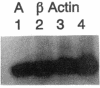
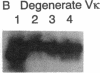
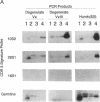
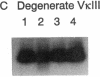
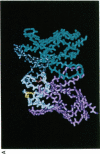
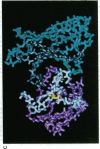
Abstract
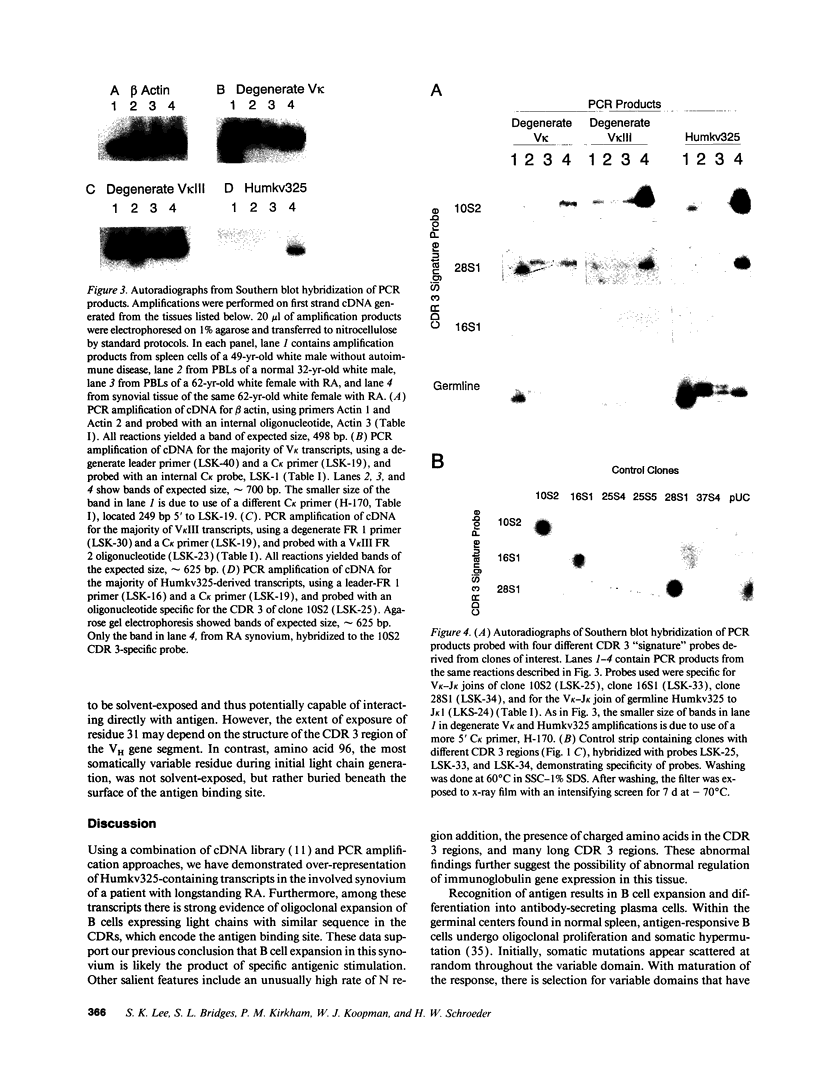
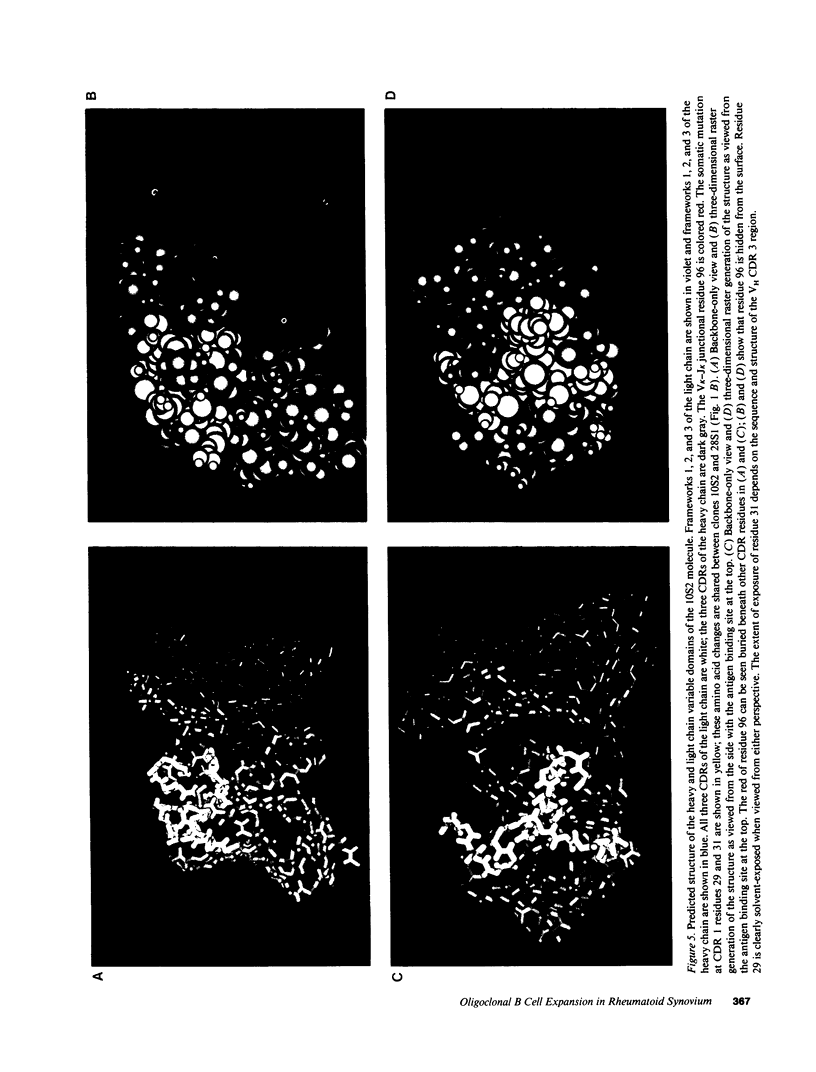
Plasma cell infiltration of synovium is common in longstanding rheumatoid arthritis (RA). The mechanism(s) underlying synovial B cell proliferation remains unclear. One theory invokes nonspecific polyclonal stimuli; another implicates antigen as the driving force. Antigen-driven repertoires are characteristically enriched for related sets of V gene segments containing similar sequence in the antigen binding site (complementarity-determining regions; CDRs). To study the forces shaping B cell proliferation, we analyzed V kappa transcripts expressed in the synovium of an RA patient. We found Humkv325, a developmentally regulated V kappa III gene segment associated with autoantibody reactivity, in > 10% of randomly-chosen synovial C kappa cDNAs. Two sets of sequences contained identical charged amino acid residues at the V kappa-J kappa join, apparently due to N region addition. We generated "signature" oligonucleotides from these CDR3s and probed PCR amplified V kappa products from the synovium and PBLs of the same patient, and from PBLs and spleen of individuals without rheumatic disease. Significant expression of transcripts containing these unique CDR3 sequences occurred only in the patient's synovium. Thus, in this synovium there is expansion of a limited set of B cell clones expressing antigen receptors that bear evidence of antigen selection.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Baltimore D. Joining of immunoglobulin heavy chain gene segments: implications from a chromosome with evidence of three D-JH fusions. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4118–4122. doi: 10.1073/pnas.79.13.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankhurst A. D., Husby G., Williams R. C., Jr Predominance of T cells in the lymphocytic infiltrates of synovial tissues in rheumatoid arthritis. Arthritis Rheum. 1976 May-Jun;19(3):555–562. doi: 10.1002/art.1780190307. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Chen P. P., Albrandt K., Orida N. K., Radoux V., Chen E. Y., Schrantz R., Liu F. T., Carson D. A. Genetic basis for the cross-reactive idiotypes on the light chains of human IgM anti-IgG autoantibodies. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8318–8322. doi: 10.1073/pnas.83.21.8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dersimonian H., McAdam K. P., Mackworth-Young C., Stollar B. D. The recurrent expression of variable region segments in human IgM anti-DNA autoantibodies. J Immunol. 1989 Jun 1;142(11):4027–4033. [PubMed] [Google Scholar]
