandez L. A., Granger D. N. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol. 1986 Oct;251(4 Pt 1):G567–G574. doi: 10.1152/ajpgi.1986.251.4.G567. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Maidt L., Poyer L. Superoxide dismutase and Fenton chemistry. Reaction of ferric-EDTA complex and ferric-bipyridyl complex with hydrogen peroxide without the apparent formation of iron(II). Biochem J. 1990 Jul 1;269(1):169–174. doi: 10.1042/bj2690169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986 May 1;246(2):501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. The importance of free radicals and catalytic metal ions in human diseases. Mol Aspects Med. 1985;8(2):89–193. doi: 10.1016/0098-2997(85)90001-9. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Protection against tissue damage in vivo by desferrioxamine: what is its mechanism of action? Free Radic Biol Med. 1989;7(6):645–651. doi: 10.1016/0891-5849(89)90145-7. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts is a feasible source of hydroxy radicals in vivo. Biochem J. 1982 Aug 1;205(2):461–463. doi: 10.1042/bj2050461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M. Biosynthesis and regulation of superoxide dismutases. Free Radic Biol Med. 1988;5(5-6):377–385. doi: 10.1016/0891-5849(88)90111-6. [DOI] [PubMed] [Google Scholar]
- Heikkila R. E., Cabbat F. S., Cohen G. In vivo inhibition of superoxide dismutase in mice by diethyldithiocarbamate. J Biol Chem. 1976 Apr 10;251(7):2182–2185. [PubMed] [Google Scholar]
- Hiraishi H., Terano A., Ota S., Ivey K. J., Sugimoto T. Oxygen metabolite-induced cytotoxicity to cultured rat gastric mucosal cells. Am J Physiol. 1987 Jul;253(1 Pt 1):G40–G48. doi: 10.1152/ajpgi.1987.253.1.G40. [DOI] [PubMed] [Google Scholar]
- Hiraishi H., Terano A., Ota S., Ivey K. J., Sugimoto T. Regulation of prostaglandin production in cultured gastric mucosal cells. Prostaglandins. 1989 Jul;38(1):65–78. doi: 10.1016/0090-6980(89)90017-8. [DOI] [PubMed] [Google Scholar]
- Hiraishi H., Terano A., Ota S., Mutoh H., Razandi M., Sugimoto T., Ivey K. J. Role for iron in reactive oxygen species-mediated cytotoxicity to cultured rat gastric mucosal cells. Am J Physiol. 1991 Apr;260(4 Pt 1):G556–G563. doi: 10.1152/ajpgi.1991.260.4.G556. [DOI] [PubMed] [Google Scholar]
- Hiraishi H., Terano A., Ota S., Mutoh H., Sugimoto T., Razandi M., Ivey K. J. Antioxidant defenses of cultured gastric cells against oxygen metabolites: role of GSH redox cycle and endogenous catalase. Am J Physiol. 1991 Dec;261(6 Pt 1):G921–G928. doi: 10.1152/ajpgi.1991.261.6.G921. [DOI] [PubMed] [Google Scholar]
- Hiraishi H., Terano A., Razandi M., Sugimoto T., Harada T., Ivey K. J. Role of iron and superoxide in mediating hydrogen peroxide injury to cultured rat gastric cells. Gastroenterology. 1993 Mar;104(3):780–788. doi: 10.1016/0016-5085(93)91013-8. [DOI] [PubMed] [Google Scholar]
- Homan-Müller J. W., Weening R. S., Roos D. Production of hydrogen peroxide by phagocytizing human granulocytes. J Lab Clin Med. 1975 Feb;85(2):198–207. [PubMed] [Google Scholar]
- Itoh M., Guth P. H. Role of oxygen-derived free radicals in hemorrhagic shock-induced gastric lesions in the rat. Gastroenterology. 1985 May;88(5 Pt 1):1162–1167. doi: 10.1016/s0016-5085(85)80075-5. [DOI] [PubMed] [Google Scholar]
- KEBERLE H. THE BIOCHEMISTRY OF DESFERRIOXAMINE AND ITS RELATION TO IRON METABOLISM. Ann N Y Acad Sci. 1964 Oct 7;119:758–768. doi: 10.1111/j.1749-6632.1965.tb54077.x. [DOI] [PubMed] [Google Scholar]
