Abstract
Rotaviruses are a leading cause of viral acute gastroenteritis in humans and animals. They are grouped according to gene composition and antigenicity of VP6. Whereas group A, B, and C rotaviruses are found in humans and animals, group D rotaviruses have been exclusively detected in birds. Despite their broad distribution among chickens, no nucleotide sequence data exist so far. Here, the first complete genome sequence of a group D rotavirus (strain 05V0049) is presented, which was amplified using sequence-independent amplification strategies and degenerate primers. Open reading frames encoding homologues of rotavirus proteins VP1 to VP4, VP6, VP7, and NSP1 to NSP5 were identified. Amino acid sequence identities between the group D rotavirus and the group A, B, and C rotaviruses varied between 12.3% and 51.7%, 11.0% and 23.1%, and 9.5% and 46.9%, respectively. Segment 10 of the group D rotavirus has an additional open reading frame. Generally, phylogenetic analysis indicated a common evolution of group A, C, and D rotaviruses, separate from that of group B. However, the NSP4 sequence of group C has only very low identities in comparison with cogent sequences of all other groups. The avian group A NSP1 sequences are more closely related to those of group D than those of mammalian group A rotaviruses. Most interestingly, the nucleotide sequences at the termini of the 11 genome segments are identical between group D and group A rotaviruses. Further investigations should clarify whether these conserved structures allow an exchange of genome segments between group A and group D rotaviruses.
Rotaviruses are a major cause of acute gastroenteritis in young children (22, 32, 54, 55). They are also etiological agents of diarrhea in several mammalian and avian species (7, 9, 10, 39, 52, 65, 75). Rotaviruses belong to the family Reoviridae and have a nonenveloped viral capsid containing a genome of 11 double-stranded RNA (dsRNA) segments (21, 62). The outer layer of the virus particle is formed by VP4 and VP7 proteins, which possess neutralization antigens. The intermediate layer consists of VP6, a conserved protein, which defines the rotavirus groups. The inner layer is formed by VP2 surrounding a complex of VP1 and VP3, which represent the RNA-dependent RNA polymerase and the guanylyl transferase, respectively (12, 60), and the viral genomic RNAs. In addition, at least five nonstructural proteins are encoded by the rotavirus genome and have diverse functions, e.g., modulation of the host immune response (NSP1), regulation of the viral gene expression (NSP3), or the induction of diarrhea (NSP4) (4, 50, 68).
Based on the antibody reactivity and sequence identity of VP6, five rotavirus groups (A to E) have been defined (62). Two additional tentative rotavirus groups (F and G) have been described but only scarcely characterized so far. Recently, novel rotavirus strains with limited sequence homologies to group B rotaviruses (strains J19 and B219) have been identified in adults with diarrhea but are without a final classification (31, 51, 77). Group A, B, and C rotaviruses are found in both humans and animals, whereas group E rotaviruses have been detected only in pigs and group D, F, and G rotaviruses have been detected only in birds (21, 58, 59, 62, 66). So far, nucleotide sequences are available only for group A, B, and C rotaviruses. Phylogenetic analysis of the sequences confirmed the suggested grouping of these viruses (30, 31, 69, 74). In addition, group-specific conserved sequences are present at the termini of the genome segments. It is suspected that these conserved sequences influence the ability of genome segment exchanges between different rotavirus strains by reassortment (37, 45, 46), which is only possible within a rotavirus group (13, 29, 34, 41, 42, 43, 56).
In avian species, rotavirus groups A, D, F, and G have been detected so far (21, 47, 48, 67). In general, rotavirus infections are common in young birds and gradually more infrequent in older birds (28, 33). The symptoms of the disease generally include diarrhea and depression (25, 47, 48, 67). Increased mortality and the chronic runting and stunting syndrome mainly characterized by weight loss have also been linked to rotavirus infections of birds (18, 28). Among the avian rotaviruses, only that of group A has been characterized in more detail, and two complete genome sequences of isolates from pigeon and chicken are available (30, 74). Phylogenetic sequence analysis shows that the avian group A isolates form a separate clade within group A; however, the relationship with the other group A rotaviruses is evident (74).
Group D rotaviruses have been first identified in feces of chickens (49, 59, 67). Results of indirect immunofluorescence, electrophoresis of the genome segments, and Southern blot hybridization of the prototype strain D/132 showed differences to group A, B, C, and E viruses and resulted in a separate grouping of the virus (59). Further studies showed that group D rotaviruses are the most widespread rotaviruses in turkey poults with diarrhea (63, 64). The detection of group D rotaviruses in chicken feces has been documented in the United Kingdom and Germany (49, 53). Unlike the group A rotaviruses, which preferentially infect cells of the duodenum, group D rotaviruses have a predilection for the jejunum and ileum (28). In contrast to avian group A rotaviruses, group D rotaviruses cannot be propagated in MA104 cells (18). As group D-specific antisera are not commercially available and any nucleotide sequence information for group D rotaviruses does not exist so far, group D rotaviruses are mainly identified by the 5:2:2:2 electrophoretic migration pattern of their genome segments (8, 47, 48, 53, 59, 70), which is considerably different from the 5:1:3:2 pattern of avian group A rotaviruses. However, classification of a rotavirus strain only on the basis of its RNA migration profile is problematic due to the possible occurrence of genome rearrangements (17).
In order to characterize the group D rotaviruses in more detail, the first complete genome sequence of a group D rotavirus has been determined here. Its comparison to that of group A, B, and C rotaviruses should reveal the distinct phylogenetic relationships among the rotavirus groups. The availability of the group D rotavirus genome sequence should be a basis for the development of diagnostic tests, which could be used to determine the clinical significance and the distribution of these rotaviruses in poultry. In addition, it may help to assess the probability of transmission of these viruses to humans and the ability to generate reassortants with other rotaviruses.
MATERIALS AND METHODS
Rotavirus strains.
Group D rotavirus strain 05V0049 (Ch-49) originated from intestinal content of a chicken suffering from runting and stunting syndrome in 2005 in Germany. The sample used in this study was derived from an experimental infection study of chicken using strain Ch-49 (P. Otto, personal communication). Group A rotavirus Ch-2G3 was propagated in MA104 cells as described previously (74).
Electron microscopy.
The supernatants of the fecal samples were applied to polioform-carbon-coated, 400-mesh copper grids (Plano GmbH, Wetzlar, Germany) for 10 min, fixed with 2.5% aqueous glutaraldehyde solution for 1 min, stained with 2% aqueous uranyl acetate solution for 1 min, and examined by transmission electron microscopy using a JEM-1010 transmission electron microscope (JOEL, Tokyo, Japan) at 80 kV accelerated voltage.
RNA-PAGE.
RNA was extracted from the virus-containing cell culture or the suspension of intestinal contents using the QIAamp viral RNA minikit (Qiagen, Hilden, Germany) according to the instructions of the manufacturer. The samples were analyzed for their RNA pattern using PAGE and silver staining, as described previously (52). Gels were dried in a GelAir dryer (Bio-Rad Laboratories, Munich, Germany).
Isolation of double-stranded RNA.
Virus was concentrated from 750 μl of the intestinal content by ultracentrifugation at 150,000 × g for 3 h. The resulting pellet was dissolved using 1 ml Trizol (Invitrogen Limited, Paisley, Scotland) and 200 μl chloroform, and the mixture was incubated for 10 min on ice. After centrifugation at 9,000 × g for 10 min, the supernatant was mixed with 900 μl isopropanol and the total RNA was precipitated at −20°C for 20 h. The suspension was centrifuged at 14,000 rpm for 15 min, and the pellet was washed with 1 ml ice-cold ethanol (70%). The pellet was dried and resuspended in 100 μl nuclease-free water. Single-stranded RNA was precipitated by adding 100 μl 4 M LiCl in an overnight incubation followed by centrifugation at 12,000 × g for 5 min. The supernatant was mixed with 200 μl isopropanol and 50 μl 3 M sodium acetate (pH 5.6) and incubated for 2 h at −20°C in order to precipitate the dsRNA. After centrifugation at 12,000 × g for 5 min, the pellet was dried and dissolved in 10 μl nuclease-free water.