- Desiderio S. V., Yancopoulos G. D., Paskind M., Thomas E., Boss M. A., Landau N., Alt F. W., Baltimore D. Insertion of N regions into heavy-chain genes is correlated with expression of terminal deoxytransferase in B cells. Nature. 1984 Oct 25;311(5988):752–755. doi: 10.1038/311752a0. [DOI] [PubMed] [Google Scholar]
- English D., Andersen B. R. Single-step separation of red blood cells. Granulocytes and mononuclear leukocytes on discontinuous density gradients of Ficoll-Hypaque. J Immunol Methods. 1974 Aug;5(3):249–252. doi: 10.1016/0022-1759(74)90109-4. [DOI] [PubMed] [Google Scholar]
- Goodhardt M., Babinet C., Lutfalla G., Kallenbach S., Cavelier P., Rougeon F. Immunoglobulin kappa light chain gene promoter and enhancer are not responsible for B-cell restricted gene rearrangement. Nucleic Acids Res. 1989 Sep 25;17(18):7403–7415. doi: 10.1093/nar/17.18.7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harindranath N., Goldfarb I. S., Ikematsu H., Burastero S. E., Wilder R. L., Notkins A. L., Casali P. Complete sequence of the genes encoding the VH and VL regions of low- and high-affinity monoclonal IgM and IgA1 rheumatoid factors produced by CD5+ B cells from a rheumatoid arthritis patient. Int Immunol. 1991 Sep;3(9):865–875. doi: 10.1093/intimm/3.9.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990 May 3;322(18):1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- Heller M., Owens J. D., Mushinski J. F., Rudikoff S. Amino acids at the site of V kappa-J kappa recombination not encoded by germline sequences. J Exp Med. 1987 Sep 1;166(3):637–646. doi: 10.1084/jem.166.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J., Kelsoe G., Rajewsky K., Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991 Dec 5;354(6352):389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- Jirik F. R., Sorge J., Fong S., Heitzmann J. G., Curd J. G., Chen P. P., Goldfien R., Carson D. A. Cloning and sequence determination of a human rheumatoid factor light-chain gene. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2195–2199. doi: 10.1073/pnas.83.7.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukes T. H., King J. L. Evolutionary nucleotide replacements in DNA. Nature. 1979 Oct 18;281(5732):605–606. doi: 10.1038/281605a0. [DOI] [PubMed] [Google Scholar]
- Kelley D. E., Perry R. P. Transcriptional and posttranscriptional control of immunoglobulin mRNA production during B lymphocyte development. Nucleic Acids Res. 1986 Jul 11;14(13):5431–5447. doi: 10.1093/nar/14.13.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipps T. J., Fong S., Tomhave E., Chen P. P., Goldfien R. D., Carson D. A. High-frequency expression of a conserved kappa light-chain variable-region gene in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 1987 May;84(9):2916–2920. doi: 10.1073/pnas.84.9.