- Kasai H., Nishimura S. Hydroxylation of deoxyguanosine at the C-8 position by ascorbic acid and other reducing agents. Nucleic Acids Res. 1984 Feb 24;12(4):2137–2145. doi: 10.1093/nar/12.4.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelner M. J., Alexander N. M. Inhibition of erythrocyte superoxide dismutase by diethyldithiocarbamate also results in oxyhemoglobin-catalyzed glutathione depletion and methemoglobin production. J Biol Chem. 1986 Feb 5;261(4):1636–1641. [PubMed] [Google Scholar]
- Kelner M. J., Bagnell R., Hale B., Alexander N. M. Inactivation of intracellular copper-zinc superoxide dismutase by copper chelating agents without glutathione depletion and methemoglobin formation. Free Radic Biol Med. 1989;6(4):355–360. doi: 10.1016/0891-5849(89)90079-8. [DOI] [PubMed] [Google Scholar]
- Kyle M. E., Nakae D., Sakaida I., Miccadei S., Farber J. L. Endocytosis of superoxide dismutase is required in order for the enzyme to protect hepatocytes from the cytotoxicity of hydrogen peroxide. J Biol Chem. 1988 Mar 15;263(8):3784–3789. [PubMed] [Google Scholar]
- MARGOLIASH E., NOVOGRODSKY A. A study of the inhibition of catalase by 3-amino-1:2:4:-triazole. Biochem J. 1958 Mar;68(3):468–475. doi: 10.1042/bj0680468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B., Guthenberg C. Glutathione transferase (human placenta). Methods Enzymol. 1981;77:231–235. doi: 10.1016/s0076-6879(81)77030-7. [DOI] [PubMed] [Google Scholar]
- Markey B. A., Phan S. H., Varani J., Ryan U. S., Ward P. A. Inhibition of cytotoxicity by intracellular superoxide dismutase supplementation. Free Radic Biol Med. 1990;9(4):307–314. doi: 10.1016/0891-5849(90)90005-4. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968 Nov 10;243(21):5753–5760. [PubMed] [Google Scholar]
- McCord J. M., Roy R. S. The pathophysiology of superoxide: roles in inflammation and ischemia. Can J Physiol Pharmacol. 1982 Nov;60(11):1346–1352. doi: 10.1139/y82-201. [DOI] [PubMed] [Google Scholar]
- Michiels C., Toussaint O., Remacle J. Comparative study of oxygen toxicity in human fibroblasts and endothelial cells. J Cell Physiol. 1990 Aug;144(2):295–302. doi: 10.1002/jcp.1041440216. [DOI] [PubMed] [Google Scholar]
- Minotti G., Aust S. D. The requirement for iron (III) in the initiation of lipid peroxidation by iron (II) and hydrogen peroxide. J Biol Chem. 1987 Jan 25;262(3):1098–1104. [PubMed] [Google Scholar]
- Nakayama T., Kodama M., Nagata C. Generation of hydrogen peroxide and superoxide anion radical from cigarette smoke. Gan. 1984 Feb;75(2):95–98. [PubMed] [Google Scholar]
- Nayfield S. G., Kent T. H., Rodman N. F. Gastrointestinal effects of acute ferrous sulfate poisoning in rats. Arch Pathol Lab Med. 1976 Jun;100(6):325–328. [PubMed] [Google Scholar]
- Nunez M. T., Cole E. S., Glass J. The reticulocyte plasma membrane pathway of iron uptake as determined by the mechanism of alpha, alpha'-dipyridyl inhibition. J Biol Chem. 1983 Jan 25;258(2):1146–1151. [PubMed] [Google Scholar]
- Ota S., Razandi M., Sekhon S., Krause W. J., Terano A., Hiraishi H., Ivey K. J. Salicylate effects on a monolayer culture of gastric mucous cells from adult rats. Gut. 1988 Dec;29(12):1705–1714. doi: 10.1136/gut.29.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks D. A., Granger D. N. Xanthine oxidase: biochemistry, distribution and physiology. Acta Physiol Scand Suppl. 1986;548:87–99. [PubMed] [Google Scholar]
- Scott M. D., Meshnick S. R., Eaton J. W. Superoxide dismutase amplifies organismal sensitivity to ionizing radiation. J Biol Chem. 1989 Feb 15;264(5):2498–2501. [PubMed] [Google Scholar]