FLAC.
Full-length amplification of cDNAs (FLAC) is a sequence-independent method for the amplification of full-length cDNA from double-stranded RNA templates. The protocol is based on ligation of a self-priming oligonucleotide at the 3′ ends of the dsRNAs and has been adapted from the protocols of Attoui et al. (3) and Maan et al. (38). Briefly, 3 μl of double-stranded RNA was mixed with 1 μl oligonucleotide iSP9 (5′-GACCTCTGAGGATTCTAAAC/C9-phosphoamidite spacer/TCCAGTTTAGAATCC-OH3′ (TIB Molbiol, Berlin, Germany); C9-phosphoamidite spacer; TIB MOLBIOL, Berlin, Germany), 8 μl nuclease-free water, 0.5 μl bovine serum albumin (BSA; 10 mg/ml), 1.5 μl T4 RNA ligase buffer (New England Bio Labs), and 1 μl T4 RNA ligase (10 U; New England Bio Labs). After incubation overnight at 17°C, the RNA segments were separated by electrophoresis on an ethidium bromide-stained 1% agarose gel. The individual segments were excised and purified using the QIAquick gel extraction kit (Qiagen, Hilden, Germany). Sixty microliters of the purified RNA was mixed with 6 μl dimethyl sulfoxide, heated for 3 min at 95°C, and immediately cooled using an ethanol bath at −20°C. The Qiagen LongRange 2Step RT-PCR kit (Qiagen, Hilden, Germany) was used for reverse transcription according to the manufacturer's instructions, but without adding a primer, at 37°C for 50 min followed by 40°C for 10 min. The same kit was used for PCR amplification of the cDNAs using primer 5-15-1 (5′GAGGGATCCAGTTTAGAATCCTCAGAGGTC3′) with the following temperature profile: 95°C for 3 min, 40 cycles of 93°C for 30 s, 63°C for 30 s, and 68°C for 5 min, and a final incubation at 68°C for 7 min.
Degenerate primer PCR.
If the whole segment sequence could not be amplified by FLAC, primers with binding sites in conserved regions of avian and mammalian rotavirus sequences were constructed. Additional primer sequences were deduced after determination of partial genome segment sequences obtained by FLAC. The successfully used primer sequences are listed in Data S1 in the supplemental material. PCR amplification was performed with the Qiagen LongRange 2Step RT-PCR kit (Qiagen, Hilden, Germany) using the cDNAs generated as described above as the template.
Sequencing and sequence analysis.
The PCR products were cloned into the vector pCR4-TOPO using the TOPO TA cloning kit for sequencing (Invitrogen, Leek, Netherlands) and subsequently sequenced using M13 forward and M13 reverse primers (Invitrogen) as well as gene-specific primers in an ABI 3730 DNA analyzer (Applied Biosystems). The complete sequences of the genome segments were assembled from the determined sequence pieces using the SeqBuilder module of the DNASTAR software package (Lasergene, Madison, WI). Open reading frames (ORFs) were identified and amino acid sequences were deduced from the nucleotide sequences using the same module. Sequence alignments and construction of phylogenetic trees were performed using the MegAlign module of the above-mentioned software package. The accession numbers of rotavirus sequences included in the analyses are shown in Data S2 in the supplemental material or included in the labeling of branches of the phylogenetic trees. The CLUSTAL W method was used with the IUB (nucleotides) or identity (amino acids) residue weight tables (71) in alignments, and bootstrap analysis of phylogenetic trees was performed with 1,000 trials and 111 random seeds.
Nucleotide sequence accession numbers.
The genome segment sequences of the group D rotavirus strain 05V0049 were deposited in the GenBank database under accession numbers GU733443 to GU733453.
RESULTS
Identification of a group D rotavirus.
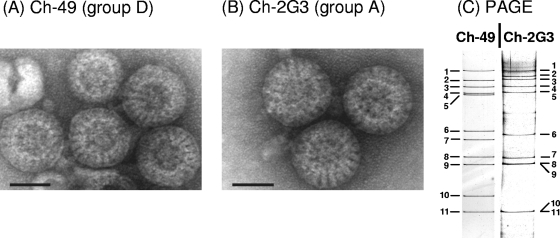
Rotavirus strain 05V0049 (Ch-49), originally derived from the intestinal content of a chicken suffering from runting and stunting syndrome, was analyzed by negative-contrast electron microscopy. As shown in Fig. 1 A, particles with the typical shape of rotaviruses and diameters of the complete virus particles of approximately 75 to 81 nm (average, 78 nm) were visible. The chicken group A rotavirus Ch-2G3 was used as a comparative control and showed similar complete particles with comparable diameters (between 75 and 81 nm; average, 78 nm) (Fig. 1B). PAGE analysis of the RNA genomes showed a different banding pattern of the two chicken rotaviruses (Fig. 1C). Strain Ch-49 has an electrophoretic migration pattern of 5:2:2:2, which has been shown to be characteristic for group D rotaviruses (8, 47, 48, 53, 59, 70), whereas isolate Ch-2G3 showed a banding pattern of 5:1:3:2, typical for avian group A rotaviruses.
FIG. 1.
Identification of a group D rotavirus (strain 05V0049 [Ch-49]) in the intestinal content of a chicken. The chicken group A rotavirus isolate 02V0002G3 (Ch-2G3) was used as a comparative control. Strains Ch-49 (A) and Ch-2G3 (B) were analyzed by negative-stain electron microscopy showing four complete particles plus one incomplete particle and three complete particles, respectively. The bar corresponds to 50 nm. (C) PAGE analysis of the RNA genome of strains Ch-49 and Ch-2G3. The numbering of the genome segments is indicated.
Genome sequence determination.
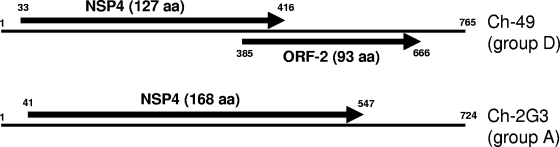
Using the FLAC technique, the full-length sequences of genome segment 6 and segments 8 to 11 could be amplified and cloned. In all other cases, additional degenerate primers were used to amplify overlapping fragments of the genome segments. The entire genome sequence of rotavirus D strain Ch-49 was assembled from a total of 33 fragments and consists of 18,500 nucleotides. The sizes of the genome segments and their coding capacity are shown in Table 1. Each genome segment contains an ORF encoding a protein with homologies to rotavirus proteins (Table 1). An ORF encoding a protein with homologies to NSP6 was not identified. Segment 10 has a relatively short NSP4-encoding ORF; however, a partially overlapping additional ORF (in alternative +1 reading) designated ORF-2 is present (Fig. 2). In order to identify possible deletions or mutations in the analyzed clone, two additional clones as well as a PCR product covering both ORFs were sequenced, but the original sequence was confirmed. ORF-2 has a coding capacity for a protein with a length of 93 amino acid residues. A BLASTp search of GenBank using the sequence encoded by ORF-2 revealed a homology to a lipoprotein of Pseudomonas entomophila; however, it had only a 33% amino acid sequence identity in the central 57 positions.
TABLE 1.
