Abstract
Early alpha interferon (IFN-α) therapy against hepatitis C virus (HCV) rescues polyfunctional, virus-specific memory CD8+ T cells, but whether immune restoration is possible during late therapy remains controversial. We compared immune restoration of HCV-specific memory T cells in patients who cleared HCV infection spontaneously and following early or late IFN therapy. Multifunctional CD4+ and CD8+ memory T cells were detected in spontaneous resolvers and in individuals treated early following an acute infection. In contrast, limited responses were detected in patients treated during chronic infection, and the phenotype of HCV-specific cells was influenced by autologous viral sequences. Our data suggest that irreversible damage to the HCV-specific memory T-cell response is associated with chronic HCV infection.
The majority of acute hepatitis C virus (HCV) infections become chronic, with persistent viremia and serious liver complications (12). Alpha interferon (IFN-α)-based therapy is the only approved treatment for chronic HCV; its success rate ranges from 40 to 90% depending on the infecting genotype (9, 18). The success of therapy is characterized by a sustained virological response (SVR), defined as undetectable HCV RNA in plasma at 6 months after termination of therapy. SVR rates are greatly enhanced if therapy is started between 3 and 6 months following acute HCV infection, but the underlying mechanisms are not well understood (27, 28). We have demonstrated that early interferon therapy for HCV can rescue and select for long-lived polyfunctional CD8+ memory T cells (1). Treatment-induced memory T cells were similar in phenotype and function to natural memory T cells generated following spontaneously resolved infection. They expressed high levels of CD127 and Bcl-2 (CD127hi, Bcl-2hi) and low levels of PD1 (PD1lo) and were polyfunctional in nature (1). However, restoration of HCV-specific memory CD4+ T cells has not been examined. Furthermore, whether immune restoration is possible following the late initiation of therapy during the chronic phase remains controversial. Kamal et al. demonstrated that SVR is associated with a recovery in HCV-specific CD4+ T-cell responses (13). In contrast, Barnes et al. and Rahman et al. demonstrated that the induction of HCV-specific immunity during therapy does not correlate with outcomes (2, 21).
Methods, results, and discussion.
The aim of this study was firstly to compare immune restoration of HCV-specific memory CD4+ and CD8+ T-cell responses in patients who received early or late treatment, irrespective of the virus genotype. Second, we wanted to compare treatment-induced/restored memory T cells to natural memory T cells generated following spontaneously resolved acute HCV infection. To address these issues, we performed cross-sectional comprehensive characterization of HCV-specific T cells using carboxyfluorescein diacetate succinimidyl ester (CFSE) proliferation assays, intracellular staining for cytokines, cytotoxicity, and polyfunctionality analysis in a cohort of 33 patients who had successfully eliminated HCV infection under different conditions. The following three subgroups were studied: (i) patients who spontaneously resolved acute HCV infection without therapeutic intervention (SpR; n = 10), (ii) patients who achieved SVR following IFN therapy initiated during acute HCV (A-SVR; n = 10), and (iii) patients who achieved SVR following IFN therapy initiated during chronic HCV infection >2 years after the first detection of HCV RNA (C-SVR; n = 13). SpR and A-SVR patients were recruited from an acute HCV cohort of intravenous drug users (IDUs) at St-Luc Hospital of the Centre Hospitalier de l'Université de Montréal (CHUM), as previously described (1, 6). A-SVR patients received 12 to 24 weeks of pegylated (PEG)-IFN-α-2a (Pegasys [Roche Diagnostics, Welwyn Garden City, Hertfordshire, United Kingdom]) (180 μg/week) and no ribavirin. C-SVR patients were recruited among patients with chronic HCV who had undergone successful treatment with the standard-of-care therapy protocol (11) at the hepatology clinics of Toronto Western Hospital, Toronto, Ontario, Canada, or St-Luc Hospital of the CHUM. The infecting genotype distribution was genotype 1a (n = 13), genotype 1b (n = 6), genotype 3a (n = 7), and undetermined genotype (n = 7). Patients' demographics and characteristics are listed in Table S1 in the supplemental material.
