Abstract
The initiation of the immune response at the cellular level relies on specific recognition molecules to rapidly signal viral infection via interferon (IFN) regulatory factor 3 (IRF-3)-dependent pathways. The absence of IRF-3 would be expected to render such pathways inoperative and thereby significantly affect viral infection. Unexpectedly, a previous study found no significant change in herpes simplex virus (HSV) pathogenesis in IRF-3−/− mice following intravenous HSV type 1 (HSV-1) challenge (K. Honda, H. Yanai, H. Negishi, M. Asagiri, M. Sato, T. Mizutani, N. Shimada, Y. Ohba, A. Takaoka, N. Yoshida, and T. Taniguchi, Nature 434:772-777, 2005). In contrast, the present study demonstrated that IRF-3−/− mice are significantly more susceptible to HSV infection via the corneal and intracranial routes. Following corneal infection with 2 × 106 PFU of HSV-1 strain McKrae, 50% of wild-type mice survived, compared to 10% of IRF-3-deficient mice. Significantly increased viral replication and inflammatory cytokine production were observed in brain tissues of IRF-3−/− mice compared to control mice, with a concomitant deficit in production of both IFN-β and IFN-α. These data demonstrate a critical role for IRF-3 in control of central nervous system infection following HSV-1 challenge. Furthermore, this work underscores the necessity to evaluate multiple routes of infection and animal models in order to fully determine the role of host resistance factors in pathogenesis.
Herpes simplex virus type I (HSV-1) is a ubiquitous pathogen of the Alphaherpesvirus family with high seroprevalence in the adult human population (70). Possessing two distinct phases, HSV-1 causes a life-long infection, with an initial lytic stage followed by a shift to latency following trafficking to sensory neurons (68). Periodically, reactivation from latency occurs and is associated with numerous diseases, ranging from the common cold sore to ocular herpetic stromal keratitis (HSK), a leading cause of infectious blindness (28, 52). Reactivation events as well as primary infections are also associated with herpes simplex encephalitis (HSE), a rare but life-threatening consequence of infection of the central nervous system (CNS) (67). Through recurrent infection in adults or maternal transmission to neonates, HSV-1 infects the brain and causes acute inflammation and significant pathological damage, resulting in nearly 70% lethality if untreated (30, 67). In developed countries, HSV remains among the most common causes of viral encephalitis (64).
Studies in mouse models and clinical studies have underscored the importance of the immune response, especially type I interferon (IFN), in protection of the host from encephalitis (10, 14, 18, 25, 29, 64, 74). In response to viral infection, type I IFN initiates a signaling cascade to stimulate the immune system and provide a first-line defense against invading pathogens (54). Consisting of IFN-β and several forms of IFN-α, newly synthesized type I IFN binds a receptor (IFNAR) and signals via the JAK/STAT pathway to induce an antiviral state through production of numerous interferon-stimulated genes (ISGs) (22, 26, 57). In the absence of type I IFN signaling, mice are very susceptible to disseminated peripheral HSV-1 infection, leading to increased viral replication and increased mortality in vivo (32, 34, 50-51).
In the CNS, type I IFN plays a critical role in control of viral infection. While peripheral tissues rely on plasmacytoid dendritic cells (pDCs) as the major IFN-producing cells, the brain is largely devoid of this cell type (2, 3, 58). Instead, the CNS relies on resident cells, including neurons, to produce and respond to type I IFN (13). In the absence of type I IFN receptors, mice are very susceptible to encephalitis caused by a variety of viral pathogens (12, 18, 25). Mice and humans with defects in type I IFN signaling were also found to be more susceptible to HSE than control groups (16). Together, these studies signal the importance of IFN signaling following CNS infection. Recent studies, however, have focused on the importance of type I IFN induction in limiting viral encephalitis. In particular, inborn disorders of IFN production, as well as Toll-like receptor 3 (TLR-3) mutations, render individuals highly susceptible to HSE (7, 74). These data suggest that recognition pathways producing type I IFN in the CNS are as important as downstream IFN signaling in controlling virus-induced encephalitis.
Work on pathogen-associated molecular patterns (PAMPs) has revealed two major recognition pathways that lead to type I IFN production (4). The toll-like receptor (TLR) pathways sample the extracellular milieu via receptors on the cell surface and within endosomes (15, 21, 41). In contrast, the RIG-I like receptor (RLR) pathways utilize a variety of sensors to recognize nucleic acid PAMPs within the cytosol of infected cells (20, 62, 72). Each pathway utilizes a variety of adaptors and signaling molecules to induce type I IFN production (27, 63, 71), yet both pathways converge onto three common signaling molecules: interferon regulatory factor 3 (IRF-3), IRF-7, and NF-κB (56). Following activation via the upstream recognition pathways, these signaling components bind the IFN-β promoter to form the “IFN enhancesome” (73). The IFN-β initially produced acts upon the IFN-αβ receptor (IFNAR) in both autocrine and paracrine manners. This leads to the induction of ISGs and the type I IFN cascade.
While NF-κB is activated via independent adaptors, IRF-3 and IRF-7 were initially thought to be interchangeable (56). The formation of IRF-3/IRF-7 homodimers or heterodimers was necessary for binding specific regions of the IFN-β promoter and production of type I IFN (22, 23). Examination of cells and animals deficient in IRF-3 or IRF-7, however, revealed distinct roles for the two signaling components. In the absence of IRF-3, mice challenged with HSV-1 showed reduced serum IFN-β production but unchanged IFN-α levels, and the mice survived intravenous challenge (24). In contrast, IRF-7 deficiency resulted in reduced serum IFN-α levels and a corresponding increase in mortality following HSV-1 intravenous infection. Therefore, IRF-7 was believed to compensate for the loss of IRF-3 and was dubbed “the master regulator” of type I IFN-dependent immune responses (24). This study, however, also indicated that the impact of IRF-3 and IRF-7 on HSV-1 replication may not be so clear. For example, replication of HSV-1 in IRF-3- or IRF-7-deficient mouse fibroblasts was unaffected relative to that in wild-type (WT) cells (24). Several studies have postulated that this may be due to tight control of IRF-3 activation by HSV during infection so that IRF-3-deficiency has little effect. For example, in the absence of viral gene expression, UV-inactivated HSV-1 induces IRF-3 activation and IFN induction to a greater extent than live virus (9, 33, 49). Viral genes, including those for ICP0, virion host shutoff protein, ICP34.5, and ICP27, have all been implicated in directly or indirectly targeting IRF-3 (17, 33, 42-44, 47, 48, 60, 65).
Recent studies from this laboratory demonstrated a significant increase in viral replication in immune cells in the absence of IRF-3 (45). The loss of IRF-3 resulted in increased viral replication in bone marrow-derived dendritic cells (BMDCs) and macrophages due to delayed and deficient type I IFN production. In the current study, the role of IRF-3 in vivo was examined. Utilizing two routes of infection, via the cornea and through direct intracranial (i.c.) inoculation, several aspects of HSV-1 infection were evaluated, including viral replication, viral tropism, lethality, and cytokine production. The study confirmed previous results showing no significant impact of IRF-3 on replication in peripheral tissues (24). In contrast to previous studies, loss of IRF-3 had a significant impact on viral replication, lethality, and cytokine production in the brain following both corneal and intracranial routes of infection. Together, the results demonstrate that IRF-3 is a pivotal determinant of the immune response and contributes to the outcome of HSV infection of the central nervous system.
MATERIALS AND METHODS
Cells, virus, and mice.
Vero cells were used for production and determination of viral stock titers as previously described (53). The HSV-1 wild-type strains were strain 17 (HSV-1 17) and strain McKrae (HSV-1 McKrae) (6, 69). Mock-treated animals were inoculated with uninfected Vero cell lysates prepared in parallel to viral stocks. The mouse strains used were control C57B6 mice as WT mice and C57B6 IRF-3-deficient (IRF-3−/−) mice (56) of either gender. Mice were housed in the Washington University School of Medicine barrier facility and infected in the Washington University School of Medicine biohazard facility. Mice were infected at between 6 and 8 weeks of age. Mice were euthanized, if necessary, in accordance with Federal and University policies.