Comparison of genome segment sizes and coding capacity of two avian rotaviruses of group D (strain Ch-49) and group A (strain Ch-2G3)
| Segment no. | Rotavirus group D strainCh-49 |
Rotavirus group A strain Ch-2G3 |
||||
|---|---|---|---|---|---|---|
| Size (nt) | Encoded protein (aa) | ORF position (nt) | Size (nt) | Encoded protein (aa) | ORF position (nt) | |
| 1 | 3,274 | VP1 (1,079) | 19-3,258 | 3,305 | VP1 (1,089) | 19-3,270 |
| 2 | 2,801 | VP2 (913) | 40-2,742 | 2,732 | VP2 (895) | 18-2,705 |
| 3 | 2,366 | VP4 (777) | 10-2,343 | 2,583 | VP3 (829) | 50-2,539 |
| 4 | 2,104 | VP3 (685) | 15-2,072 | 2,354 | VP4 (770) | 10-2,322 |
| 5 | 1,872 | NSP1 (574) | 38-1,762 | 2,122 | NSP1 (577) | 39-1,772 |
| 6 | 1,353 | VP6 (398) | 21-1,217 | 1,348 | VP6 (397) | 24-1,217 |
| 7 | 1,242 | NSP3 (370) | 43-1,155 | 1,089 | NSP3 (304) | 78-992 |
| 8 | 1,026 | NSP2 (310) | 50-982 | 1,066 | VP7 (329) | 50-1,039 |
| 9 | 1,025 | VP7 (316) | 44-994 | 1,042 | NSP2 (315) | 46-993 |
| 10 | 765 | NSP4 (127) | 33-416 | 724 | NSP4 (168) | 41-547 |
| ORF2 (93) | 385-666 | |||||
| 11 | 672 | NSP5 (195) | 30-603 | 699 | NSP5 (208) | 22-648 |
| Total genome | 18,500 | 19,064 | ||||
FIG. 2.
Schematic presentation of the structures of genome segment 10 of group D rotavirus Ch-49 and group A rotavirus Ch-2G3. Open reading frames are shown as arrows; their coding capacities are presented in parentheses (aa, amino acid residues). The numbers in small type indicate the nucleotide positions.
Phylogenetic analysis.
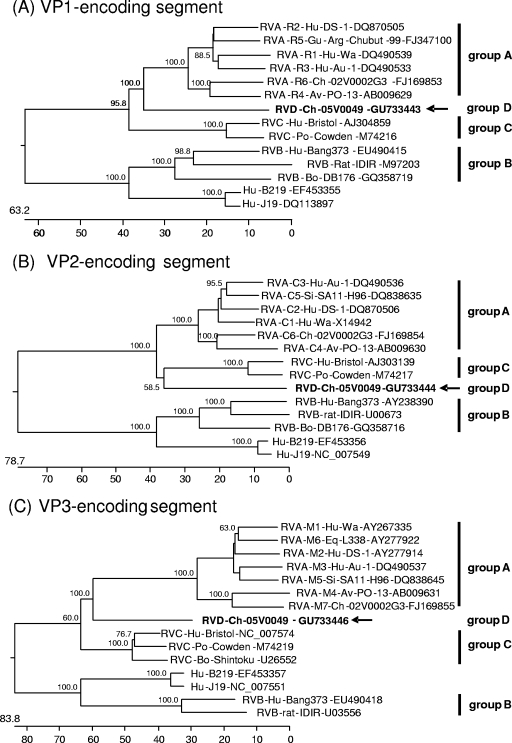
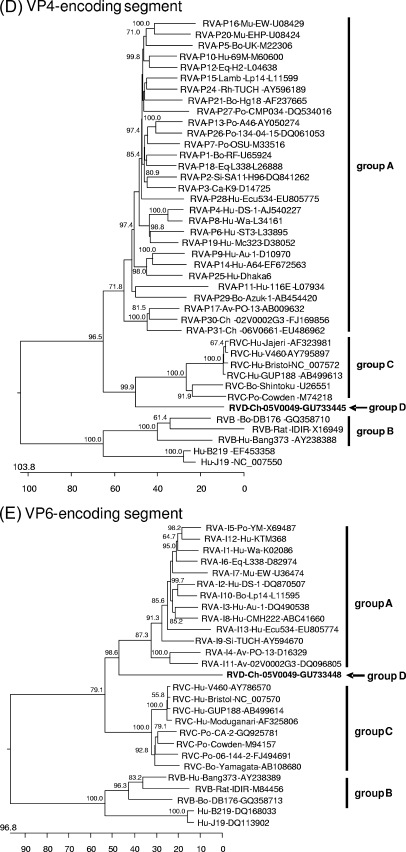
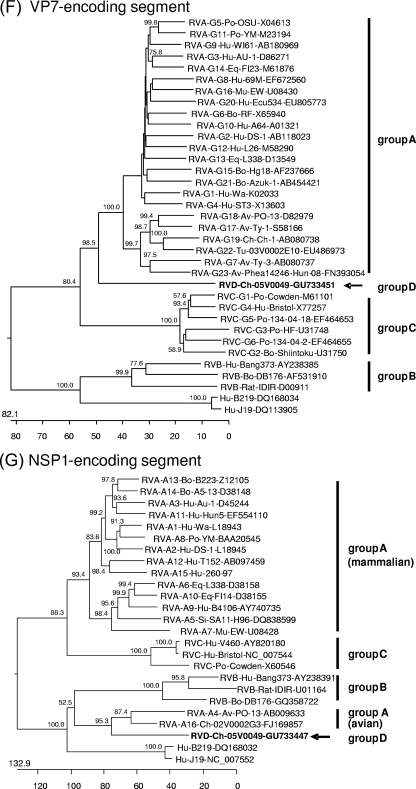
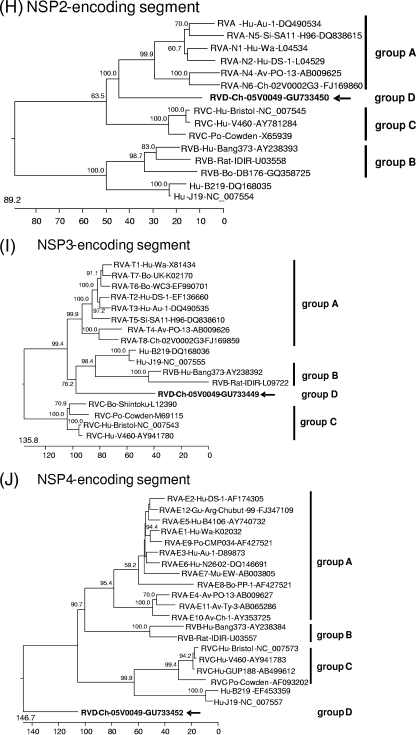
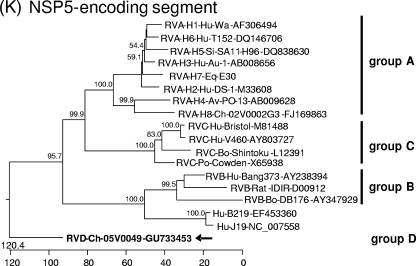
Phylogenetic trees were constructed on the basis of the entire nucleotide sequences of the genome segments (Fig. 3) or the deduced amino acid sequences (see Data S3 and S4 in the supplemental material). Most of the resulting trees showed a largely similar grouping irrespective of whether nucleotide or amino acid sequences had been used for alignment; however, different trees were obtained in the case of the NSP3-, NSP4-, and NSP5-encoding segments. All trees constructed for the segments encoding the structural proteins and NSP2 show one branch containing the group B rotaviruses together with the newly described rotavirus strains J19 and B219 and another branch containing group A, C, and D rotaviruses (Fig. 3A to F and H; see also Data S3A to F and S4B in the supplemental material). Within the latter branch, group D generally branches out close to the root. A different grouping is obtained for the NSP1 sequences, with one branch consisting of avian group A and D rotaviruses and another branch containing mammalian group A and C rotaviruses (Fig. 3G; see also Data S4A in the supplemental material). The NSP3 tree based on nucleotide sequences shows one branch consisting of groups A, B, and D and another branch containing group C (Fig. 3I), whereas the amino acid-based tree shows the groups A, C, and D in one branch and group B in the other (see Data S4C in the supplemental material). In both trees based on the nucleotide sequences of the NSP4- and NSP5-encoding segments, the group D rotavirus forms a branch well separated from the other groups, reflecting the low nucleotide sequence identities of these genome segments (Fig. 3J and K). However, the NSP4 and NSP5 sequences of the group D rotavirus are clearly grouped together with their homologues when their amino acid sequences are phylogenetically analyzed (see Data S4D and E in the supplemental material). By this, the NSP4 sequences of groups A, B, and D form one cluster separated from that of group C (see Data S4D in the supplemental material), and the NSP5 sequences of group A, C, and D form one cluster separated from that of group B (see item S4E in the supplemental material).