The time point studied in this cross-sectional analysis was >6 months after spontaneous resolution or the end of antiviral therapy. Immune responses were monitored using four peptide pools representing the NS3 and NS5B regions, the most immunodominant regions of HCV (8, 15-17). Each pool consisted of 42 to 49 overlapping peptides, 18 amino acids (aa) long, overlapping by 11 aa as follows: pool 1 (P1) NS3 (aa 1,027 to 1,339), pool 2 (P2) NS3 (aa 1,340 to 1,658), pool 3 (P3) NS5B (aa 2,421 to 2,716), and pool 4 (P4) NS5B (aa 2,717 to 3,012). Peptides were obtained from the Biodefense and Emerging Infections Research Resources Repository (BEI Resources), Manassas, VA, and matched with the infecting viral genotype in each patient (1a, 1b, or 3a). For patients with unknown genotypes, all within the SpR group, genotype 1a peptides were used. All statistical analyses were performed by Mann-Whitney U tests using GraphPad Prism software version 5.02.
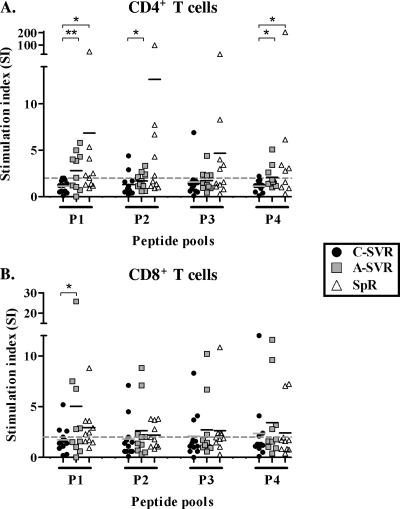
Cross-sectional proliferative responses were first monitored using a CFSE dilution assay as previously described (1). Representative data and the gating strategy are presented in Fig. S1 in the supplemental material. CD4+ T-cell proliferation showed a trend of higher means of responses in SpR and A-SVR patients compared to C-SVR patients. This difference was statistically significant in many instances (P < 0.05 [Mann-Whitney U test]) (Fig. 1A). Furthermore, the means of CD8+ T-cell proliferative responses were consistently higher in A-SVR patients than in C-SVR patients in response to all peptide pools, although they were not statistically significant except for peptide pool 1 (Fig. 1B). There was no statistically significant difference in CD4+ or CD8+ T-cell proliferative responses between A-SVR and SpR patients, suggesting similar trends. It is possible that in patients of the SpR group, where the infecting virus genotype was unknown, our capacity to detect memory T cells was compromised because of a mismatch between the infecting genotype and the genotype 1a peptides used.
FIG. 1.
Limited proliferation of HCV-specific CD4+ and CD8+ T cells in HCV patients achieving SVR following late therapy (C-SVR) compared to patients who resolved following early IFN therapy (A-SVR) or who resolved spontaneously (SpR). Cumulative data from CFSE proliferation assays performed on the different patient groups are represented as stimulation indices (SI) in response to different HCV peptide pools in CD4+ (A) and CD8+ (B) T cells. *, P < 0.05; **, P < 0.01 (Mann-Whitney U test). PBMCs (2.5 × 106) were stimulated with HCV peptide pools at 1 μg/ml of each peptide for 6 days. IL-2 was added at the end of day 3 at a concentration of 20 IU/ml. The dashed line represents the cutoff for positive responses (SI = 2).