Animal infection procedures.
For corneal infection, mice were anesthetized intraperitoneally with ketamine (87 mg/kg of body weight) and xylazine (13 mg/kg). Corneas were bilaterally scarified with a 25-gauge syringe needle, and virus was inoculated by adding 2 × 106 PFU HSV-1 per eye in a volume of 5 μl. Mice were sacrificed at specified times postinfection for tissue harvest or observed daily for 21 days to evaluate survival.
For intracranial (i.c.) infections, mice were anesthetized as described above, injected intracranially with 100 PFU or 1 × 106 PFU of HSV 17, or mock infected in a volume of 20 μl Dulbecco modified Eagle medium (DMEM) using a Hamilton syringe with a 26-gauge needle. Mice were sacrificed at specific times postinfection for tissue harvest or observed until day 21 postinfection to evaluate survival.
Tissue titers.
Following in vivo cornea infection, the following tissues were harvested and titers determined as previously described (53): corneal swabs, periocular skin, trigeminal ganglia, brain, and brain stem. Briefly, tissues were harvested and stored at −80°C until processing. Tissues were mechanically disrupted and sonicated, and titers were determined via standard plaque assay on Vero cells.
Histological analysis.
WT and IRF-3−/− mice were infected and harvested at days 3 and 5 postinfection as described above. Briefly, mice were sacrificed and whole brains were harvested into 4 ml of 10% formalin solution for fixation. Paraffin-embedded brains were then sectioned sagitally and every 10th section stained using an anti-HSV-1 rabbit polyclonal antibody (Dako, Denmark); staining was detected with streptavidin-horseradish peroxidase (HRP) and diaminobenzidine (DAB) followed by a hematoxylin counterstain. Each section was divided into five regions (olfactory bulb, central brain, mid-brain, cerebellum, and brain stem) and scored as either positive or negative for HSV antigen staining in a masked fashion. Total positive regions were then divided by total sections counted to obtain percent antigen-positive regions.
Bead-based cytokine analysis.
Brains and brain stems were isolated and assayed following corneal and intracranial infections. A single brain or brain stem was harvested from mice and mechanically disrupted in 1 ml of phosphate-buffered saline (PBS). Samples were then sonicated on ice twice for 30 s and centrifuged for 4 min at 515 × g at 4°C. Supernatants were transferred to a 1.5-ml Eppendorf tube and spun in a minicentrifuge for 5 min at 7,500 rpm at 4°C. Supernatants were then transferred to new tube and diluted 1:1 with serum sample diluent (BioPlex mouse Serum sample kit; Bio-Rad, Hercules, CA). The samples were then stored at −80°C until assayed. The BioPlex assay (Bio-Rad) was preformed as described in the kit protocol. Briefly, equivalent amounts of protein, as measured by Bradford assay, were added to each well of a multiplex mouse cytokine BioPlex array. Cytokine concentrations were determined by comparison to a standard curve provided by Bio-Rad, and the results are reported as pg/ml/μg protein. The results shown are the averages from two experiments, with each experiment using three or more mice per data point.
IFN ELISA.
Following a high-dose intracranial infection, brains were harvested at 12, 18, and 48 h postinfection. The brains were mechanically disrupted and sonicated two times in 1 ml PBS. Brain samples were then spun at 515 × g in a tabletop centrifuge. The supernatant were then harvested and spun at 7,500 rpm in a minicentrifuge for 10 min. The clarified supernatants were harvested and stored at −80°C until processing by enzyme-linked immunosorbent assay (ELISA). For both IFN-β and IFN-α ELISA, 100 μl of sample was assayed per the kit protocol (PBL InterferonSource, Piscataway, NJ). Protein levels were normalized via Bradford assay, and the results were expressed pg/ml/μg protein.
Real time reverse transcription-PCR (RT-PCR) of brain tissue.
At the indicated time postinfection, brains were harvested into 2 ml of solution D (4 M guanidine thiocyanate, 25 ml of sodium citrate, 0.5% sarcosyl, 0.1 M 2-mercaptoethanol) (8) and stored at −80°C. Total RNA was harvested as previously described (50) and resuspended in a small volume of nuclease-free water. cDNA was generated using the iScript cDNA synthesis kit as per the kit protocol (Bio-Rad). PCR mixtures were prepared with iQ SYBR green supermix (Bio-Rad), 5% acetamide, primers (IDT, Coralville, IA), and 2 μl cDNA. Each PCR was performed in duplicate, and each infection condition was replicated in at least four mice from three independent experiments. Actin primer sequences 5′-TGGTACGACCAGAGGCATACAG (forward) and 5′-CCAACTGGGACGACATGGAG (reverse) and IFN-β primer sequences 5′-CAGCTCCAAGAAAGGACGAAC and 5′-GGCAGTGTAACTCTTCTGCAT (reverse) were used.
Statistics.
Statistical calculations were done by Student's t test and are relative to the WT control group unless otherwise stated. Statistical analysis of survival curves utilized the log rank test.
RESULTS
IRF-3−/− mice have no defect in controlling HSV-1 replication in the cornea and trigeminal ganglia following peripheral infection.
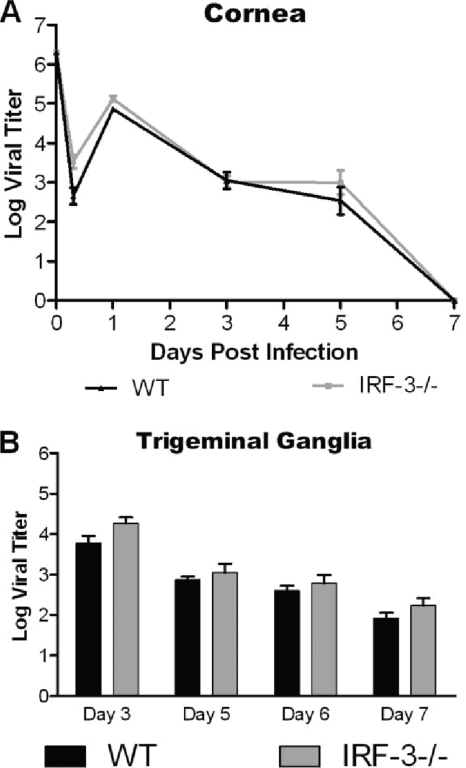
Following ocular challenge with HSV-1 McKrae, examination of corneal eye swabs from IRF-3−/− and WT mice demonstrated no significant change in viral replication following infection (Fig. 1 A). Similarly, the periocular skin and trigeminal ganglia titers also showed no major change in viral replication (Fig. 1B and data not shown). There was, however, a slight trend for higher replication in the IRF-3−/− mice. Similar results were observed in these tissues utilizing another, less virulent lab strain, HSV-1 17 (data not shown). The data together supported the previous findings by Honda et al. and suggest that IRF-3-mediated pathways play only a minor role in controlling HSV-1 during peripheral infection (24).
FIG. 1.
Loss of IRF-3 has minimal impact on HSV-1 replication in peripheral tissues following cornea infection. WT and IRF-3−/− mice were infected with 2 × 106 PFU HSV-1 McKrae per eye. Cornea swabs and trigeminal ganglia were harvested and titers determined at the specified days. The graphs represent averages and standard deviations for several mice at each time point from two independent experiments (n ≥ 6).