FIG. 3.
Phylogenetic relationship of group A, B, C, and D rotaviruses based on the entire nucleotide sequences of genome segments encoding VP1 (A), VP2 (B), VP3 (C), VP4 (D), VP6 (E), VP7 (F), NSP1 (G), NSP2 (H), NSP3 (I), NSP4 (J), and NSP5 (K). The branches are labeled with the rotavirus group and genotype (if applicable), the specific host abbreviation, strain abbreviation, and GenBank accession number. Clustering into the rotavirus groups is indicated at the right and the group D rotavirus 05V0049 is marked in boldface and with an arrow. The trees are scaled in nucleotide substitution units and were constructed using MEGALIGN software (CLUSTAL W method; IUB DNA weight matrix; 1,000 trials and 111 random seeds in bootstrap analysis; bootstrap values of >50% are indicated at the knots of the branches).
Analysis of coding regions.
The deduced amino acid sequences of the proteins encoded by the Ch-49 genome were compared to the homologues of 10 group A, B, and C rotaviruses and strain J19 (all strains are listed in Data S2 in the supplemental material). The percentage of amino acid sequence identity between Ch-49 and group A, B, and C rotaviruses ranged between 12.3% and 51.7%, 11.0% and 23.1%, and 9.5% and 46.9%, respectively. As shown in Table 2, the structural proteins are generally more conserved compared to the nonstructural proteins. The NSP1 sequence of the group D rotavirus has 34.7% to 35.5% sequence identity to that of the avian group A rotaviruses but only 6.7% to 15.8% sequence identity to that of all other groups, including the mammalian group A rotaviruses and rotavirus strains J19 and B219. Based on the results of phylogenetic analysis and the determined percentage of sequence identity, the amino acid sequences encoded by Ch-49 were compared in more detail to that of mammalian group A and C rotaviruses. For the NSP1 and NSP4 sequences, avian group A rotavirus and group B rotavirus sequences were additionally included, respectively.
TABLE 2.
Comparison of the amino acid sequences of the group D rotavirus (strain Ch-49) proteins with that of group A, B, and C rotaviruses
| Protein | % amino acid sequence identity ofa: |
|||
|---|---|---|---|---|
| Group D and: |
Group A and: |
|||
| Group A | Group B | Group C | Group C | |
| VP1 | 50.5-51.7 | 21.5-23.1 | 46.6-46.5 | 47.5-48.6 |
| VP2 | 46.6-46.8 | 16.2-17.2 | 42.5-45.2 | 46.1-49.5 |
| VP3 | 33.8-35.5 | 18.4-20.0 | 30.2-30.9 | 35.4-38.3 |
| VP4 | 33.5-35.9 | 12.4-13.5 | 30.7-32.4 | 31.7-34.3 |
| VP6 | 36.4-38.1 | 12.6-14.2 | 35.0-35.7 | 41.5-43.2 |
| VP7 | 35.6-40.0 | 14.9-17.4 | 28.3-29.3 | 32.4-35.2 |
| NSP1 | 12.3-35.2 | 11.0-11.0 | 15.1-15.8 | 14.3-25.3 |
| NSP2 | 36.6-38.6 | 18.8-19.2 | 33.0-34.0 | 33.0-36.0 |
| NSP3 | 29.1-30.6 | 15.2-16.8 | 21.3-21.7 | 25.2-26.5 |
| NSP4 | 15.2-19.2 | 11.5-16.4 | 9.5-12.7 | 9.7-15.8 |
| NSP5 | 20.7-23.6 | 17.5-17.9 | 23.7-24.0 | 28.9-32.2 |
Amino acid sequence identities were calculated using the CLUSTAL W method with the identity residue weight table. The strains used in alignments are listed in Data S2 in the supplemental material.
VP1.
An analysis of predicted functionally important domains in Ch-49 revealed a conservation of polymerase elements in VP1 (76), with only a few amino acid exchanges in the motifs A, B, C, D, and F, and the strictly conserved catalytic sequence GDD in motif C. The sequence alignment shows that all of these motifs are highly conserved among group A, C, and D rotaviruses (see Data S5A in the supplemental material). Eight of the 10 amino acid residues involved in recognition of the 3′-terminal genome segment sequence UGUGACC in the group A rotavirus VP1 (37) are identical to that in the group D rotavirus VP1.
VP2.
Conserved amino acid positions are scattered throughout the whole sequence of VP2 (see Data S5B in the supplemental material). As evident from the sequence alignment, the group D VP2 has insertions of approximately 25 amino acids in the N-terminal region compared to the VP2 molecules of group A and C.
VP3.
The motif KX[D/N]G, which is required for guanylyltransferase activity (23), is found in the VP3 sequence of Ch-49 at position 532 to 535 (see Data S5C in the supplemental material). Only the first five amino acid positions of the motif KXTAMDXEXP, which is found in group A and C rotaviruses and possibly also involved in enzymatic activity (15), are conserved in the group D rotavirus VP3.
VP4.
The VP4 region corresponding to that carrying the major trypsin cleavage sites is less conserved in Ch-49 (positions 251 to 267) and contains only one of the arginine positions (Arg263) together with group A rotaviruses (24); however, three other Arg/Lys residues potentially serving as cleavage sites are present in this region (see Data S5D in the supplemental material). Sequence conservation is generally low in the N-terminal region of VP4, which also contains a sialic acid binding sequence in some of the group A rotaviruses (19) that is not evident in the group D rotavirus.
VP6.
The amino acid exchanges in the VP6 sequence of group A, C, and D rotaviruses are scattered over the whole sequence. As expected, the sequences of the group-specific antigenic regions identified in group A rotaviruses (11) are not identical to that of the other groups (see Data S5E in the supplemental material). The histidine residue at position 153 of VP6 involved in zinc binding in group A rotaviruses (40) is also conserved in Ch-49.
VP7.
The regions A, B, and C of VP7, which have been implicated in neutralization of group A rotaviruses (14), are not conserved in the group C and D rotaviruses (see Data S5F in the supplemental material). The sequence Asn-Ser-Thr, which serves as a carbohydrate acceptor site in group A rotaviruses (2), is not found in the Ch-49 VP7. Eight of the cysteine residues involved in the stabilization of the protein structure by the formation of disulfide bonds (21) are conserved among the VP7 sequences of group A, C, and D rotaviruses.
NSP1.
An alignment of the NSP1 sequence of Ch-49 with that of mammalian group A and C rotaviruses reveals nearly no sequence conservation (see Data S5G in the supplemental material). In contrast, sequence conservation is evident throughout the whole NSP1 sequence for the group D rotavirus and an avian group A rotavirus. A search of the Conserved Domain Database (NCBI) revealed a conserved domain of the TFA-1 (transcription initiation factor IIE, alpha subunit) superfamily in the central part of the avian group A and D NSP1 sequences but not in those of the NSP1 molecules of the other rotavirus groups. However, the degree of sequence conservation between the entire TFA-1 sequence and the avian rotavirus NSP1 molecules is relatively low (18.7 to 35.2% amino acid sequence identity). All NSP1 sequences of the group A, C, and D rotaviruses contain conserved cysteine and histidine residues within the functionally important zinc-binding RING finger domain (26).