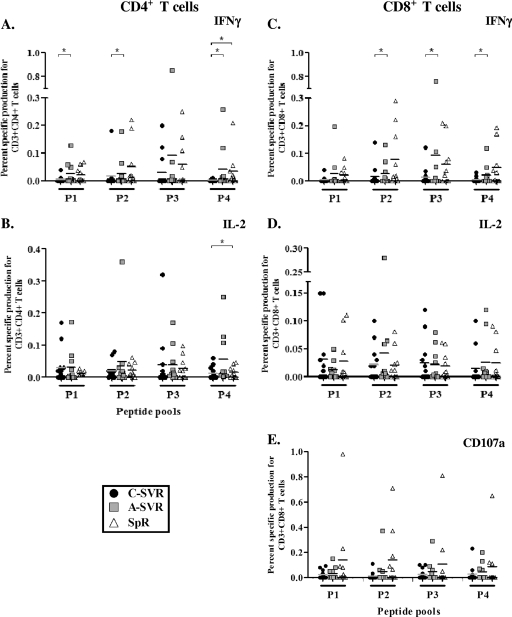
Next, we tested the functionality of HCV-specific CD4+ and CD8+ T cells by simultaneously examining the production of IFN-γ and interleukin 2 (IL-2) as well as the degranulation capacity of CD8+ T cells using the CD107a degranulation assay (4) in response to the four HCV peptide pools, as previously described (1). Representative data and the gating strategy are presented in Fig. S2 in the supplemental material. IFN-γ responses in both CD4+ and CD8+ T cells were higher in response to several peptide pools in the A-SVR patients than in the C-SVR patients (Fig. 2A and C). Furthermore, in CD8+ T cells, CD107a responses demonstrated a trend of being higher in the SpR and A-SVR patients than in the C-SVR patients in response to most peptide pools (Fig. 2E). In contrast, very limited cytokine responses and/or CD107a responses were detected in the C-SVR patients. The CD8+ T cells from the SpR group expressed the highest levels of CD107a, suggesting that the degranulation capacity often used as a surrogate marker for cytotoxicity (4) might be more crucial in spontaneous viral clearance compared to treatment-induced clearance.
FIG. 2.
Higher IFN-γ responses in both CD4+ and CD8+ T cells in SpR and A-SVR patients than in C-SVR patients. Cumulative data from the different patient groups for IFN-γ and IL-2 production and CD107a expression in a standard intracellular staining for cytokines (ICS) assay in response to stimulation with different HCV peptide pools. *, P < 0.05 (Mann-Whitney U test). PBMCs (2 × 106) were stimulated with HCV peptide pools at 1 μg/ml of each peptide for 12 h. Data are presented as the percentages of specific cytokine production. Specific cytokine production of 0.01% was used as a cutoff for positive responses.
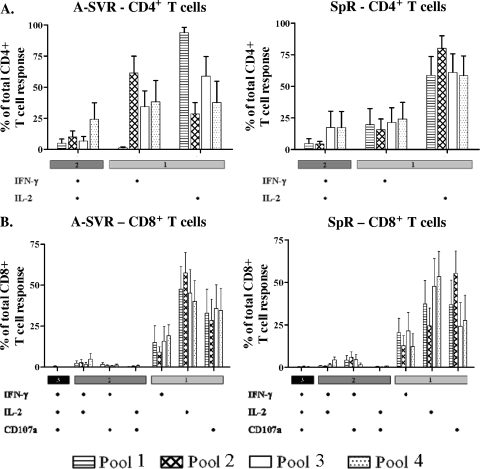
We analyzed the polyfunctionality of HCV-specific T cells. Functionality data were exported as Boolean gates using FlowJo software (version 9.0.1; TreeStar, Portland, OR), and polyfunctionality was analyzed using a mathematical algorithm for the calculation of the contribution of all possible combinations of mono-, dual, and trifunctional cells. Polyfunctionality analysis was not performed for C-SVR patients, as frequencies of cytokine-producing cells were too low. The analysis showed overall similar memory T-cell profiles in A-SVR and SpR patients. The CD4+ T-cell responses in A-SVR and SpR patients were mostly single IL-2 producers (Fig. 3A), consistent with earlier reports documenting that IL-2 production is one of the earliest functions lost upon dysfunction of virus-specific CD4+ T cells (7, 24, 29). Nevertheless, 4.3 to 24.2% of HCV-specific cells we detected exhibited dual function. CD8+ T cells in both A-SVR and SpR patients were primarily single positive, with a similar profile. However, the dual-function cells in A-SVR patients were more biased toward IFN-γ+ and IL-2+, while in SpR patients they were more biased toward IFN-γ+ and CD107a+ (Fig. 3B). These results suggest that despite an overall similar memory T-cell profile, minor functional difference may exist between natural T-cell memory and treatment-induced memory. It is also possible that IFN therapy, by virtue of its immune modulatory role, can influence the functional profile of virus-specific memory T cells.