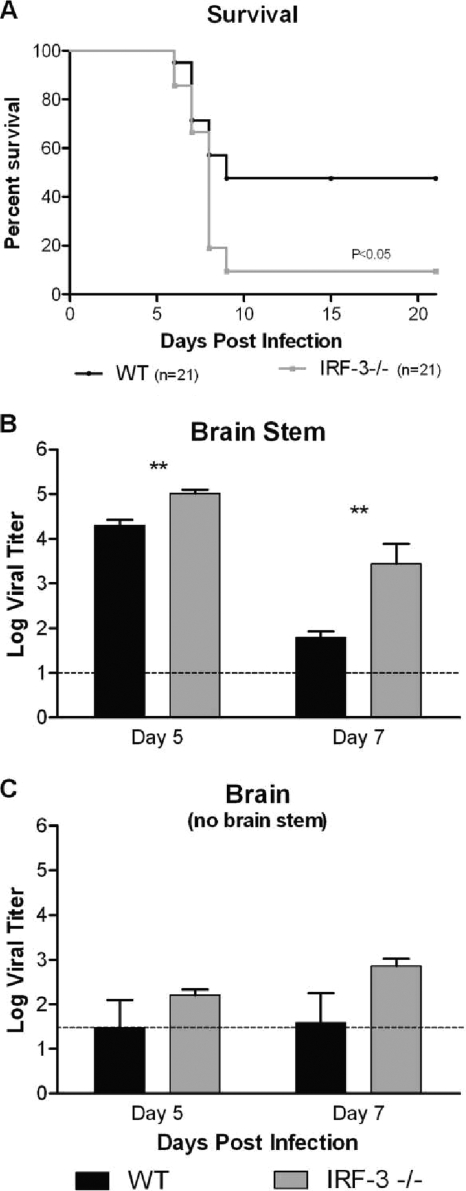
IRF-3−/− mice show increased mortality following cornea infection and an associated increase in viral replication in the brain stem.
Previous reports had demonstrated reduced virulence and limited CNS penetration following peripheral infection with HSV-1 strain 17 in the C57/BL6 mouse background strain due to a variety of factors (36-38). A similar result, including limited CNS titers and no lethality in either group, was observed following cornea infection with HSV-1 17 (data not shown). Therefore, to evaluate CNS infection and lethality in IRF-3−/− mice following cornea challenge, infection with the neurovirulent McKrae strain was utilized and resulted in approximately 50% survival in WT mice yet less than 10% survival in IRF-3−/− mice (P < 0.05) (Fig. 2 A). Throughout these studies, no differences in susceptibility between male and female mice were noted. Evaluating viral titers, IRF-3−/− brain stems were found to have a statistically significant increase in viral replication compared to wild-type controls following corneal challenge (Fig. 2B). Although the differences were not statistically significant, whole-brain titers from IRF-3−/− mice were also increased compared to those from controls (Fig. 2C). Together, these data suggest that IRF-3−/− mice have a deficit in their ability to control lethal brain infection.
FIG. 2.
IRF-3−/− mice have decreased survival and increased viral replication in brain tissues following HSV-1 McKrae cornea infection. (A) Survival plot following infection of WT and IRF-3−/− mice with 2 × 106 PFU HSV-1 McKrae per eye. Survival experiments were conducted independently of the other experiments and represent the sum of multiple experiments (n = 21). (B and C) Brains and brain stems were harvested and titers determined at the specified days following infection of WT and IRF-3−/− mice with 2 × 106 PFU HSV-1 McKrae per eye. The graphs represent the averages and standard deviations for several mice from two independent experiments (n ≥ 5). The dotted line represents the limit of detection for this assay. **, P < 0.01.
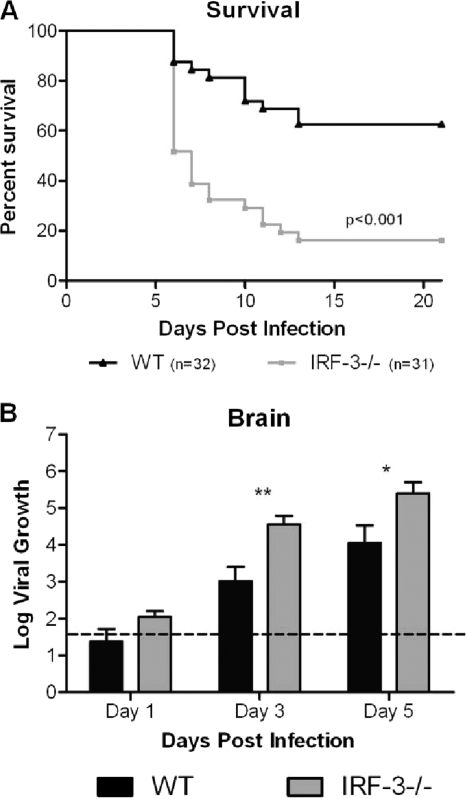
Loss of IRF-3 results in increased viral replication and increased mortality following intracranial HSV-1 infection.
The cornea model of HSV-1 infection mimics the physiological course of eye disease seen in humans and permits evaluation of viral replication in peripheral tissues. However, many factors can affect the ability of the virus to replicate in peripheral tissues and also affect its ability to enter the brain and replicate therein. Therefore, while not strictly speaking physiologically relevant, direct intracerebral infection was used to bypass the impact of IRF3 in peripheral tissues and isolate its role in protection of the CNS. One hundred PFU of HSV-1 was inoculated directly into the brain to evaluate the role of IRF-3 in replication and survival following viral challenge. HSV-1 strain 17 was utilized as the background strain for these experiments since HSV-1 McKrae is too virulent in the i.c. model (the 50% lethal dose [LD50] is <10 PFU). Following infection, there was a significant increase in lethality of HSV in the IRF-3−/− mice compared to the controls (Fig. 3 A). While over 60% of the WT mice survived i.c. injection, fewer than 20% of the IRF-3−/− mice survived the same challenge. Correspondingly, beginning at day 3 and continuing at day 5, IRF-3−/− brains permitted a 10- to 100-fold increase in viral replication compared to control mice (Fig. 3B). These results suggest that IRF-3-mediated pathways are important in controlling HSV-1 replication in brain tissues following direct intracranial injection.
FIG. 3.
IRF-3−/− mice have reduced survival and increased viral titers in the brain following HSV-1 intracranial infection. (A) Survival plot of IRF-3−/− and WT mice following intracranial (i.c.) infection with 100 PFU HSV-1 17. Survival experiments were conducted independent of the other experiments and represents the sum of two experiments (n ≥ 31). (B) Viral titers in whole brain tissue harvested at the specified days. Data represent the averages and standard deviations for several mice from two independent experiments (n ≥ 9). The dotted line represents the limit of detection for this assay. *, P < 0.05; **, P < 0.01.
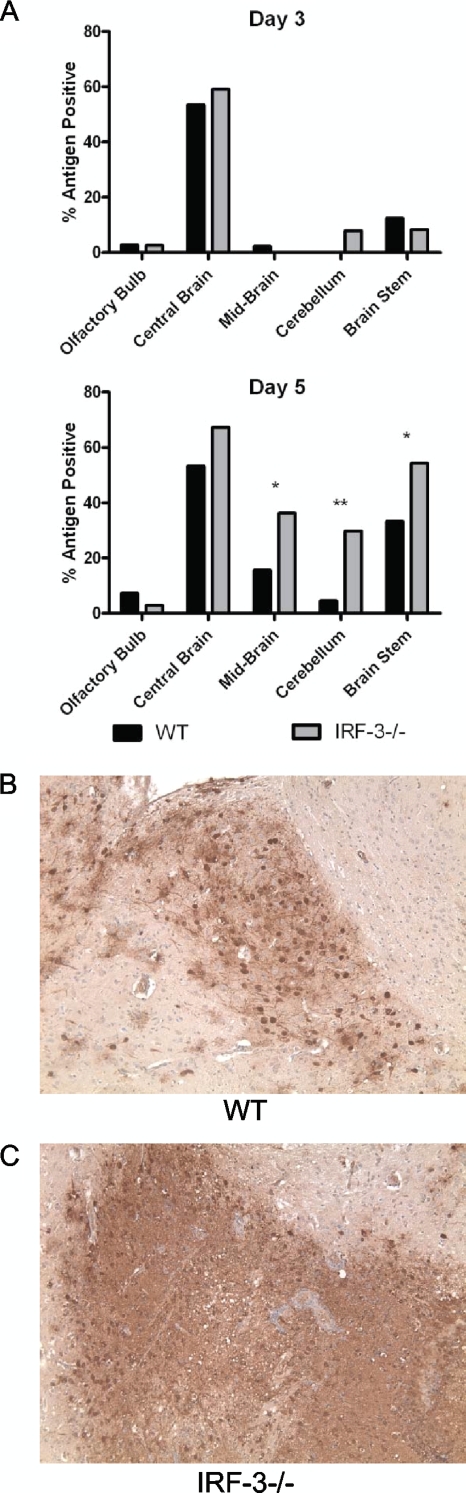
IRF-3−/− mice have increased and altered antigen staining following i.c. challenge with HSV-1.
