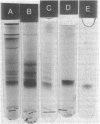
Abstract
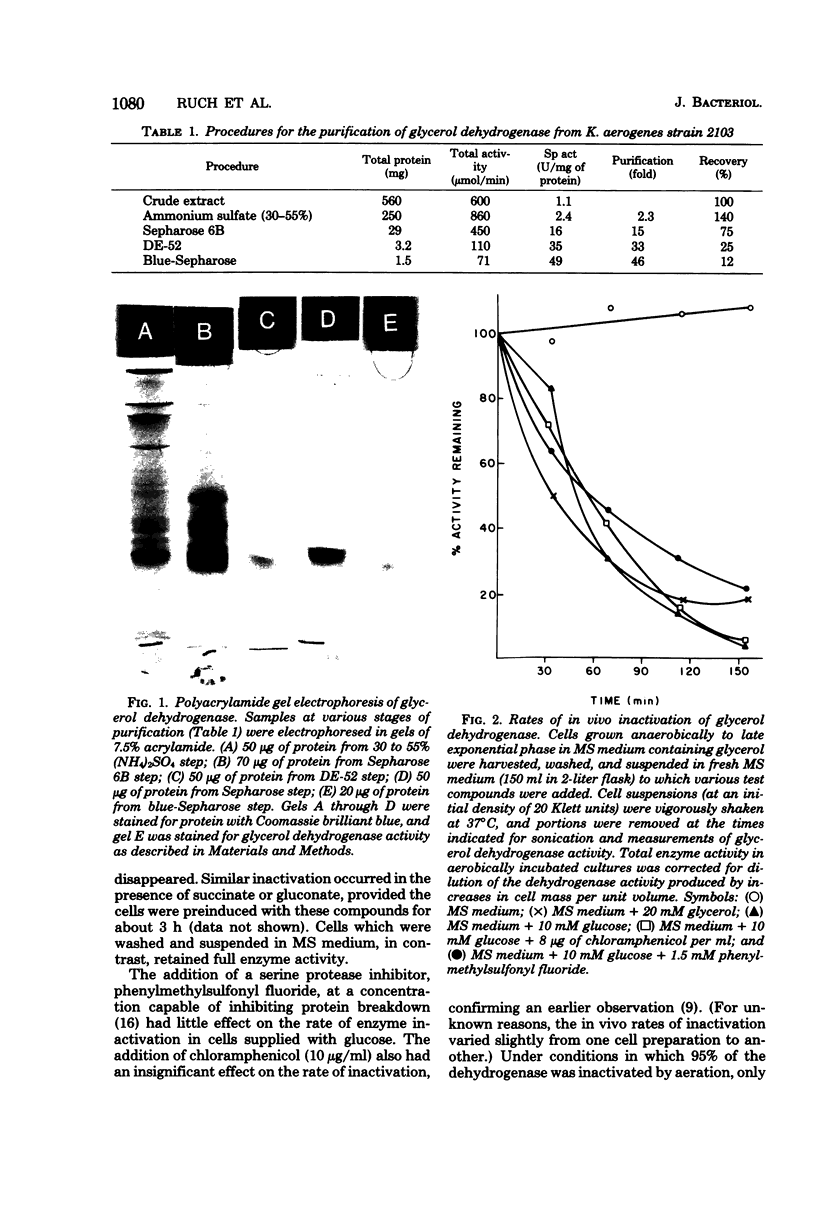
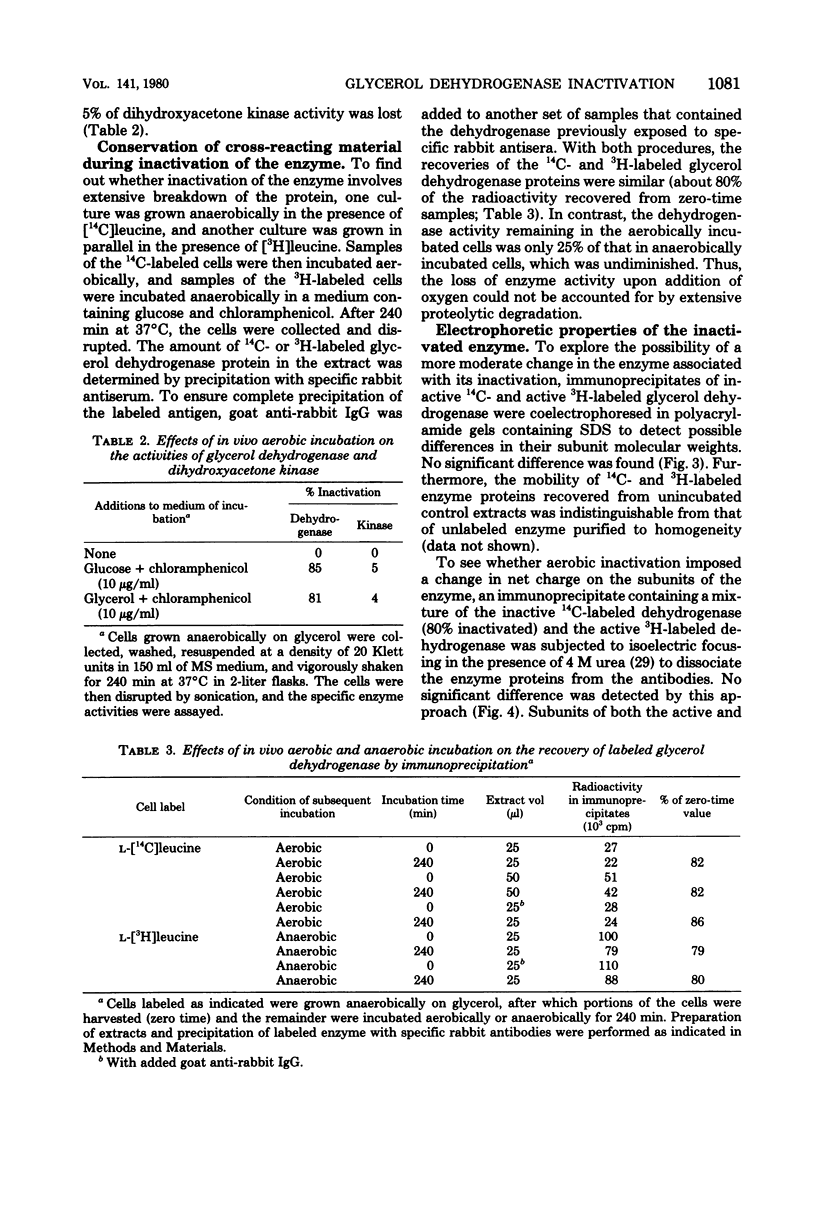
Glycerol:oxidized nicotinamide adenine dinucleotide (NAD+) 2-oxidoreductase (EC 1.1.1.6), an inducible enzyme for anaerobic glycerol catabolism in Klebsiella aerogenes, was purified and found to have a molecular weight of 79,000 by gel electrophoresis. The protein seemed to be enzymatically active either as a dimer of a 40,000-dalton peptide at pH 8.6 or as a tetramer of 160,000 molecular weight at pH 7.0. The enzyme activity was present at high levels in cells growing anaerobically on glycerol, but disappeared with a half-life of about 45 min if molecular oxygen was introduced to the culture. In contrast, no such phenomenon occurred with dihydroxyacetone kinase activity, the second enzyme in the pathway. Immunochemical analysis showed that the inactivation of the oxidoreductase did not involve degradation of the protein. Furthermore, subunits of the active and inactive forms of the enzyme were indistinguishable in size on polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate and had similar isoelectric points (pH 4.7). Inactivation did, however, alter the gel filtration properties of the enzyme protein and, more importantly, reduced its affinity for the dye Cibacron F3GA and the coenzyme NAD+.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz H., Weiser U. Protein degradation during yeast sporulation. Enzyme and cytochrome patterns. Eur J Biochem. 1976 Nov 15;70(2):385–395. doi: 10.1111/j.1432-1033.1976.tb11028.x. [DOI] [PubMed] [Google Scholar]
- Böhme H. J., Kopperschläger G., Schulz J., Hofmann E. Affinity chromatography of phosphofructokinase using Cibacron blue F3G-A. J Chromatogr. 1972 Jun 28;69(1):209–214. doi: 10.1016/s0021-9673(00)83103-9. [DOI] [PubMed] [Google Scholar]