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipps T. J., Robbins B. A., Carson D. A. Uniform high frequency expression of autoantibody-associated crossreactive idiotypes in the primary B cell follicles of human fetal spleen. J Exp Med. 1990 Jan 1;171(1):189–196. doi: 10.1084/jem.171.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipps T. J., Robbins B. A., Kuster P., Carson D. A. Autoantibody-associated cross-reactive idiotypes expressed at high frequency in chronic lymphocytic leukemia relative to B-cell lymphomas of follicular center cell origin. Blood. 1988 Aug;72(2):422–428. [PubMed] [Google Scholar]
- Klobeck H. G., Bornkamm G. W., Combriato G., Mocikat R., Pohlenz H. D., Zachau H. G. Subgroup IV of human immunoglobulin K light chains is encoded by a single germline gene. Nucleic Acids Res. 1985 Sep 25;13(18):6515–6529. doi: 10.1093/nar/13.18.6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobeck H. G., Combriato G., Zachau H. G. N segment insertion and region-directed somatic hypermutation in a kappa gene of a t(2;8) chromosomal translocation. Nucleic Acids Res. 1987 Jun 25;15(12):4877–4888. doi: 10.1093/nar/15.12.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobeck H. G., Meindl A., Combriato G., Solomon A., Zachau H. G. Human immunoglobulin kappa light chain genes of subgroups II and III. Nucleic Acids Res. 1985 Sep 25;13(18):6499–6513. doi: 10.1093/nar/13.18.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman W. J., Schrohenloher R. E., Crago S. S., Spalding D. M., Mestecky J. IgA rheumatoid factor synthesis by dissociated synovial cells. Characterization and relationship to IgM rheumatoid factor synthesis. Arthritis Rheum. 1985 Nov;28(11):1219–1227. doi: 10.1002/art.1780281105. [DOI] [PubMed] [Google Scholar]
- Landau N. R., Schatz D. G., Rosa M., Baltimore D. Increased frequency of N-region insertion in a murine pre-B-cell line infected with a terminal deoxynucleotidyl transferase retroviral expression vector. Mol Cell Biol. 1987 Sep;7(9):3237–3243. doi: 10.1128/mcb.7.9.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrick J. W., Danielsson L., Brenner C. A., Abrahamson M., Fry K. E., Borrebaeck C. A. Rapid cloning of rearranged immunoglobulin genes from human hybridoma cells using mixed primers and the polymerase chain reaction. Biochem Biophys Res Commun. 1989 May 15;160(3):1250–1256. doi: 10.1016/s0006-291x(89)80138-x. [DOI] [PubMed] [Google Scholar]
- Ledford D. K., Goñi F., Pizzolato M., Franklin E. C., Solomon A., Frangione B. Preferential association of kappa IIIb light chains with monoclonal human IgM kappa autoantibodies. J Immunol. 1983 Sep;131(3):1322–1325. [PubMed] [Google Scholar]