WT and IRF-3−/− mice were infected intracranially with 100 PFU of HSV-1 strain 17 and harvested at days 3 and 5 postinfection. Total antigen-positive regions were then assessed as described in Materials and Methods (Fig. 4). In general, IRF-3−/− mice displayed a higher percentage of antigen-positive regions than WT mice. The central brain region (cerebral cortex, hippocampus, septum, thalamus, and hypothalamus) was the site of inoculation and displayed a consistent and high percentage of antigen-positive regions at both days 3 and 5 in both WT and IRF-3−/− mice. In contrast, the mid-brain, cerebellum, and brain stem displayed little antigen staining (<10%) in either WT or IRF-3−/− mice at day 3 (Fig. 4A). By day 5, however, IRF-3−/− mice displayed a significant increase in antigen-positive sections compared to WT mice in mid-brain, cerebellum, and brain stem (Fig. 4A). In addition to increased antigen-positive regions, IRF-3−/− mice displayed a distinct antigen staining pattern compared to WT mice (Fig. 4B and C). IRF-3−/− brain sections showed generalized antigen-positive lesions with diffuse areas appearing uniformly stained. In contrast, HSV staining of WT lesions showed focal staining in cells with neuronal morphology. This altered staining was consistent in each IRF-3−/− antigen-positive region examined and suggest increased antigen production and spread in IRF-3−/− brains compared to control brains. Together, the increase in antigen production and distribution correlates with the previously observed increased viral titers in IRF-3−/− brains (Fig. 2 and 3).
FIG. 4.
IRF-3−/− brain sections have increased antigen staining following intracranial HSV-1 infection. Following i.c. infection with 100 PFU HSV-1 strain 17, brains were harvested on days 3 and 5 postinfection, formalin fixed, sectioned sagitally, and stained with a polyclonal anti-HSV antibody. Sections were divided into five regions (olfactory bulb, central brain, mid-brain cerebellum, and pons/medulla/brain stem) and scored as either positive or negative for HSV antigen staining in a masked fashion. (A) Following scoring, total antigen-positive regions were then divided by total sections counted in order to calculate a percentage of antigen-positive regions for day 3 and day 5. (B and C) Representative anti-HSV-1 peroxidase staining images from the central brain regions of WT and IRF-3−/− mice. *, P < 0.05; **, P < 0.01.
IRF-3−/− mice produce increased amounts of inflammatory cytokines following direct intracranial infection.
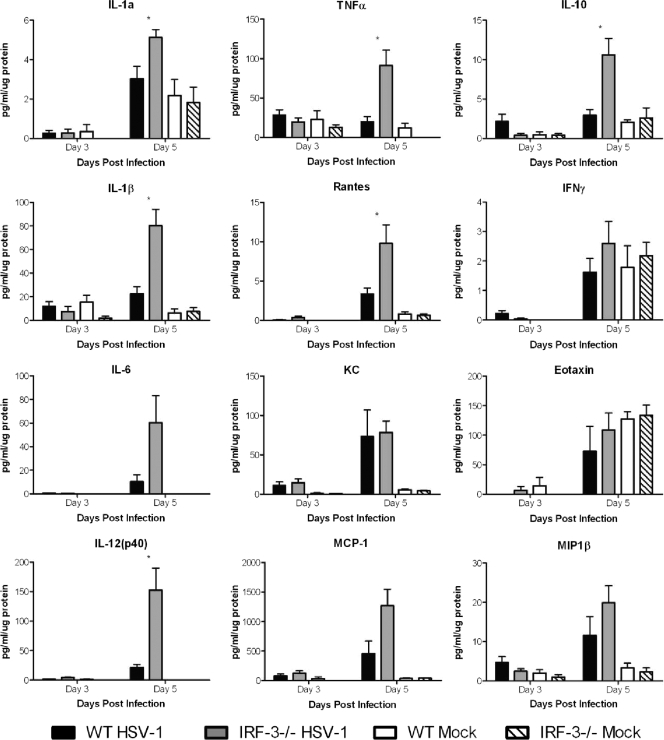
In addition to HSV-1 replication, several studies have implicated inflammatory cytokines as contributing to increased lethality following CNS infection (35, 36). To assess inflammatory cytokine production in IRF-3−/− mice, total brain homogenates were prepared and cytokines were assayed by a bead-based multiplex array following i.c. infection with 100 PFU of HSV-1 strain 17 or mock treatment (Fig. 5). In both the IRF-3−/− and WT brains, cytokine samples taken on day 3 showed minimal induction of cytokines, with little or no variation between the virus-infected and mock-treated groups. At day 5, however, there was a significant increase in several inflammatory cytokines in infected IRF-3−/− brains compared to WT brains. IRF-3−/− brains showed a 3.5-fold increase in interleukin-1β (IL-1β), a 4.6-fold increase in tumor necrosis factor alpha (TNF-α), and a 5.8-fold increase in IL-6 levels compared to infected WT brains. This trend also extended to IL-12 (7.1 fold), IL-10 (3.6 fold), and several chemokines, including MCP-1, Rantes, and MIP1β. In contrast, one cytokine (KC) demonstrated similar levels of production in the WT and IRF-3−/− groups. In addition, several (IL-5, IL-13, and granulocyte-macrophage colony-stimulating factor [GM-CSF]) were globally upregulated in both WT and IRF-3−/− mice following virus or mock infection, suggesting that injection of virus-free cell extract or the mechanical damage of injection alone was sufficient to induce their expression (data not shown). Together, the data suggest that in response to HSV-1 infection, IRF-3−/− mice produce a stronger inflammatory response as measured by cytokine production. The timing of this increase in inflammatory cytokines, on day 5, also coincided with the lethality seen in this model of infection.
FIG. 5.
IRF-3−/− mice show increased inflammatory cytokine production following intracranial infection with HSV-1. Following i.c. infection with 100 PFU HSV-1 17, brains were harvested on days 3 and 5 postinfection, processed, and assayed via a bead-based cytokine assay (BioPlex; Bio-Rad). The results shown are the averages and standard deviations for four to six mouse brains per group per time point. Statistical calculations were based on infected WT and infected IRF-3−/− mice. *, P < 0.05.
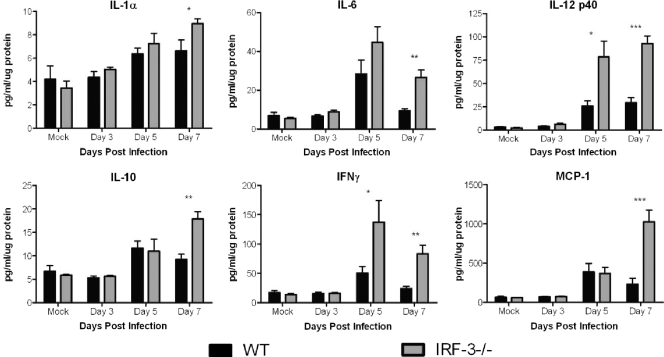
IRF-3−/− mice have increased cytokine expression in the brain stem following peripheral infection.
Having shown increased cytokine expression in brains following direct intracranial injection, it was of interest to observe changes in cytokine levels following peripheral infection. Examination of the brain stem revealed increased production of several cytokines in the IRF-3−/− mice compared to control mice (Fig. 6). While maintaining similar levels at day 3, several cytokines, including IL-6, IL-12, and IFN-γ, had increased expression at both days 5 and 7 in IRF-3-deficient mice. IL-10, MCP-1, and G-CSF levels were increased only on day 7 (data not shown). The increased cytokine production at this late time corresponded with the peak in lethality seen following corneal infection, consistent with the idea that inflammation contributes to the increased mortality in IRF-3−/− mice.