- Freedberg W. B., Kistler W. S., Lin E. C. Lethal synthesis of methylglyoxal by Escherichia coli during unregulated glycerol metabolism. J Bacteriol. 1971 Oct;108(1):137–144. doi: 10.1128/jb.108.1.137-144.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giffhorn F., Gottschalk G. Inactivation of citrate lyase from Rhodopseudomonas gelatinosa by a specific deacetylase and inhibition of this inactivation by L-(+1-glutamate. J Bacteriol. 1975 Dec;124(3):1052–1061. doi: 10.1128/jb.124.3.1052-1061.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson G. L., Kong W. Y., Wright B. E. Analysis of uridine diphosphate-glucose pyrophosphorylase synthesis during differentiation in Dictyostelium discoideum. J Biol Chem. 1973 Jul 25;248(14):5188–5196. [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- John P. C., Thurston C. F., Syrett P. J. Disappearance of isocitrate lyase enzyme from cells of Chlorella pyrenoidosa. Biochem J. 1970 Oct;119(5):913–919. doi: 10.1042/bj1190913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN E. C., LEVIN A. P., MAGASANIK B. The effect of aerobic metabolism on the inducible glycerol dehydrogenase of Aerobacter aerogenes. J Biol Chem. 1960 Jun;235:1824–1829. [PubMed] [Google Scholar]
- LIN E. C., MAGASANIK B. The activation of glycerol dehydrogenase from Aerobacter aerogenes by monovalent cations. J Biol Chem. 1960 Jun;235:1820–1823. [PubMed] [Google Scholar]
- MUNKRES K. D., RICHARDS F. M. THE PURIFICATION AND PROPERTIES OF NEUROSPORA MALATE DEHYDROGENASE. Arch Biochem Biophys. 1965 Mar;109:466–479. doi: 10.1016/0003-9861(65)90391-7. [DOI] [PubMed] [Google Scholar]
- Magni G., Pallotta G., Natalini P., Ruggieri S., Santarelli I., Vita A. Inactivation of uridine nucleosidase in yeast. Purification and properties of an inactivating protein. J Biol Chem. 1978 Apr 25;253(8):2501–2503. [PubMed] [Google Scholar]
- McGregor W. G., Phillips J., Suelter C. H. Purification and kinetic characterization of a monovalent cation-activated glycerol dehydrogenase from Aerobacter aerogenes. J Biol Chem. 1974 May 25;249(10):3132–3139. [PubMed] [Google Scholar]