- Scott M. D., Meshnick S. R., Eaton J. W. Superoxide dismutase-rich bacteria. Paradoxical increase in oxidant toxicity. J Biol Chem. 1987 Mar 15;262(8):3640–3645. [PubMed] [Google Scholar]
- Smith S. M., Grisham M. B., Manci E. A., Granger D. N., Kvietys P. R. Gastric mucosal injury in the rat. Role of iron and xanthine oxidase. Gastroenterology. 1987 Apr;92(4):950–956. doi: 10.1016/0016-5085(87)90969-3. [DOI] [PubMed] [Google Scholar]
- Smith S. M., Holm-Rutili L., Perry M. A., Grisham M. B., Arfors K. E., Granger D. N., Kvietys P. R. Role of neutrophils in hemorrhagic shock-induced gastric mucosal injury in the rat. Gastroenterology. 1987 Sep;93(3):466–471. doi: 10.1016/0016-5085(87)90907-3. [DOI] [PubMed] [Google Scholar]
- Spragg R. G., Hinshaw D. B., Hyslop P. A., Schraufstätter I. U., Cochrane C. G. Alterations in adenosine triphosphate and energy charge in cultured endothelial and P388D1 cells after oxidant injury. J Clin Invest. 1985 Oct;76(4):1471–1476. doi: 10.1172/JCI112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke P. E., Farber J. L. Ferric iron and superoxide ions are required for the killing of cultured hepatocytes by hydrogen peroxide. Evidence for the participation of hydroxyl radicals formed by an iron-catalyzed Haber-Weiss reaction. J Biol Chem. 1985 Aug 25;260(18):10099–10104. [PubMed] [Google Scholar]
- Sutton H. C., Winterbourn C. C. On the participation of higher oxidation states of iron and copper in Fenton reactions. Free Radic Biol Med. 1989;6(1):53–60. doi: 10.1016/0891-5849(89)90160-3. [DOI] [PubMed] [Google Scholar]
- Takahashi M. A., Asada K. Superoxide anion permeability of phospholipid membranes and chloroplast thylakoids. Arch Biochem Biophys. 1983 Oct 15;226(2):558–566. doi: 10.1016/0003-9861(83)90325-9. [DOI] [PubMed] [Google Scholar]
- Taylor A. E., Martin D. Oxygen radicals and the microcirculation. Physiologist. 1983 Jun;26(3):152–155. [PubMed] [Google Scholar]
- Terano A., Ivey K. J., Stachura J., Sekhon S., Hosojima H., McKenzie W. N., Jr, Krause W. J., Wyche J. H. Cell culture of rat gastric fundic mucosa. Gastroenterology. 1982 Dec;83(6):1280–1291. [PubMed] [Google Scholar]
- Thomas E. L., Bates K. P., Jefferson M. M. Hypothiocyanite ion: detection of the antimicrobial agent in human saliva. J Dent Res. 1980 Sep;59(9):1466–1472. doi: 10.1177/00220345800590090201. [DOI] [PubMed] [Google Scholar]
- Thurman R. G., Ley H. G., Scholz R. Hepatic microsomal ethanol oxidation. Hydrogen peroxide formation and the role of catalase. Eur J Biochem. 1972 Feb;25(3):420–430. doi: 10.1111/j.1432-1033.1972.tb01711.x. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Trombetta L. D., Toulon M., Jamall I. S. Protective effects of glutathione on diethyldithiocarbamate (DDC) cytotoxicity: a possible mechanism. Toxicol Appl Pharmacol. 1988 Mar 30;93(1):154–164. doi: 10.1016/0041-008x(88)90035-x. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C. Comparison of superoxide with other reducing agents in the biological production of hydroxyl radicals. Biochem J. 1979 Aug 15;182(2):625–628. doi: 10.1042/bj1820625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steveninck J., van der Zee J., Dubbelman T. M. Site-specific and bulk-phase generation of hydroxyl radicals in the presence of cupric ions and thiol compounds. Biochem J. 1985 Nov 15;232(1):309–311. doi: 10.1042/bj2320309. [DOI] [PMC free article] [PubMed] [Google Scholar]