FIG. 6.
IRF-3−/− mice show increased inflammatory cytokine production following peripheral infection. Following ocular infection with 1 × 106 PFU HSV-1 McKrae per eye, brains were harvested on days 3 and 5 postinfection, processed, and assayed via a bead-based cytokine assay (BioPlex; Bio-Rad). The results shown are the averages and standard deviations for four to six mouse brain stems per group per time point. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
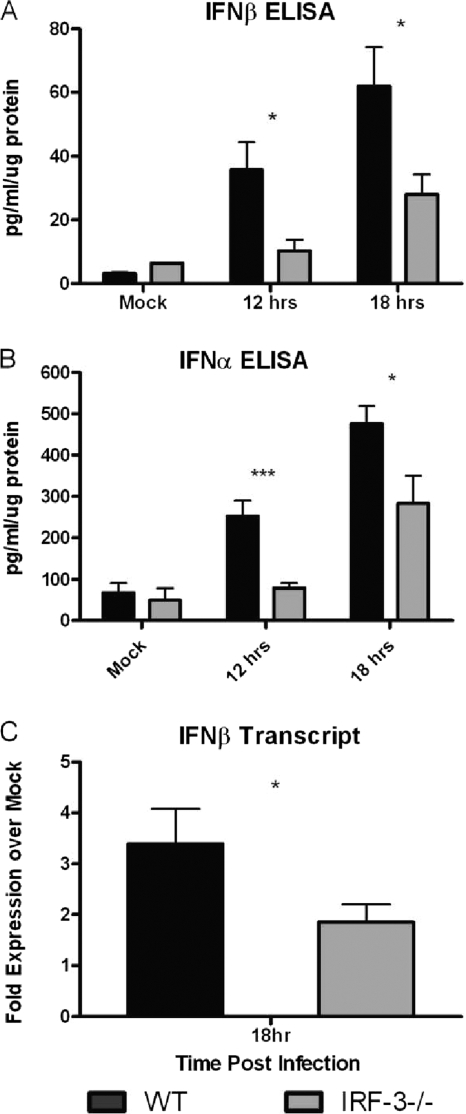
IRF-3-deficient mice have a deficit in type I IFN production.
Previous work demonstrated a deficit in the production of IFN-β following infection of IRF-3−/− bone marrow-derived dendritic cells compared to control cells (45). The current experiments sought to determine whether IRF-3-deficient mice displayed a similar IFN-β production deficit in brain tissues following i.c. infection. WT and IRF-3−/− mice were challenged with a high dose of HSV-1, and brains were harvested at 12 and 18 h postinfection. At both 12 and 18 h postinfection there was a statistically significant difference in IFN-β protein levels in IRF-3−/− mice compared to WT control mice (Fig. 7 A). At 12 h postinfection, WT mice produced nearly 3.5-fold more IFN-β than IRF-3−/− mice, and they produced 2.2-fold more IFN-β at 18 h postinfection. The 12- and 18-h results therefore recapitulated the results previously reported for BMDCs (45), consistent with the idea that IRF-3−/− mice permit increased viral replication and show increased susceptibility to infection.
FIG. 7.
IRF-3−/− mice have a deficit in type I IFN production following intracranial infection with HSV-1. WT and IRF-3−/− mice were infected i.c. with 1 × 106 PFU HSV-1 strain 17. (A and B) Whole brain tissue was harvested at the specified times, processed, and analyzed for IFN-β (A) and IFN-α (B) by ELISA (PBL Laboratories). The results shown represent the averages and standard deviations for 10 to 14 mice per group per time point from two separate experiments. (C) Following infection with 100 PFU HSV-1 strain 17, brain tissue, excluding brain stem and olfactory bulb, was harvested for RNA at 18 h postinfection. Samples were assayed by real-time RT-PCR, and results are expressed as fold expression over that for mock-infected samples. The results shown are the average fold expression and standard deviation from six or seven mice per group per time point from two separate experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Previous studies in vivo showed no change in serum IFN-α levels relative to those in control mice following intravenous infection of IRF-3−/− mice with HSV-1 (24). The authors concluded that IRF-7 was primarily responsible for IFN-α production in vivo. To further assess that idea in this work, brain samples were assayed by ELISA for IFN-α (Fig. 7B). The data showed a defect in IFN-α production in IRF-3−/− mice compared to WT control mice at all time points tested. At 12 h postinfection, WT mice have nearly 5-fold more IFN-α than IRF-3-deficient brains, in which IFN-α remained at minimal levels. However, by 18 h, IFN-α production from IRF-3−/− brains was significantly above background levels, suggesting an IRF-3-independent response to viral challenge, consistent with a role for IRF-7. While this IFN-α production is still notably deficient compared to that in WT mice, it does suggest that IRF-3−/− mice are capable of inducing the type I IFN cascade. Together, the results confirm a deficit and delay in the induction of type I IFN in IRF-3−/− mice in vivo.
The previous results demonstrated a significant difference between IRF-3−/− and WT mice in terms of type I IFN production. However, those experiments required a higher dose of HSV-1; attempts with a lower dose had failed to detect IFN within the linear range of the ELISA. Therefore, following i.c. infection with 100 PFU of HSV-1, WT and IRF-3−/− brains were removed, the olfactory bulb and brain stem discarded, and RNA harvested from the remaining brain for analysis at 18 h postinfection. The results demonstrated a statistically significant decrease in fold expression of IFN-β RNA in IRF-3−/− mice compared to controls (Fig. 7C). The WT brains averaged a 3.4-fold increase in IFN-β transcript compared to mock samples, while in contrast, IRF-3−/− brains averaged a 1.9-fold increase. These results, coupled with the type I IFN ELISA results following high-dose infection, demonstrate an immediate deficiency in type I IFN production in the brains of IRF-3−/− mice.
DISCUSSION
The data in this study show that IRF-3 plays a critical role in the control of HSV-1 CNS infection. A deficient IFN response most likely permitted HSV-1 to establish a foothold for infection, resulting in increased viral replication and antigen staining in IRF-3−/− brains. Concomitant with increased viral replication, the immune system induced an increased inflammatory cytokine response in IRF-3-deficient mice, and these factors combined to result in significantly increased lethality. Together, these results highlight the importance of the IRF-3-dependent immune response in preventing lethal CNS infection following HSV-1 challenge. Similar to previous reports on immune cells (45), IRF-3−/− mice had reduced type I IFN production in the brain following HSV-1 challenge, and delayed or reduced type I IFN production has broad implications for susceptibility to viral infection of the CNS. Deficient type I IFN production in brains contributes to encephalitis in a variety of RNA virus infections, including West Nile virus, Semliki virus, and mouse hepatitis virus (11, 12, 18, 25). The results also correspond with genetic studies in humans demonstrating a deficiency in TLR signaling, specifically TLR-3, which results in increased susceptibility to HSE (7, 74). While TLR-3 is dispensable for protection of mice from viral infection, IRF-3 is apparently required for protection. IRF-3 is downstream of TLR-3 in the signaling pathway, and these findings emphasize the importance of this type I IFN induction pathway in controlling HSE in vivo (59).
In addition to controlling viral replication, the inflammatory response to CNS infection is also thought to contribute to lethality following HSV-1 challenge. Indeed, the inflammatory response is both protective and harmful to the host during HSE. Deletion or inhibition of parts of the inflammatory response results in the host succumbing to HSV-1 infection (5, 38, 59). In contrast, antagonizing other inflammatory elements has positive results in terms of morbidity and mortality (35, 36, 46). In the absence of type I IFN signaling, several viruses have been reported to induce increased CNS inflammation in addition to increased viral replication (25, 61). A similar pattern emerges in these studies, as IRF-3−/− mice have increased inflammatory cytokine production in the brains following i.c. and corneal infection. The increase in inflammatory cytokine production in IRF-3−/− mice preceded the major peak in lethality in both models. These data cannot definitively show whether increased inflammatory cytokine production, increased viral replication, or a combination of both results in the increased mortality seen in IRF-3−/− mice, although ongoing studies in this laboratory will help to distinguish these possibilities.