- Lee S. K., Bridges S. L., Jr, Koopman W. J., Schroeder H. W., Jr The immunoglobulin kappa light chain repertoire expressed in the synovium of a patient with rheumatoid arthritis. Arthritis Rheum. 1992 Aug;35(8):905–913. doi: 10.1002/art.1780350809. [DOI] [PubMed] [Google Scholar]
- Marks J. D., Tristem M., Karpas A., Winter G. Oligonucleotide primers for polymerase chain reaction amplification of human immunoglobulin variable genes and design of family-specific oligonucleotide probes. Eur J Immunol. 1991 Apr;21(4):985–991. doi: 10.1002/eji.1830210419. [DOI] [PubMed] [Google Scholar]
- Martin T., Blaison G., Levallois H., Pasquali J. L. Molecular analysis of the V kappa III-J kappa junctional diversity of polyclonal rheumatoid factors during rheumatoid arthritis frequently reveals N addition. Eur J Immunol. 1992 Jul;22(7):1773–1779. doi: 10.1002/eji.1830220716. [DOI] [PubMed] [Google Scholar]
- Meindl A., Klobeck H. G., Ohnheiser R., Zachau H. G. The V kappa gene repertoire in the human germ line. Eur J Immunol. 1990 Aug;20(8):1855–1863. doi: 10.1002/eji.1830200834. [DOI] [PubMed] [Google Scholar]
- Panush R. S., Bianco N. E., Schur P. H. Serum and synovial fluid IgG, IgA and IgM antigammaglobulins in rheumatoid arthritis. Arthritis Rheum. 1971 Nov-Dec;14(6):737–747. doi: 10.1002/art.1780140609. [DOI] [PubMed] [Google Scholar]
- Pons-Estel B., Goñi F., Solomon A., Frangione B. Sequence similarities among kappa IIIb chains of monoclonal human IgM kappa autoantibodies. J Exp Med. 1984 Sep 1;160(3):893–904. doi: 10.1084/jem.160.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L. F., Rassenti L., Larrick J., Robbins B., Banks P. M., Kipps T. J. Ig V region gene expression in small lymphocytic lymphoma with little or no somatic hypermutation. J Immunol. 1989 Jul 15;143(2):699–705. [PubMed] [Google Scholar]
- Radoux V., Chen P. P., Sorge J. A., Carson D. A. A conserved human germline V kappa gene directly encodes rheumatoid factor light chains. J Exp Med. 1986 Dec 1;164(6):2119–2124. doi: 10.1084/jem.164.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz I., Capra J. D. V kappa and J kappa gene segments of A/J Ars-A antibodies: somatic recombination generates the essential arginine at the junction of the variable and joining regions. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1085–1089. doi: 10.1073/pnas.84.4.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz I. Multiple mechanisms participate in the generation of diversity of human H chain CDR3 regions. J Immunol. 1991 Sep 1;147(5):1720–1729. [PubMed] [Google Scholar]
- Scott M. G., Tarrand J. J., Crimmins D. L., McCourt D. W., Siegel N. R., Smith C. E., Nahm M. H. Clonal characterization of the human IgG antibody repertoire to Haemophilus influenzae type b polysaccharide. II. IgG antibodies contain VH genes from a single VH family and VL genes from at least four VL families. J Immunol. 1989 Jul 1;143(1):293–298. [PubMed] [Google Scholar]