FIG. 3.
Minor differences in polyfunctionality profiles between SpR and A-SVR patients. Polyfunctionality data gated on viable, CD3+, CD4+ T cells (A) or CD8+ T cells (B). Data are represented as the means of responses in all patients plus the standard errors (SE). There was no significant difference in the percentages of polyfunctional cells between SpR and A-SVR patients. For C-SVR patients, the individual production of cytokines was too low to analyze polyfunctionality.
To confirm that indeed there was no short-lived recovery in HCV-specific memory T cells during therapy in the C-SVR group, we examined HCV-specific immune responses longitudinally in 8 of the C-SVR patients for whom longitudinal samples were available in an IFN-γ enzyme-linked immunospot (ELISPOT) assay using a panel of 12 peptide pools spanning the entire HCV polyprotein. Three time points were examined: at baseline, at 3 to 4 months after the start of therapy, and at termination of therapy with PEG-IFN plus ribavirin. Earlier studies suggested an immunomodulatory role for ribavirin as a mechanism for enhanced HCV clearance with PEG-IFN-ribavirin combination therapy (22, 25). However, we did not observe any de novo HCV-specific T-cell responses or statistically significant enhancement in any of the responses that existed at baseline except in one patient (C-SVR-7) (see Fig. S3 in the supplemental material). It is important to note that memory T-cell responses in the A-SVR group (treated without ribavirin) were consistently higher than those of the C-SVR group in the cross-sectional analysis (Fig. 1 and 2). Together, these results argue against an immune modulatory role for ribavirin.
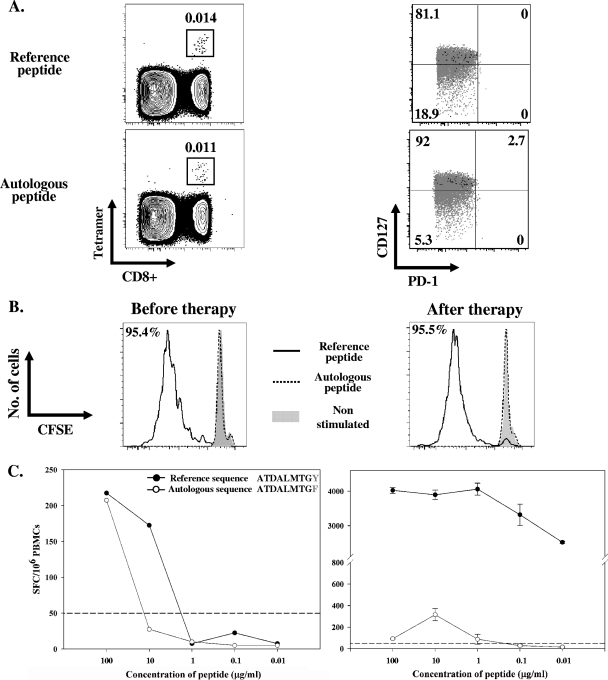
Finally, to confirm that HCV-specific responses were limited or severely defective from the start in chronically infected patients, we screened C-SVRs prior to starting interferon therapy with a panel of 10 major histocompatibility complex (MHC) class I tetramers corresponding to the most dominant HCV class I epitopes (see Table S2 in the supplemental material). No responses were detected except in 1/13 patients (patient C-SVR-5), who reacted to an A1/NS3-1406 tetramer at a frequency of 0.014% of CD8+ T cells (Fig. 4A, top panels). Despite the fact that this patient was chronically infected for over 2 years with a viral load of 12 × 106 IU/ml, HCV tetramer-specific cells were CD127hi and PD1lo (Fig. 4A, top panels), consistent with a memory phenotype previously observed only in spontaneously resolved individuals (1, 3, 10, 19, 20, 26). Furthermore, these HCV-specific cells proliferated efficiently at ∼95% of tetramer-positive cells (Fig. 4B) and produced cytokines in response to the specific peptide epitope in an ELISPOT assay (Fig. 4C). In order to understand this confusing result, we sequenced the autologous virus circulating in this patient, and 8/8 molecular clone sequences contained a Y→F change at residue 9. We synthesized a new HLA-A1 tetramer containing the autologous sequence variant (GenBank accession no. HM044663). This tetramer recognized HCV-specific T cells at a frequency of 0.011% and exhibited the same phenotype, CD127hi and PD1lo (Fig. 4A, bottom panels). However, the patients' peripheral blood mononuclear cells (PBMCs) did not