Previous work with other viruses has suggested an alteration in viral distribution or viral tropism in the context of defective or antagonized type I IFN signaling (19, 25, 55). In this study, assessment of viral antigen distribution revealed that while initially limited to the central brain region, HSV-1 was distributed in the brain stem, cerebellum, and mid-brain in both WT and IRF-3−/− mice by day 5 following i.c. infection. In each region, IRF-3−/− brains exhibited a higher percentage of antigen-positive regions, but the overall location of the virus was similar in the WT and IRF-3−/− mice. There was, however, a distinct antigen staining pattern in IRF-3−/− and WT brain sections. IRF-3−/− mice showed lesions with uniform antigen-positive regions, while WT lesions showed HSV-1 staining foci in cells with neuronal morphology. This observation is consistent with the hypothesis that IRF-3−/− mice permit initial uncontrolled viral replication resulting in wide, uniform antigen staining. The data also suggest a possible shift in cell tropism within the CNS of IRF-3−/− mice; microglia, astrocytes, and other glial cells have been shown to respond to type I IFN and may be more susceptible to HSV-1 infection in the absence of IRF-3 (1, 25, 39, 40, 66).
The data presented in this study demonstrate a more complex role for IRF-3 than previously shown (24). There is consistency between the previous study and the current data examining replication in corneas, trigeminal ganglia, and periocular skin (data not shown), but there are also some sharp distinctions when considering the current observation of increased lethality and brain titers. A possible explanation is the nature of the immune response in the CNS. In peripheral tissues, the type I IFN response is driven primarily by plasmacytoid dendritic cells in an IRF-7-dependent manner; high levels of IFN-α are produced, which can compensate for the loss of IRF-3-dependent pathways (31). This model is supported by the previous intravenous challenge data (24) and by data in this study. However, in the brain, examination of type I IFN production revealed a deficit in IRF-3−/− brains compared to control brains, providing a mechanism for increased HSV-1 replication and suggesting a CNS-specific necessity for IRF-3. While peripheral tissues primarily utilize type I IFN production by plasmacytoid dendritic cells (pDCs), the brain is largely devoid of this cell type (2, 3, 58). Instead, the CNS relies on resident cells to produce and respond to type I IFN (13). In the absence of IRF-3, the CNS fails to produce an immediate type I IFN response, and HSV-1 establishes a foothold for infection. Augmented viral replication follows, which leads to increased cytokine production and increased lethality. In the periphery, the loss of IRF-3 affects local production of type I IFN as demonstrated by reduced IFN-β (24, 56). However, infiltration by immune cells and IRF-7-mediated production of IFN-α likely rescues the type I IFN cascade and prevents the virus from establishing a foothold in peripheral tissues. This exogenous IFN production by infiltrating cells is not available in the CNS, as few pDCs are found in the brain and type I IFN has not been shown to pass through the blood-brain barrier (13, 14). Therefore, the CNS requires local production of type I IFN, and IRF-3 is critical for a timely and efficient response. In the absence of IRF-3, the virus gains its foothold, and the result is increased susceptibility to HSV-1 CNS infection.
Taken together, these data demonstrate a critical role for IRF-3 in the brain following HSV-1 challenge. The results demonstrate a major delineation between the peripheral and CNS innate immune responses. The data also underscore the importance of testing multiple infection models and measuring multiple parameters to fully ascertain the roles of host resistance factors in viral infection. Ongoing experiments in our laboratory seek to evaluate changes in viral tropism and inflammatory infiltrates in the brains of IRF-3−/− mice. Further experiments will determine the precise pathways and molecules responsible for HSV-1 recognition. Several candidates involved in the early recognition pathways have been implicated, and cells and mice lacking these components are being evaluated both in vitro and in vivo.
Acknowledgments
This work was supported by NIH grants to David Leib (EY 09083) and the Department of Ophthalmology and Visual Sciences (P30EY02687) from the National Eye Institute. The project was also supported by award no. P20RR016437 from the National Center for Research Resources. Vineet Menachery was supported by Research Training Grant in the Visual Sciences NIH T32 EY013360-09. Support from the Research to Prevent Blindness to the Department of Ophthalmology and Visual Sciences and a Senior Scientific Investigator Award to David A. Leib are gratefully acknowledged.
IRF-3−/− and IRF-7−/− mice were generously provided by T. Tanaguchi. Thanks go to Belinda McMahan and the ImmunoMorphology & Digital Imaging CORE Lab for preparation of histology samples. We also thank M. Diamond for helpful feedback and provision of mice and members of the Leib lab for useful discussions.
Footnotes
Published ahead of print on 21 July 2010.
REFERENCES
- 1.Aravalli, R. N., S. Hu, T. N. Rowen, J. M. Palmquist, and J. R. Lokensgard. 2005. TLR2-mediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. J. Immunol. 175:4189-4193. [DOI] [PubMed] [Google Scholar]
- 2.Asselin-Paturel, C., A. Boonstra, M. Dalod, I. Durand, N. Yessaad, C. Dezutter-Dambuyant, A. Vicari, A. O'Garra, C. Biron, F. Briere, and G. Trinchieri. 2001. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2:1144-1150. [DOI] [PubMed] [Google Scholar]
- 3.Bjorck, P. 2001. Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood 98:3520-3526. [DOI] [PubMed] [Google Scholar]
- 4.Bowie, A. G., and L. Unterholzner. 2008. Viral evasion and subversion of pattern-recognition receptor signalling. Nat. Rev. Immunol. 8:911-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradford, R. D., A. C. Pettit, P. W. Wright, M. J. Mulligan, L. W. Moreland, D. A. McLain, J. W. Gnann, and K. C. Bloch. 2009. Herpes simplex encephalitis during treatment with tumor necrosis factor-α inhibitors. Clin. Infect. Dis. 49:924-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, S. M., D. A. Ritchie, and J. H. Subak-Sharpe. 1973. Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J. Gen. Virol. 18:329-346. [DOI] [PubMed] [Google Scholar]
- 7.Casrouge, A., S. Y. Zhang, C. Eidenschenk, E. Jouanguy, A. Puel, K. Yang, A. Alcais, C. Picard, N. Mahfoufi, N. Nicolas, L. Lorenzo, S. Plancoulaine, B. Senechal, F. Geissmann, K. Tabeta, K. Hoebe, X. Du, R. L. Miller, B. Heron, C. Mignot, T. B. de Villemeur, P. Lebon, O. Dulac, F. Rozenberg, B. Beutler, M. Tardieu, L. Abel, and J. L. Casanova. 2006. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science 314:308-312. [DOI] [PubMed] [Google Scholar]