NSP2.
This protein shows the highest degree of sequence conservation among the nonstructural proteins of the group A, C, and D rotaviruses (see Data S5H in the supplemental material). The NTPase domain is highly conserved in the NSP2 sequences, with His218 most likely representing the catalytic residue (35) in the case of Ch-49.
NSP3.
Sequence conservation in group A, C, and D rotaviruses is only evident within the RNA-binding domain of this protein (see Data S5I in the supplemental material). NSP3 of group A and C rotaviruses preferentially binds to slightly different sequences, which are present at the 3′-terminal ends of their mRNAs (16). One of the amino acid positions responsible for the different sequence binding (Arg105 in group A rotaviruses) is identical in group A and D NSP3, whereas the other (Gln115 in group A rotaviruses) is identical in group C and D NSP3.
NSP4.
NSP4 of Ch-49 shows only low sequence identities compared to that of group A rotaviruses; however, potential glycosylation sites (Asn11 and Asn20) (36) are evident in both groups (see Data S5K in the supplemental material). Almost no sequence identities are found between group C and D NSP4. More identical amino acid positions could be found by comparison of the Ch-49 NSP4 with that of group B; however, several large insertions are evident in the group B sequence compared to that of group D.
NSP5.
As in group A rotaviruses, the NSP5 protein of Ch-49 is a serine/threonine-rich protein (23 serine residues and 9 threonine residues out of 195 amino acids). Only one of the four casein kinase II phosphorylation sites identified in group A rotavirus NSP5 (20) is also found in Ch-49 NSP5 (Ser166) (see Data S5L in the supplemental material). Most sequence conservation between group A, C, and D rotavirus NSP5 is found in the carboxy-terminal region, which is responsible for dimerization of this protein (72).
Analysis of genome segment ends.
The noncoding regions of the genome segments of strain Ch-49 have a length of 9 to 49 nucleotides in the 5′ region and 16 to 136 nucleotides in the 3′ region (Table 1). Only the 3′ noncoding region of the NSP1-encoding genome segment 5 of Ch-49 shows an accumulation of adenine residues (54 adenine residues out of 110 nucleotides), which is similar to the corresponding region of the NSP1-encoding segment of the chicken group A rotavirus Ch-2G3 and the function of which is so far not known (74). The terminal nucleotide sequences are conserved among the group D rotavirus genome segments with consensus sequences of GGUUUUUAAA at the 5′ end and UUGUGACC at the 3′ end. As evident from Table 3, these consensus sequences are nearly identical to that of group A rotaviruses (only the 5′-terminal nucleotide position 3 is more variable in group D) but different from those of group B and C rotaviruses.
TABLE 3.
Comparison of 5′- and 3′-terminal sequences of group D rotavirus strain Ch-49 genome segments to those of group A, B and C rotavirusesa
| Segment or group | 5′-terminal sequence | 3′-terminal sequence |
|---|---|---|
| 1 | GGCAAUUAAAGUGGU | CACUUACUUGUGACC |
| 2 | GGCAUAUAAACUGCG | UGUGCCUUUGUGACC |
| 3 | GGCAAUAAAAUGGCU | CGUACUAUUGUGACC |
| 4 | GGCUUUUAAAGCACA | CCCCUUUAUGUGACC |
| 5 | GGUUUUUAAAAGACC | GCAUAAAAUGUGACC |
| 6 | GGUUUUAAAUAGUAA | CUAUUAUCUUUGACC |
| 7 | GGUUUUAAAAUUCAG | GGGCACCAUGCGACC |
| 8 | GGUUUUUAAAAGUUU | ACUUUUAAUGUGACC |
| 9 | GGCAUUUAAAGGCAU | UACUUUAAUAUGACC |
| 10 | GGUUUUAAAAUUUAU | UAAUAAAUUGUGACC |
| 11 | GGUUUUAAAUUGCUA | ACCCACCUUGUGACC |
| Group D | GGUUUUUAAA | UUGUGACC |
| Ch-49 | CAAAA U | A AC |
| Consensus | C U | |
| Group A | GGCUUUUAAA | AUGUGACC |
| Ch-2G3 | AA A | U A |
| Consensus | U | |
| Group B | GGUAUAAAAA | UAAAAACCC |
| Bang373 | CUAUUUUU | AU GG |
| Consensus | C G C | |
| G | ||
| Group C | GGCUUUUAAA | AUGUGGCU |
| Bristol | AAAA U | U |
| Consensus | C | |
| G |
Consensus sequences are in bold.
DISCUSSION
Rotaviruses are genetically highly diverse and can be differentiated into 7 groups according to the antigenicity and gene composition of their VP6 (21, 62). In addition, several group A, B, and C rotaviruses have been characterized on the basis of their genome sequences, allowing a deeper insight into the evolutionary relationship of these viruses (1, 31, 42, 43). The first genome sequence of the group D rotavirus presented here enables a more comprehensive classification of this virus compared to its former grouping based on serological criteria and limited terminal fingerprinting analysis (59). Generally, the obtained sequence data support the classification of this virus in a separate rotavirus group, as the sequence identities to the other rotavirus groups are relatively low. This is not only restricted to the VP6 sequence commonly used for group classification but also accounts for the other proteins. In the phylogenetic trees, the group D rotavirus does not consistently branch with one of the other groups, indicating a separate evolution of this group. In most of the trees, group A, C, and D rotaviruses cluster together, indicating a common ancestor for these groups which has been previously separated from the common ancestor of group B and strain J19. Analysis of more group D rotavirus strains is necessary in order to reconstruct the evolutionary relationship of these viruses in more detail.
The genome of the group D rotavirus shows typical features common to all rotaviruses in regard to the encoded proteins and the conserved sequences at the genome segment ends. However, homologous proteins have been identified only on the basis of sequence comparisons so far; therefore, functional studies have to be performed in further studies. Generally, each genome segment was found to encode one protein, with the exception of segment 10, which possesses two partially overlapping ORFs. Additional ORFs have also been found in other rotavirus genes, e.g., the ORF encoding NSP6 in many of the group A rotaviruses (61, 72) and an additional ORF in the NSP1-encoding segment of group B rotaviruses (1). The functional significance of ORF-2 of the group D rotavirus is so far not known. The search for homologous proteins revealed only low percentages of sequence identity to known proteins. Further investigations on other group D rotaviruses may help to identify whether this ORF is present only incidentally in the investigated strain or it represents a typical feature of this rotavirus group.
A closer examination of the identified proteins revealed many conserved amino acid positions in functionally important domains of the proteins throughout mammalian and avian group A, C, and D rotaviruses. However, an unexpected high sequence identity between the NSP1 sequences was found only between the group D rotavirus and the avian group A rotaviruses. The close phylogenetic relationship of the NSP1 sequences of these avian rotaviruses are comparable to that observed within the mammalian group A rotavirus NSP1 sequences or within the other rotavirus groups (Fig. 3G; see also Data S4A in the supplemental material). In addition, the NSP1-encoding segments of avian group A and D rotaviruses contain a large number of A residues in their 3′ untranslated region, which is unique for these viruses. The low sequence identities between the avian and mammalian group A rotavirus NSP1 sequences have been described previously (30, 74). Based on the results presented here, it may be speculated that in former times the avian group A rotaviruses received their NSP1-encoding genome segment from a virus related to group D. Alternatively, as the function of NSP1 in modulation of the host immune response (5, 6, 27) requires a close interaction with the host proteins, the close relationship of both groups may simply reflect an adaptation on the same host. Until now, the mechanism of action of avian rotavirus NSP1 is not known. The identification of a conserved functional domain in this protein, which is also present in several transcription factors, may be a starting point for further investigations, revealing the function of avian rotavirus NSP1.