proliferate in response to the autologous peptide (Fig. 4B). Moreover, when the recognition of the tetramer peptide sequence and the autologous viral sequence were compared over severalfold dilutions in an ELISPOT assay, we observed decreased recognition of the autologous viral sequence (Fig. 4C). The same result was obtained prior to therapy and following IFN therapy-mediated viral clearance. These results suggest that, at an earlier time point, this epitope probably underwent mutation to the current autologous sequence that is recognized less efficiently. This diminished recognition most likely prevented exhaustion of T cells specific for the original peptide and facilitated their transformation or preservation into long-lived memory T cells. This is consistent with recent results demonstrating that expression of the exhaustion marker PD-1 is likely influenced by the degree of recognition of the autologous viral sequence (23) and might be lost following viral escape mutations (5) and change into CD127hi memory T cells (14).
FIG. 4.
Virus-specific memory CD8+ T cells with a long-lived memory phenotype in chronic HCV have limited functionality against the autologous viral sequence. Thirteen chronic HCV patients were screened using a panel of 10 MHC class I tetramers. Patient C-SVR-5 was the only patient in which HCV-specific tetramer-positive CD8+ T cells were detected using the A1/NS3-1436 tetramer with the reference sequence. (A) Detection and phenotyping (PD-1, CD127 expression) of T cells reactive to the A1/NS3-1436 tetramer containing either the reference sequence (top panels) or the autologous sequence (bottom panels). Cells are gated on viable, CD3+, CD8+ T cells. Black represents tetramer-positive cells and gray represents total CD3+ CD8+ T cells in the same donor. Percentages of tetramer-positive cells in each quadrant are shown. (B) Proliferation of A1/NS3-1436 tetramer-positive cells in response to the reference peptide (solid line) in a CFSE dilution assay in comparison to the autologous peptide (dotted line), performed for two time points, before starting therapy and at 3 months after the end of therapy. Gated on CD3+, CD8+, A1/NS3-1436 tetramer-posituve cells. (C) Dose response for the reference tetramer peptide sequence versus the autologous virus sequence for the NS3-1436 epitope in patient C-SVR-5 using IFN-γ ELISPOT assay performed for the two time points, before starting therapy and 3 months after the end of therapy. Results are represented as specific spot forming cells (SFC)/106 PBMCs.
Our results suggest that both HCV-specific CD4+ and CD8+ T cells become persistently defective with prolonged infection. Nevertheless, there is a narrow window of time early during the acute phase when some functional aspects of the HCV-specific T-cell response might be rescued or preserved. Early therapeutic intervention during this period and subsequent viral clearance seems to prevent T-cell exhaustion, as is seen with chronic hepatitis C, thereby allowing their development into long-lived memory T cells. Similarly, variations in viral sequences leading to loss or diminished recognition of the autologous viral sequence by T cells decrease T-cell exhaustion and permit such T cells to develop into a functional memory T-cell pool.