- 8.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 9.Collins, S. E., R. S. Noyce, and K. L. Mossman. 2004. Innate cellular response to virus particle entry requires IRF3 but not virus replication. J. Virol. 78:1706-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conrady, C. D., D. A. Drevets, and D. J. J. Carr. 2010. Herpes simplex type I (HSV-1) infection of the nervous system: is an immune response a good thing? J. Neuroimmunol. 220:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daffis, S., M. A. Samuel, B. C. Keller, M. Gale, and M. S. Diamond. 2007. Cell-specific IRF-3 responses protect against West Nile virus infection by interferon-dependent and -independent mechanisms. PLoS Pathog. 3:e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daffis, S., M. A. Samuel, M. S. Suthar, B. C. Keller, M. Gale, and M. S. Diamond. 2008. Interferon regulatory factor IRF-7 induces the antiviral alpha interferon response and protects against lethal West Nile virus infection. J. Virol. 82:8465-8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delhaye, S., S. Paul, G. Blakqori, M. Minet, F. Weber, P. Staeheli, and T. Michiels. 2006. Neurons produce type I interferon during viral encephalitis. Proc. Natl. Acad. Sci. U. S. A. 103:7835-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Detje, C. N., T. Meyer, H. Schmidt, D. Kreuz, J. K. Rose, I. Bechmann, M. Prinz, and U. Kalinke. 2009. Local type I IFN receptor signaling protects against virus spread within the central nervous system. J. Immunol. 182:2297-2304. [DOI] [PubMed] [Google Scholar]
- 15.Diebold, S. S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529-1531. [DOI] [PubMed] [Google Scholar]
- 16.Dupuis, S., E. Jouanguy, S. Al-Hajjar, C. Fieschi, I. Z. Al-Mohsen, S. Al-Jumaah, K. Yang, A. Chapgier, C. Eidenschenk, P. Eid, A. Al Ghonaium, H. Tufenkeji, H. Frayha, S. Al-Gazlan, H. Al-Rayes, R. D. Schreiber, I. Gresser, and J. L. Casanova. 2003. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat. Genet. 33:388-391. [DOI] [PubMed] [Google Scholar]
- 17.Eidson, K. M., W. E. Hobbs, B. J. Manning, P. Carlson, and N. A. DeLuca. 2002. Expression of herpes simplex virus ICP0 inhibits the induction of interferon-stimulated genes by viral infection. J. Virol. 76:2180-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fragkoudis, R., L. Breakwell, C. McKimmie, A. Boyd, G. Barry, A. Kohl, A. Merits, and J. K. Fazakerley. 2007. The type I interferon system protects mice from Semliki Forest virus by preventing widespread virus dissemination in extraneural tissues, but does not mediate the restricted replication of avirulent virus in central nervous system neurons. J. Gen. Virol. 88:3373-3384. [DOI] [PubMed] [Google Scholar]
- 19.Gardner, C. L., C. W. Burke, M. Z. Tesfay, P. J. Glass, W. B. Klimstra, and K. D. Ryman. 2008. Eastern and Venezuelan equine encephalitis viruses differ in their ability to infect dendritic cells and macrophages: impact of altered cell tropism on pathogenesis. J. Virol. 82:10634-10646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gitlin, L., W. Barchet, S. Gilfillan, M. Cella, B. Beutler, R. A. Flavell, M. S. Diamond, and M. Colonna. 2006. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. U. S. A. 103:8459-8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 22.Honda, K., A. Takaoka, and T. Taniguchi. 2006. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity 25:349-360. [DOI] [PubMed] [Google Scholar]
- 23.Honda, K., and T. Taniguchi. 2006. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 6:644-658. [DOI] [PubMed] [Google Scholar]
- 24.Honda, K., H. Yanai, H. Negishi, M. Asagiri, M. Sato, T. Mizutani, N. Shimada, Y. Ohba, A. Takaoka, N. Yoshida, and T. Taniguchi. 2005. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434:772-777. [DOI] [PubMed] [Google Scholar]
- 25.Ireland, D. D., S. A. Stohlman, D. R. Hinton, R. Atkinson, and C. C. Bergmann. 2008. Type I interferons are essential in controlling neurotropic coronavirus infection irrespective of functional CD8 T cells. J. Virol. 82:300-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katze, M. G., Y. He, and M. Gale. 2002. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2:675-687. [DOI] [PubMed] [Google Scholar]
- 27.Kawai, T., K. Takahashi, S. Sato, C. Coban, H. Kumar, H. Kato, K. J. Ishii, O. Takeuchi, and S. Akira. 2005. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6:981-988. [DOI] [PubMed] [Google Scholar]
- 28.Kaye, S., and A. Choudhary. 2006. Herpes simplex keratitis. Prog. Retin Eye Res. 25:355-380. [DOI] [PubMed] [Google Scholar]
- 29.Kimberlin, D. 2004. Herpes simplex virus, meningitis and encephalitis in neonates. Herpes 11(Suppl 2):65A–76A. [PubMed] [Google Scholar]
- 30.Kimberlin, D. W., F. D. Lakeman, A. M. Arvin, C. G. Prober, L. Corey, D. A. Powell, S. K. Burchett, R. F. Jacobs, S. E. Starr, and R. J. Whitley. 1996. Application of the polymerase chain reaction to the diagnosis and management of neonatal herpes simplex virus disease. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. J. Infect. Dis. 174:1162-1167. [DOI] [PubMed] [Google Scholar]
- 31.Lang, P. A., L. Cervantes-Barragan, A. Verschoor, A. A. Navarini, M. Recher, M. Pellegrini, L. Flatz, A. Bergthaler, K. Honda, B. Ludewig, P. S. Ohashi, and K. S. Lang. 2009. Hematopoietic cell-derived interferon controls viral replication and virus-induced disease. Blood 113:1045-1052. [DOI] [PubMed] [Google Scholar]
- 32.Leib, D. A., T. E. Harrison, K. M. Laslo, M. A. Machalek, N. J. Moorman, and H. W. Virgin. 1999. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J. Exp. Med. 189:663-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin, R., R. S. Noyce, S. E. Collins, R. D. Everett, and K. L. Mossman. 2004. The herpes simplex virus ICP0 RING finger domain inhibits IRF3- and IRF7-mediated activation of interferon-stimulated genes. J. Virol. 78:1675-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luker, G. D., J. L. Prior, J. Song, C. M. Pica, and D. A. Leib. 2003. Bioluminescence imaging reveals systemic dissemination of herpes simplex virus type 1 in the absence of interferon receptors. J. Virol. 77:11082-11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lundberg, P., H. Openshaw, M. Wang, H. J. Yang, and E. Cantin. 2007. Effects of CXCR3 signaling on development of fatal encephalitis and corneal and periocular skin disease in HSV-infected mice are mouse-strain dependent. Invest. Ophthalmol. Vis Sci. 48:4162-4170. [DOI] [PubMed] [Google Scholar]
- 36.Lundberg, P., C. Ramakrishna, J. Brown, J. M. Tyszka, M. Hamamura, D. R. Hinton, S. Kovats, O. Nalcioglu, K. Weinberg, H. Openshaw, and E. M. Cantin. 2008. The immune response to herpes simplex virus type 1 infection in susceptible mice is a major cause of central nervous system pathology resulting in fatal encephalitis. J. Virol. 82:7078-7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lundberg, P., P. Welander, H. Openshaw, C. Nalbandian, C. Edwards, L. Moldawer, and E. Cantin. 2003. A locus on mouse chromosome 6 that determines resistance to herpes simplex virus also influences reactivation, while an unlinked locus augments resistance of female mice. J. Virol. 77:11661-11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lundberg, P., P. V. Welander, C. K. Edwards, 3rd, N. van Rooijen, and E. Cantin. 2007. Tumor necrosis factor (TNF) protects resistant C57BL/6 mice against herpes simplex virus-induced encephalitis independently of signaling via TNF receptor 1 or 2. J. Virol. 81:1451-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marques, C. P., M. C. Cheeran, J. M. Palmquist, S. Hu, S. L. Urban, and J. R. Lokensgard. 2008. Prolonged microglial cell activation and lymphocyte infiltration following experimental herpes encephalitis. J. Immunol. 181:6417-6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marques, C. P., S. Hu, W. Sheng, and J. R. Lokensgard. 2006. Microglial cells initiate vigorous yet non-protective immune responses during HSV-1 brain infection. Virus Res. 121:1. [DOI] [PubMed] [Google Scholar]
- 41.Matsumoto, M., S. Kikkawa, M. Kohase, K. Miyake, and T. Seya. 2002. Establishment of a monoclonal antibody against human Toll-like receptor 3 that blocks double-stranded RNA-mediated signaling. Biochem. Biophys. Res. Commun. 293:1364-1369. [DOI] [PubMed] [Google Scholar]