Although the encoded proteins have only low sequence identities, the sequences at the termini of the group D rotavirus genome segments are nearly identical to that of group A. A relatively high degree of sequence conservation between the genome segment termini of group A and D rotaviruses, but not between other groups, has been already found in earlier studies using terminal fingerprinting analysis (59). So far, each of the molecularly characterized rotavirus groups possesses unique terminal sequences, which are most evident at the 3′-terminal genome segment ends (62). These sequences are specifically bound by the group-specific replication proteins and contribute also to gene expression and packaging of the genome segments into the viral particles (37, 44, 45, 46, 57, 73). It has been proposed that these conserved sequences influence the ability of genome segment exchanges between different rotavirus strains by reassortment (21, 44, 62). It may be therefore speculated that the finding of nearly identical terminal sequences in group A and group D rotaviruses may allow reassortment of genome segments between viruses of these groups. Further investigations should focus on using in vitro experiments to try to generate those reassortants. If such reassortment could occur in the field, the generation of novel group A rotavirus strains with the largely different antigenicity of group D rotaviruses may be theoretically possible. So far, no group D rotavirus sequences have been detected in human or mammalian rotaviruses, which might indicate that the reassortment events as suggested above did not occur. However, it may also reflect an insensitivity of the used methods with respect to the detection of group D rotaviruses. Based on the genome sequence determined here, group D rotavirus-specific diagnostic methods could now be developed. Such methods will be useful not only for investigations of group D rotavirus sequences in human or mammalian samples but may also help to assess the distribution of group D rotaviruses within the poultry and possibly other avian populations and to investigate their involvement in poultry diseases.
Supplementary Material
Acknowledgments
We thank Silke Apelt, Ute Polster-Brylla, and Maria M. Vargas for excellent technical assistance and Jelle Matthijnssens (Rega Institute, University of Leuven, Belgium) for providing the nucleotide sequences of prototype strains for group A rotavirus genotypes.
This work was funded in part by a grant from the Deutsche Forschungsgemeinschaft (JO 369/4-1).
Footnotes
Published ahead of print on 14 July 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Ahmed, M. U., N. Kobayashi, M. Wakuda, T. Sanekata, K. Taniguchi, A. Kader, T. N. Naik, M. Ishino, M. M. Alam, K. Kojima, and A. Sumi. 2004. Genetic analysis of group B human rotaviruses detected in Bangladesh in 2000 and 2001. J. Med. Virol. 1:149-155. [DOI] [PubMed] [Google Scholar]
- 2.Arias, C. F., S. López, J. R. Bell, and J. H. Strauss. 1984. Primary structure of the neutralization antigen of simian rotavirus SA11 as deduced from cDNA sequence. J. Virol. 50:657-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attoui, H., F. Billoir, J. F. Cantaloube, P. Biagini, P. De Micco, and X. De Lamballerie. 2000. Strategies for the sequence determination of viral dsRNA genomes. J. Virol. Methods 89:147-158. [DOI] [PubMed] [Google Scholar]
- 4.Ball, J. M., P. Tian, C. Q. Zeng, A. P. Morris, and M. K. Estes. 1996. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science 272:101-104. [DOI] [PubMed] [Google Scholar]
- 5.Barrow, M., and J. T. Patton. 2005. Rotavirus nonstructural protein 1 subverts innate immune response by inducing degradation of IFN regulatory factor 3. Proc. Natl. Acad. Sci. U. S. A. 102:4114-4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrow, M., and J. T. Patton. 2007. Rotavirus NSP1 inhibits expression of type I interferon by antagonizing the function of interferon regulatory factors IPF3, IRF5, and IRF7. J. Virol. 81:4473-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergeland, M. E., J. P. McAdaragh, and J. Stotz. 1977. Rotaviral enteritis in turkey poults, p. 129-130. In Proceedings of the 26th Western Poultry Disease Conference. University of California, Davis, CA.
- 8.Bridger, J. C. 1994. Non-group A rotaviruses, p. 369-407. In A. Z. Kapikian (ed.), Viral infections of the gastrointestinal tract, 2nd ed. Marcel Dekker Inc., New York, NY.
- 9.Browning, G. F., and A. P. Begg. 1996. Prevalence of G and P serotypes among equine rotaviruses in the faeces of diarrhoeic foals. Arch. Virol. 141:1077-1089. [DOI] [PubMed] [Google Scholar]
- 10.Brussow, H., O. Nakagomi, G. Gerna, and W. Eichhorn. 1992. Isolation of an avianlike group A rotavirus from a calf with diarrhea. J. Clin. Microbiol. 30:67-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buragohain, M., S. S. Cherian, G. Prabhakar, and S. D. Chitambar. 2008. VP6 capsid protein of chicken rotavirus strain CH2: sequence, phylogeny and in silico antigenic analyses. Virus Res. 137:173-178. [DOI] [PubMed] [Google Scholar]
- 12.Chen, D., C. L. Luongo, M. L. Nibert, and J. T. Patton. 1999. Rotavirus open cores catalyze 5′-capping and methylation of exogenous RNA: evidence that VP3 is a methyltransferase. Virology 265:120-130. [DOI] [PubMed] [Google Scholar]
- 13.Chizhikov, V., and J. T. Patton. 2000. A four-nucleotide translation enhancer in the 3′-terminal consensus sequence of the nonpolyadenylated mRNAs of rotavirus. RNA 6:814-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciarlet, M., M. Hidalgo, M. Gorziglia, and F. Liprandi. 1994. Characterization of neutralization epitopes on the VP7 surface protein of serotype G11 porcine rotaviruses. J. Gen. Virol. 75:1867-1873. [DOI] [PubMed] [Google Scholar]
- 15.Cook, J. P., and M. A. McCrae. 2004. Sequence analysis of the guanylyltransferase (VP3) of group A rotaviruses. J. Gen. Virol. 85:929-932. [DOI] [PubMed] [Google Scholar]
- 16.Deo, R. C., C. M. Groft, K. R. Rajashankar, and S. K. Burley. 2002. Recognition of the rotavirus mRNA 3′ consensus by an asymmetric NSP3 homodimer. Cell 108:71-81. [DOI] [PubMed] [Google Scholar]
- 17.Desselberger, U. 1996. Genome rearrangements of rotaviruses. Adv. Virus Res. 46:69-95. [DOI] [PubMed] [Google Scholar]
- 18.Devitt, C. M., and D. L. Reynolds. 1993. Characterization of a group D rotavirus. Avian Dis. 37:749-755. [PubMed] [Google Scholar]
- 19.Dormitzer, P. R., Z. J. Sun, G. Wagner, and S. C. Harrison. 2002. The rhesus rotavirus VP4 sialic acid binding domain has a galectin fold with a novel carbohydrate binding site. EMBO J. 21:885-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eichwald, C., F. Vascotto, E. Fabbretti, and O. R. Burrone. 2002. Rotavirus NSP5: mapping phosphorylation sites and kinase activation and viroplasm localization domains. J. Virol. 76:3461-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Estes, M. K., and A. Z. Kapikian. 2007. Rotaviruses, p. 1917-1974. In D. M. Kniepe, P. M. Howley, D. E. Griffin, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed. Lippincott, Williams and Wilkins, Philadelphia, PA.