- Shlomchik M. J., Aucoin A. H., Pisetsky D. S., Weigert M. G. Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9150–9154. doi: 10.1073/pnas.84.24.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik M. J., Marshak-Rothstein A., Wolfowicz C. B., Rothstein T. L., Weigert M. G. The role of clonal selection and somatic mutation in autoimmunity. 1987 Aug 27-Sep 2Nature. 328(6133):805–811. doi: 10.1038/328805a0. [DOI] [PubMed] [Google Scholar]
- Shlomchik M. J., Nemazee D. A., Sato V. L., Van Snick J., Carson D. A., Weigert M. G. Variable region sequences of murine IgM anti-IgG monoclonal autoantibodies (rheumatoid factors). A structural explanation for the high frequency of IgM anti-IgG B cells. J Exp Med. 1986 Aug 1;164(2):407–427. doi: 10.1084/jem.164.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley J. D., Sachs C., Ziff M. In vitro synthesis of immunoglobulin by rheumatoid synovial membrane. J Clin Invest. 1968 Mar;47(3):624–632. doi: 10.1172/JCI105758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straubinger B., Huber E., Lorenz W., Osterholzer E., Pargent W., Pech M., Pohlenz H. D., Zimmer F. J., Zachau H. G. The human VK locus. Characterization of a duplicated region encoding 28 different immunoglobulin genes. J Mol Biol. 1988 Jan 5;199(1):23–34. doi: 10.1016/0022-2836(88)90376-2. [DOI] [PubMed] [Google Scholar]
- Suh S. W., Bhat T. N., Navia M. A., Cohen G. H., Rao D. N., Rudikoff S., Davies D. R. The galactan-binding immunoglobulin Fab J539: an X-ray diffraction study at 2.6-A resolution. Proteins. 1986 Sep;1(1):74–80. doi: 10.1002/prot.340010112. [DOI] [PubMed] [Google Scholar]
- Tillman D. M., Jou N. T., Hill R. J., Marion T. N. Both IgM and IgG anti-DNA antibodies are the products of clonally selective B cell stimulation in (NZB x NZW)F1 mice. J Exp Med. 1992 Sep 1;176(3):761–779. doi: 10.1084/jem.176.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Uematsu Y. A novel and rapid cloning method for the T-cell receptor variable region sequences. Immunogenetics. 1991;34(3):174–178. doi: 10.1007/BF00205820. [DOI] [PubMed] [Google Scholar]
- Victor K. D., Randen I., Thompson K., Forre O., Natvig J. B., Fu S. M., Capra J. D. Rheumatoid factors isolated from patients with autoimmune disorders are derived from germline genes distinct from those encoding the Wa, Po, and Bla cross-reactive idiotypes. J Clin Invest. 1991 May;87(5):1603–1613. doi: 10.1172/JCI115174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withrington R. H., Teitsson I., Valdimarsson H., Seifert M. H. Prospective study of early rheumatoid arthritis. II. Association of rheumatoid factor isotypes with fluctuations in disease activity. Ann Rheum Dis. 1984 Oct;43(5):679–685. doi: 10.1136/ard.43.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]