Our data suggest that adaptive immunity can be preserved when IFN therapy is initiated early, and although, in general, frequencies of HCV-specific memory T cells continue to decline, there is a selection for polyfunctional and long-lived memory T cells. In contrast, irreversible damage and exhaustion of virus-specific memory T cells precipitates with chronic HCV infection. The question of whether immune restoration during antiviral treatment of acute hepatitis C is a cause or effect of an enhanced response to therapy remains unresolved. It is possible that such enhanced quality of the immune response may play a role in instigating viral clearance, but we favor the hypothesis that the reconstitution of memory T cells is an effect of viral clearance. Our data also suggest that there could be minor differences between natural memory and therapy-reconstituted memory T cells, and whether they would have the same protective capacity upon reexposure to the virus requires further investigation.
Nucleotide sequence accession number.
A new HCV sequence containing the autologous HCV sequence variant isolated from patient C-SVR-5 was deposited into GenBank under accession no. HM044663.
Supplementary Material
Acknowledgments
This study was funded by the Canadian Institutes for Health Research (CIHR) (MOP-74524), Fonds de la Recherche en Santé du Quebec (FRSQ) (FRSQ-12428), and the FRSQ-AIDS and Infectious Disease Network (SIDA-MI). M.S. Abdel-Hakeem received a graduate fellowship from the Université de Montréal and currently holds a doctoral fellowship from the National Canadian Research Training Programme in Hepatitis C (NCRTP-Hep C). G. Badr received a postdoctoral fellowship from FRSQ. J. Bruneau holds a senior clinical research award from FRSQ. N.H. Shoukry holds a joint New Investigator Award from the Canadian Foundation for Infectious Diseases and CIHR.
Footnotes
Published ahead of print on 28 July 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Badr, G., N. Bedard, M. S. Abdel-Hakeem, L. Trautmann, B. Willems, J. P. Villeneuve, E. K. Haddad, R. P. Sekaly, J. Bruneau, and N. H. Shoukry. 2008. Early interferon therapy for hepatitis C virus infection rescues polyfunctional, long-lived CD8+ memory T cells. J. Virol. 82:10017-10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes, E., G. Harcourt, D. Brown, M. Lucas, R. Phillips, G. Dusheiko, and P. Klenerman. 2002. The dynamics of T-lymphocyte responses during combination therapy for chronic hepatitis C virus infection. Hepatology 36:743-754. [DOI] [PubMed] [Google Scholar]
- 3.Bengsch, B., H. C. Spangenberg, N. Kersting, C. Neumann-Haefelin, E. Panther, F. von Weizsacker, H. E. Blum, H. Pircher, and R. Thimme. 2007. Analysis of CD127 and KLRG1 expression on hepatitis C virus-specific CD8+ T cells reveals the existence of different memory T-cell subsets in the peripheral blood and liver. J. Virol. 81:945-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betts, M. R., J. M. Brenchley, D. A. Price, S. C. De Rosa, D. C. Douek, M. Roederer, and R. A. Koup. 2003. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 281:65-78. [DOI] [PubMed] [Google Scholar]