- 22.Fischer, T. K., C. Viboud, U. Parashar, M. Malek, C. Steiner, R. Glass, and L. Simonsen. 2007. Hospitalizations and deaths from diarrhea and rotavirus among children <5 years of age in the United States, 1993-2003. J. Infect. Dis. 195:1117-1125. [DOI] [PubMed] [Google Scholar]
- 23.Fresco, L. D., and S. Buratowski. 1994. Active site of the mRNA-capping enzyme guanylyltransferase from Saccharomyces cerevisiae: similarity to the nucleotidyl attachment motif of DNA and RNA ligases. Proc. Natl. Acad. Sci. U. S. A. 91:6624-6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilbert, J. M., and H. B. Greenberg. 1998. Cleavage of rhesus rotavirus VP4 after arginine 247 is essential for rotavirus-like particle-induced fusion from without. J. Virol. 72:5323-5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gough, R. E., M. S. Collins, D. J. Alexander, and W. J. Cox. 1990. Viruses and virus-like particles detected in samples from diseased game birds in Great Britain during 1988. Avian Pathol. 19:331-343. [DOI] [PubMed] [Google Scholar]
- 26.Graff, J. W., J. Ewen, K. Ettayebi, and M. E. Hardy. 2007. Zinc-binding domain of rotavirus NSP1 is required for proteasome-dependent degradation of IRF3 and autoregulatory NSP1 stability. J. Gen. Virol. 88:613-620. [DOI] [PubMed] [Google Scholar]
- 27.Graff, J. W., K. Ettayebi, and M. E. Hardy. 2009. Rotavirus NSP1 inhibits NFκB activation by inducing proteasome-dependent degradation of β-TrCP: a novel mechanism of IFN antagonism. PLoS Pathog. 5.1:e1000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haynes, J. S., D. L. Reynolds, J. A. Fagerland, and A. S. Fix. 1994. Morphogenesis of enteric lesions induced by group D rotavirus in ringneck pheasant chicks (Phasianus colchicus). Vet. Pathol. 31:74-81. [DOI] [PubMed] [Google Scholar]
- 29.Imai, M., K. Akatani, N. Ikegami, and Y. Furuichi. 1983. Capped and conserved terminal structures in human rotavirus genome double-stranded RNA segments. J. Virol. 47:125-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ito, H., M. Sugiyama, K. Masubuchi, Y. Mori, and N. Minamoto. 2001. Complete nucleotide sequence of a group A avian rotavirus genome and a comparison with its counterparts of mammalian rotaviruses. Virus Res. 75:123-138. [DOI] [PubMed] [Google Scholar]
- 31.Jiang, S., S. Ji, Q. Tang, X. Cui, H. Yang, B. Kan, and S. Gao. 2008. Molecular characterization of a novel adult diarrhoea rotavirus strain J19 isolated in China and its significance for the evolution and origin of group B rotaviruses. J. Gen. Virol. 89:2622-2629. [DOI] [PubMed] [Google Scholar]
- 32.Kapikian, A. Z., Y. Hoshino, and R. M. Chanock. 2001. Rotaviruses, p. 1787-1833. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott, Williams and Wilkins, Philadelphia, PA.
- 33.Karim, M. R., F. I. Rume, M. M. Alam, and U. Ahmed. 2007. Molecular epidemiologic study on avian rotavirus prevailing in Bangladesh. Bangl. J. Vet. Med. 5:43-48. [Google Scholar]
- 34.Khamrin, P., N. Maneekarn, S. Peerakome, F. Yagyu, S. Okitsu, and H. Ushijima. 2006. Molecular characterization of a rare G3P[3] human rotavirus reassortant strain reveals evidence for multiple human-animal interspecies transmissions. J. Med. Virol. 78:986-994. [DOI] [PubMed] [Google Scholar]
- 35.Kumar, M., H. Jayaram, R. Vasquez-Del Carpio, X. Jiang, Z. F. Taraporewala, R. H. Jacobson, J. T. Patton, and B. V. Prasad. 2007. Crystallographic and biochemical analysis of rotavirus NSP2 with nucleotides reveals a nucleoside diphosphate kinase-like activity. J. Virol. 81:12272-12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin, S. L., and P. Tian. 2003. Detailed computational analysis of a comprehensive set of group A rotavirus NSP4 proteins. Virus Genes 26:271-282. [DOI] [PubMed] [Google Scholar]
- 37.Lu, X., S. M. McDonald, M. A. Tortorici, Y. J. Tao, R. Vasquez-Del Carpio, M. L. Nibert, J. T. Patton, and S. C. Harrison. 2008. Mechanism for coordinated RNA packaging and genome replication by rotavirus polymerase VP1. Structure 16. 11:1678-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maan, S., S. Rao, N. S. Maan, S. J. Anthony, H. Attoui, A. R. Samuel, and P. P. Mertens. 2007. Rapid cDNA synthesis and sequencing techniques for the genetic study of bluetongue and other dsRNA viruses. J. Virol. Methods 143:132-139. [DOI] [PubMed] [Google Scholar]
- 39.Martella, V., K. Bányai, E. Lorusso, A. L. Bellacicco, N. Decaro, M. Camero, G. Bozzo, P. Moschidou, S. Arista, G. Pezzotti, A. Lavazza, and C. Buonavoglia. 2007. Prevalence of group C rotaviruses in weaning and post-weaning pigs with enteritis. Vet. Microbiol. 123:26-33. [DOI] [PubMed] [Google Scholar]
- 40.Mathieu, M., I. Petitpas, J. Navaza, J. Lepault, E. Kohli, P. Pothier, B. V. V. Prasad, J. Cohen, and F. A. Rey. 2001. Atomic structure of the major capsid protein of rotavirus: implications for the architecture of the virion. EMBO J. 20:1485-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matthijnssens, J., M. Rahman, V. Martella, Y. Xuelei, S. De Vos, K. De Leener, M. Ciarlet, C. Buonavoglia, and M. Van Ranst. 2006. Full genomic analysis of human rotavirus strain B4106 and lapine rotavirus strain 30/96 provides evidence for interspecies transmission. J. Virol. 80:3801-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matthijnssens, J., M. Ciarlet, E. Heiman, I. Arijs, T. Delbeke, S. M. McDonald, E. A. Palombo, M. Iturriza-Gómara, P. Maes, J. T. Patton, M. Rahman, and M. Van Ranst. 2008. Full genome-based classification of rotaviruses reveals a common origin between human Wa-like and porcine rotavirus strains and human DS-1-like and bovine rotavirus strains. J. Virol. 82:3204-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matthijnssens, J., M. Ciarlet, M. Rahman, H. Attoui, K. Bányai, M. K. Estes, J. R. Gentsch, M. Iturriza-Gómara, C. D. Kirkwood, V. Martella, P. P. Mertens, O. Nakagomi, J. T. Patton, F. M. Ruggeri, L. J. Saif, N. Santos, A. Steyer, K. Taniguchi, U. Desselberger, and M. Van Ranst. 2008. Recommendations for the classification of group A rotaviruses using all 11 genomic RNA segments. Arch. Virol. 153:1621-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDonald, S. M., and J. T. Patton. 2008. Molecular characterization of a subgroup specificity associated with the rotavirus inner capsid protein VP2. J. Virol. 82:2752-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDonald, S. M., D. Aguayo, F. D. Gonzalez-Nilo, and J. T. Patton. 2009. Shared and group-specific features of the rotavirus RNA polymerase reveal potential determinants of gene reassortment restriction. J. Virol. 83:6135-6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McDonald, S. M., Y. J. Tao, and J. T. Patton. 2009. The ins and outs of four-tunneled Reoviridae RNA-dependent RNA polymerases. Curr. Opin. Struct. Biol. 19:775-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McNulty, M. S. 2008. Rotavirus infections, p. 338-350. In Y. M. Saif (ed.), Diseases of poultry, 12th ed. Iowa State University Press, Ames, IA.