- 42.Melchjorsen, J., J. Siren, I. Julkunen, S. R. Paludan, and S. Matikainen. 2006. Induction of cytokine expression by herpes simplex virus in human monocyte-derived macrophages and dendritic cells is dependent on virus replication and is counteracted by ICP27 targeting NF-kappaB and IRF-3. J. Gen. Virol. 87:1099-1108. [DOI] [PubMed] [Google Scholar]
- 43.Melroe, G. T., N. A. DeLuca, and D. M. Knipe. 2004. Herpes simplex virus 1 has multiple mechanisms for blocking virus-induced interferon production. J. Virol. 78:8411-8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melroe, G. T., L. Silva, P. A. Schaffer, and D. M. Knipe. 2007. Recruitment of activated IRF-3 and CBP/p300 to herpes simplex virus ICP0 nuclear foci: potential role in blocking IFN-beta induction. Virology 360:305-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menachery, V. D., and D. A. Leib. 2009. Control of herpes simplex virus replication is mediated through an interferon regulatory factor 3-dependent pathway. J. Virol. 83:12399-12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Milatovic, D., Y. Zhang, S. J. Olson, K. S. Montine, L. J. Roberts, 2nd, J. D. Morrow, T. J. Montine, T. S. Dermody, and T. Valyi-Nagy. 2002. Herpes simplex virus type 1 encephalitis is associated with elevated levels of F2-isoprostanes and F4-neuroprostanes. J. Neurovirol. 8:295-305. [DOI] [PubMed] [Google Scholar]
- 47.Mossman, K. L., H. A. Saffran, and J. R. Smiley. 2000. Herpes simplex virus ICP0 mutants are hypersensitive to interferon. J. Virol. 74:2052-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mossman, K. L., and J. R. Smiley. 2002. Herpes simplex virus ICP0 and ICP34.5 counteract distinct interferon-induced barriers to virus replication. J. Virol. 76:1995-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paladino, P., D. T. Cummings, R. S. Noyce, and K. L. Mossman. 2006. The IFN-independent response to virus particle entry provides a first line of antiviral defense that is independent of TLRs and retinoic acid-inducible gene I. J. Immunol. 177:8008-8016. [DOI] [PubMed] [Google Scholar]
- 50.Pasieka, T. J., B. Lu, S. D. Crosby, K. M. Wylie, L. A. Morrison, D. E. Alexander, V. D. Menachery, and D. A. Leib. 2008. Herpes simplex virus virion host shutoff attenuates establishment of the antiviral state. J. Virol. 82:5527-5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pasieka, T. J., B. Lu, and D. A. Leib. 2008. Enhanced pathogenesis of an attenuated herpes simplex virus for mice lacking Stat1. J. Virol. 82:6052-6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pepose, J. S., T. L. Keadle, and L. A. Morrison. 2006. Ocular herpes simplex: changing epidemiology, emerging disease patterns, and the potential of vaccine prevention and therapy. Am. J. Ophthalmol. 141:547-557. [DOI] [PubMed] [Google Scholar]
- 53.Rader, K. A., C. E. Ackland-Berglund, J. K. Miller, J. S. Pepose, and D. A. Leib. 1993. In vivo characterization of site-directed mutations in the promoter of the herpes simplex virus type 1 latency-associated transcripts. J. Gen. Virol. 74:1859-1869. [DOI] [PubMed] [Google Scholar]
- 54.Samuel, C. E. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14:778-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samuel, M. A., and M. S. Diamond. 2005. Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J. Virol. 79:13350-13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sato, M., H. Suemori, N. Hata, M. Asagiri, K. Ogasawara, K. Nakao, T. Nakaya, M. Katsuki, S. Noguchi, N. Tanaka, and T. Taniguchi. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 13:539-548. [DOI] [PubMed] [Google Scholar]
- 57.Sen, G. C. 2001. Viruses and interferons. Annu. Rev. Microbiol. 55:255-281. [DOI] [PubMed] [Google Scholar]
- 58.Serafini, B., S. Columba-Cabezas, F. Di Rosa, and F. Aloisi. 2000. Intracerebral recruitment and maturation of dendritic cells in the onset and progression of experimental autoimmune encephalomyelitis. Am. J. Pathol. 157:1991-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sergerie, Y., S. Rivest, and G. Boivin. 2007. Tumor necrosis factor-alpha and interleukin-1 beta play a critical role in the resistance against lethal herpes simplex virus encephalitis. J. Infect. Dis. 196:853-860. [DOI] [PubMed] [Google Scholar]
- 60.Sobol, P. T., and K. L. Mossman. 2006. ICP0 prevents RNase L-independent rRNA cleavage in herpes simplex virus type 1-infected cells. J. Virol. 80:218-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suthar, M. S., D. Y. Ma, S. Thomas, J. M. Lund, N. Zhang, S. Daffis, A. Y. Rudensky, M. J. Bevan, E. A. Clark, M.-K. Kaja, M. S. Diamond, and M. Gale, Jr. 2010. IPS-1 is essential for the control of West Nile virus infection and immunity. PLoS Pathog. 6:e1000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takaoka, A., Z. Wang, M. K. Choi, H. Yanai, H. Negishi, T. Ban, Y. Lu, M. Miyagishi, T. Kodama, K. Honda, Y. Ohba, and T. Taniguchi. 2007. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448:501-505. [DOI] [PubMed] [Google Scholar]
- 63.Takeuchi, O., and S. Akira. 2002. MyD88 as a bottle neck in Toll/IL-1 signaling. Curr. Top. Microbiol. Immunol. 270:155-167. [DOI] [PubMed] [Google Scholar]
- 64.Tyler, K. L. 2004. Herpes simplex virus infections of the central nervous system: encephalitis and meningitis, including Mollaret's. Herpes 11(Suppl 2):57A-64A. [PubMed] [Google Scholar]
- 65.Verpooten, D., Y. Ma, S. Hou, Z. Yan, and B. He. 2009. Control of TANK-binding kinase 1-mediated signaling by the gamma(1)34.5 protein of herpes simplex virus 1. J. Biol. Chem. 284:1097-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vital, C., E. Monlun, A. Vital, M. Martin-Negrier, V. Cales, F. Leger, M. Longy-Boursier, M. Le Bras, and B. Bloch. 1995. Concurrent herpes simplex type 1 necrotizing encephalitis, cytomegalovirus ventriculoencephalitis and cerebral lymphoma in an AIDS patient. Acta Neuropathol. 89:105-108. [DOI] [PubMed] [Google Scholar]
- 67.Whitley, R. J. 2006. Herpes simplex encephalitis: adolescents and adults. Antiviral Res. 71:141-148. [DOI] [PubMed] [Google Scholar]
- 68.Whitley, R. J. (ed.). 1996. Herpes simplex viruses. Lippencott-Raven Publishers, Philadelphia, PA.
- 69.Williams, L. E., A. B. Nesburn, and H. E. Kaufman. 1965. Experimental induction of disciform keratitis. Arch. Ophthalmol. 73:112-114. [DOI] [PubMed] [Google Scholar]
- 70.Xu, F., M. R. Sternberg, B. J. Kottiri, G. M. McQuillan, F. K. Lee, A. J. Nahmias, S. M. Berman, and L. E. Markowitz. 2006. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 296:964-973. [DOI] [PubMed] [Google Scholar]
- 71.Yamamoto, M., S. Sato, K. Mori, K. Hoshino, O. Takeuchi, K. Takeda, and S. Akira. 2002. A novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J. Immunol. 169:6668-6672. [DOI] [PubMed] [Google Scholar]
- 72.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]
- 73.Yoneyama, M., W. Suhara, Y. Fukuhara, M. Fukuda, E. Nishida, and T. Fujita. 1998. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 17:1087-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang, S.-Y., E. Jouanguy, S. Ugolini, A. Smahi, G. Elain, P. Romero, D. Segal, V. Sancho-Shimizu, L. Lorenzo, A. Puel, C. Picard, A. Chapgier, S. Plancoulaine, M. Titeux, C. Cognet, H. von Bernuth, C.-L. Ku, A. Casrouge, X.-X. Zhang, L. Barreiro, J. Leonard, C. Hamilton, P. Lebon, B. Heron, L. Vallee, L. Quintana-Murci, A. Hovnanian, F. Rozenberg, E. Vivier, F. Geissmann, M. Tardieu, L. Abel, and J.-L. Casanova. 2007. TLR3 deficiency in patients with herpes simplex encephalitis. Science 317:1522-1527. [DOI] [PubMed] [Google Scholar]