- 5.Blattman, J. N., E. J. Wherry, S. J. Ha, R. G. van der Most, and R. Ahmed. 2009. Impact of epitope escape on PD-1 expression and CD8 T-cell exhaustion during chronic infection. J. Virol. 83:4386-4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox, A. L., K. Page, J. Bruneau, N. H. Shoukry, G. M. Lauer, A. Y. Kim, H. R. Rosen, H. Radziewicz, A. Grakoui, D. S. Fierer, A. D. Branch, D. E. Kaplan, and K. M. Chang. 2009. Rare birds in North America: acute hepatitis C cohorts. Gastroenterology 136:26-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Day, C. L., and B. D. Walker. 2003. Progress in defining CD4 helper cell responses in chronic viral infections. J. Exp. Med. 198:1773-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diepolder, H. M., R. Zachoval, R. M. Hoffmann, E. A. Wierenga, T. Santantonio, M. C. Jung, D. Eichenlaub, and G. R. Pape. 1995. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet 346:1006-1007. [DOI] [PubMed] [Google Scholar]
- 9.Fried, M. W., M. L. Shiffman, K. R. Reddy, C. Smith, G. Marinos, F. L. Goncales, Jr., D. Haussinger, M. Diago, G. Carosi, D. Dhumeaux, A. Craxi, A. Lin, J. Hoffman, and J. Yu. 2002. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347:975-982. [DOI] [PubMed] [Google Scholar]
- 10.Golden-Mason, L., J. R. Burton, Jr., N. Castelblanco, J. Klarquist, S. Benlloch, C. Wang, and H. R. Rosen. 2006. Loss of IL-7 receptor alpha-chain (CD127) expression in acute HCV infection associated with viral persistence. Hepatology 44:1098-1109. [DOI] [PubMed] [Google Scholar]
- 11.Hadziyannis, S. J., H. Sette, Jr., T. R. Morgan, V. Balan, M. Diago, P. Marcellin, G. Ramadori, H. Bodenheimer, Jr., D. Bernstein, M. Rizzetto, S. Zeuzem, P. J. Pockros, A. Lin, and A. M. Ackrill. 2004. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann. Intern. Med. 140:346-355. [DOI] [PubMed] [Google Scholar]
- 12.Hoofnagle, J. H. 2002. Course and outcome of hepatitis C. Hepatology 36:S21-S29. [DOI] [PubMed] [Google Scholar]
- 13.Kamal, S. M., J. Fehr, B. Roesler, T. Peters, and J. W. Rasenack. 2002. Peginterferon alone or with ribavirin enhances HCV-specific CD4 T-helper 1 responses in patients with chronic hepatitis C. Gastroenterology 123:1070-1083. [DOI] [PubMed] [Google Scholar]
- 14.Kasprowicz, V., Y. H. Kang, M. Lucas, J. Schulze zur Wiesch, T. Kuntzen, V. Fleming, B. E. Nolan, S. Longworth, A. Berical, B. Bengsch, R. Thimme, L. Lewis-Ximenez, T. M. Allen, A. Y. Kim, P. Klenerman, and G. M. Lauer. 2010. Hepatitis C virus (HCV) sequence variation induces an HCV-specific T-cell phenotype analogous to spontaneous resolution. J. Virol. 84:1656-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lauer, G. M., E. Barnes, M. Lucas, J. Timm, K. Ouchi, A. Y. Kim, C. L. Day, G. K. Robbins, D. R. Casson, M. Reiser, G. Dusheiko, T. M. Allen, R. T. Chung, B. D. Walker, and P. Klenerman. 2004. High resolution analysis of cellular immune responses in resolved and persistent hepatitis C virus infection. Gastroenterology 127:924-936. [DOI] [PubMed] [Google Scholar]
- 16.Lauer, G. M., M. Lucas, J. Timm, K. Ouchi, A. Y. Kim, C. L. Day, J. Schulze Zur Wiesch, G. Paranhos-Baccala, I. Sheridan, D. R. Casson, M. Reiser, R. T. Gandhi, B. Li, T. M. Allen, R. T. Chung, P. Klenerman, and B. D. Walker. 2005. Full-breadth analysis of CD8+ T-cell responses in acute hepatitis C virus infection and early therapy. J. Virol. 79:12979-12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lauer, G. M., K. Ouchi, R. T. Chung, T. N. Nguyen, C. L. Day, D. R. Purkis, M. Reiser, A. Y. Kim, M. Lucas, P. Klenerman, and B. D. Walker. 2002. Comprehensive analysis of CD8+-T-cell responses against hepatitis C virus reveals multiple unpredicted specificities. J. Virol. 76:6104-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McHutchison, J. G., S. C. Gordon, E. R. Schiff, M. L. Shiffman, W. M. Lee, V. K. Rustgi, Z. D. Goodman, M. H. Ling, S. Cort, and J. K. Albrecht for the Hepatitis Interventional Therapy Group. 1998. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N. Engl. J. Med. 339:1485-1492. [DOI] [PubMed] [Google Scholar]