- 48.McNulty, M. S., G. M. Allan, D. Todd, J. B. McFerran, E. R. McKillop, D. S. Collins, and R. M. McCracken. 1980. Isolation of rotaviruses from turkeys and chickens: demonstration of distinct serotypes and RNA electropherotypes. Avian Pathol. 9:363-375. [DOI] [PubMed] [Google Scholar]
- 49.McNulty, M. S., D. Todd, G. M. Allan, J. B. McFerran, and J. A. Green. 1984. Epidemiology of rotavirus infection in broiler chickens: recognition of four serogroups. Arch. Virol. 81:113-121. [DOI] [PubMed] [Google Scholar]
- 50.Mori, Y., M. A. Borgan, N. Ito, M. Sugiyama, and N. Minamoto. 2002. Diarrhea-inducing activity of avian rotavirus NSP4 glycoproteins, which differ greatly from mammalian rotavirus NSP4 glycoproteins in deduced amino acid sequence in suckling mice. J. Virol. 76:5829-5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagashima, S., N. Kobayashi, M. Ishino, M. M. Alam, M. U. Ahmed, S. K. Paul, B. Ganesh, M. Chawla-Sarkar, T. Krishnan, T. N. Naik, and Y. H. Wang. 2008. Whole genomic characterization of a human rotavirus strain B219 belonging to a novel group of the genus Rotavirus. J. Med. Virol. 80:2023-2033. [DOI] [PubMed] [Google Scholar]
- 52.Otto, P., P. Schulze, and W. Herbst. 1999. Demonstration of group C rotaviruses in fecal samples of diarrheic dogs from Germany. Arch. Virol. 144:2467-2473. [DOI] [PubMed] [Google Scholar]
- 53.Otto, P., E. M. Liebler-Tenorio, M. Elschner, J. Reetz, U. Löhren, and R. Diller. 2006. Detection of rotaviruses and intestinal lesions in broiler chicks from flocks with runting and stunting syndrome (RSS). Avian Dis. 50:411-418. [DOI] [PubMed] [Google Scholar]
- 54.Parashar, U. D., E. G. Hummelman, J. S. Bresee, M. A. Miller, and R. I. Glass. 2003. Global illness and deaths caused by rotavirus disease in children. Emerg. Infect. Dis. 9:565-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parashar, U. D., C. J. Gibson, J. S. Bresee, and R. I. Glass. 2006. Rotavirus and severe childhood diarrhea. Emerg. Infect. Dis. 12:304-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patton, J. T., M. Wentz, J. Xiaobo, and R. F. Ramig. 1996. cis-Acting signals that promote genome replication in rotavirus mRNA. J. Virol. 70:3961-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patton, J. T., and E. Spencer. 2000. Genome replication and packaging of segmented double-stranded RNA viruses. Virology 277:217-225. [DOI] [PubMed] [Google Scholar]
- 58.Pedley, S., J. C. Bridger, J. F. Brown, and M. A. McCrae. 1983. Molecular characterization of rotaviruses with distinct group antigens. J. Gen. Virol. 64:2093-2101. [DOI] [PubMed] [Google Scholar]
- 59.Pedley, S., J. C. Bridger, D. Chasey, and M. A. McCrae. 1986. Definition of two new groups of atypical rotaviruses. J. Gen. Virol. 67:131-137. [DOI] [PubMed] [Google Scholar]
- 60.Pizarro, J. L., A. M. Sandino, J. M. Pizarro, J. Fernandez, and E. Spencer. 1991. Characterization of rotavirus guanylyltransferase activity associated with polypeptide VP3. J. Gen. Virol. 72:325-332. [DOI] [PubMed] [Google Scholar]
- 61.Rainsford, E. W., and M. A. McCrae. 2007. Characterization of the NSP6 protein product of rotavirus gene 11. Virus Res. 130:193-201. [DOI] [PubMed] [Google Scholar]
- 62.Ramig, R. F., M. Ciarlet, P. P. C. Mertens, and T. S. Dermody. 2005. Genus Rotavirus, p. 484-496. In C. M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, and L. A. Ball (ed.), Virus taxonomy. Eighth report of the ICTV. Elsevier Academic Press, Amsterdam, Netherlands.
- 63.Reynolds, D. L., Y. M. Saif, and K. W. Theil. 1987. A survey of enteric viruses of turkey poults. Avian Dis. 31:89-98. [PubMed] [Google Scholar]
- 64.Reynolds, D. L., K. W. Theil, and Y. M. Saif. 1987. Demonstration of rotavirus and rotavirus-like virus in the intestinal contents of diarrheic pheasant chicks. Avian Dis. 31:376-379. [PubMed] [Google Scholar]
- 65.Saif, L. J., E. H. Bohl, K. W. Theil, R. F. Cross, and J. A. House. 1980. Rotavirus-like, calicivirus-like, and 23-nm virus-like particles associated with diarrhea in young pigs. J. Clin. Microbiol. 12:105-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saif, L. J., and B. Jiang. 1994. Nongroup A rotaviruses of humans and animals. Curr. Top. Microbiol. Immunol. 185:339-371. [DOI] [PubMed] [Google Scholar]
- 67.Saif, L. J., Y. M. Saif, and K. W. Theil. 1985. Enteric viruses in diarrheic turkey poults. Avian Dis. 29:798-811. [PubMed] [Google Scholar]
- 68.Sasaki, S., Y. Horie, T. Nakagomi, M. Oseto, and O. Nakagomi. 2001. Group C rotavirus NSP4 induces diarrhea in neonatal mice. Arch. Virol. 146:801-806. [DOI] [PubMed] [Google Scholar]
- 69.Schumann, T., H. Hotzel, P. Otto, and R. Johne. 2009. Evidence of interspecies transmission and reassortment among avian group A rotaviruses. Virology 386:334-343. [DOI] [PubMed] [Google Scholar]
- 70.Theil, K. W., D. L. Reynolds, and Y. M. Saif. 1986. Genomic variation among avian rotavirus-like viruses detected by polyacrylamide gel electrophoresis. Avian Dis. 30:829-834. [PubMed] [Google Scholar]
- 71.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Torres-Vega, M. A., R. A. Gonzalez, M. Duarte, D. Poncet, S. Lopez, and C. F. Arias. 2000. The C-terminal domain of rotavirus NSP5 is essential for its multimerization, hyperphosphorylation and interaction with NSP6. J. Gen. Virol. 81:821-830. [DOI] [PubMed] [Google Scholar]
- 73.Tortorici, M. A., B. A. Shapiro, and J. T. Patton. 2006. A base-specific recognition signal in the 5′ consensus sequence of rotavirus plus-strand RNAs promotes replication of the double-stranded RNA genome segments. RNA 12:133-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Trojnar, E., P. Otto, and R. Johne. 2009. The first complete genome sequence of a chicken group A rotavirus indicates independent evolution of mammalian and avian strains. Virology 386:325-333. [DOI] [PubMed] [Google Scholar]
- 75.Tzipori, S. 1985. The relative importance of enteric pathogens affecting neonates of domestic animals. Adv. Vet. Sci. Comp. Med. 29:103-206. [PubMed] [Google Scholar]
- 76.Vásquez-del Carpió, R., J. L. Morales, M. Barro, A. Ricardo, and E. Spencer. 2006. Bioinformatic prediction of polymerase elements in the rotavirus VP1 protein. Biol. Res. 39:649-659. [DOI] [PubMed] [Google Scholar]
- 77.Yang, H., E. V. Makeyey, Z. Kang, S. Ji, D. H. Bamford, and A. A. Van Dijk. 2004. Cloning and sequence analysis of dsRNA segments 5, 6 and 7 of a novel non-group A, B, C adult rotavirus that caused an outbreak of gastroenteritis in China. Virus Res. 106:15-26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.