- 19.Penna, A., M. Pilli, A. Zerbini, A. Orlandini, S. Mezzadri, L. Sacchelli, G. Missale, and C. Ferrari. 2007. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology 45:588-601. [DOI] [PubMed] [Google Scholar]
- 20.Radziewicz, H., C. C. Ibegbu, M. L. Fernandez, K. A. Workowski, K. Obideen, M. Wehbi, H. L. Hanson, J. P. Steinberg, D. Masopust, E. J. Wherry, J. D. Altman, B. T. Rouse, G. J. Freeman, R. Ahmed, and A. Grakoui. 2007. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J. Virol. 81:2545-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahman, F., T. Heller, Y. Sobao, E. Mizukoshi, M. Nascimbeni, H. Alter, S. Herrine, J. Hoofnagle, T. J. Liang, and B. Rehermann. 2004. Effects of antiviral therapy on the cellular immune response in acute hepatitis C. Hepatology 40:87-97. [DOI] [PubMed] [Google Scholar]
- 22.Rigopoulou, E. I., W. G. Abbott, R. Williams, and N. V. Naoumov. 2007. Direct evidence for immunomodulatory properties of ribavirin on T-cell reactivity to hepatitis C virus. Antiviral Res. 75:36-42. [DOI] [PubMed] [Google Scholar]
- 23.Rutebemberwa, A., S. C. Ray, J. Astemborski, J. Levine, L. Liu, K. A. Dowd, S. Clute, C. Wang, A. Korman, A. Sette, J. Sidney, D. M. Pardoll, and A. L. Cox. 2008. High-programmed death-1 levels on hepatitis C virus-specific T cells during acute infection are associated with viral persistence and require preservation of cognate antigen during chronic infection. J. Immunol. 181:8215-8225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semmo, N., C. L. Day, S. M. Ward, M. Lucas, G. Harcourt, A. Loughry, and P. Klenerman. 2005. Preferential loss of IL-2-secreting CD4+ T helper cells in chronic HCV infection. Hepatology 41:1019-1028. [DOI] [PubMed] [Google Scholar]
- 25.Tam, R. C., B. Pai, J. Bard, C. Lim, D. R. Averett, U. T. Phan, and T. Milovanovic. 1999. Ribavirin polarizes human T cell responses towards a type 1 cytokine profile. J. Hepatol. 30:376-382. [DOI] [PubMed] [Google Scholar]
- 26.Urbani, S., B. Amadei, P. Fisicaro, D. Tola, A. Orlandini, L. Sacchelli, C. Mori, G. Missale, and C. Ferrari. 2006. Outcome of acute hepatitis C is related to virus-specific CD4 function and maturation of antiviral memory CD8 responses. Hepatology 44:126-139. [DOI] [PubMed] [Google Scholar]
- 27.Wedemeyer, H., E. Jackel, J. Wiegand, M. Cornberg, and M. P. Manns. 2004. Whom? When? How? Another piece of evidence for early treatment of acute hepatitis C. Hepatology 39:1201-1203. [DOI] [PubMed] [Google Scholar]
- 28.Wiegand, J., P. Buggisch, W. Boecher, S. Zeuzem, C. M. Gelbmann, T. Berg, W. Kauffmann, B. Kallinowski, M. Cornberg, E. Jaeckel, H. Wedemeyer, and M. P. Manns. 2006. Early monotherapy with pegylated interferon alpha-2b for acute hepatitis C infection: the HEP-NET acute-HCV-II study. Hepatology 43:250-256. [DOI] [PubMed] [Google Scholar]
- 29.Younes, S. A., B. Yassine-Diab, A. R. Dumont, M. R. Boulassel, Z. Grossman, J. P. Routy, and R. P. Sekaly. 2003. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J. Exp. Med. 198:1909-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.