- Neeff J., Mecke D. Inaktivierung von Malat-Dehydrogenase in Hefe. Hoppe Seylers Z Physiol Chem. 1972 Oct;353(10):1552–1553. [PubMed] [Google Scholar]
- Orlowski M., White D. Inactivation of isocitrate lyase during myxospore development in Myxococcus xanthus. J Bacteriol. 1974 Apr;118(1):96–102. doi: 10.1128/jb.118.1.96-102.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prouty W. F., Goldberg A. L. Effects of protease inhibitors on protein breakdown in Escherichia coli. J Biol Chem. 1972 May 25;247(10):3341–3352. [PubMed] [Google Scholar]
- Ruch F. E., Lengeler J., Lin E. C. Regulation of glycerol catabolism in Klebsiella aerogenes. J Bacteriol. 1974 Jul;119(1):50–56. doi: 10.1128/jb.119.1.50-56.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruch F. E., Lin E. C. Independent constitutive expression of the aerobic and anaerobic pathways of glycerol catabolism in Klebsiella aerogenes. J Bacteriol. 1975 Oct;124(1):348–352. doi: 10.1128/jb.124.1.348-352.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims A. P., Toone J., Box V. The regulation of glutamine synthesis in the food yeast Candida utilis: the purification and subunit structure of glutamine synthetase and aspects of enzyme deactivation. J Gen Microbiol. 1974 Feb;80(2):485–499. doi: 10.1099/00221287-80-2-485. [DOI] [PubMed] [Google Scholar]
- Sorger G. J., Debanne M. T., Davies J. Effect of nitrate on the synthesis and decay of nitrate reductase of Neurospora. Biochem J. 1974 Jun;140(3):395–403. doi: 10.1042/bj1400395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland J. E., Miller O. N. Inhibition of glycerol dehydrogenase from Aerobacter aerogenes by dihydroxyacetone, high ionic strength, and monovalent cations. Biochim Biophys Acta. 1968 Jun 4;159(2):221–225. doi: 10.1016/0005-2744(68)90070-3. [DOI] [PubMed] [Google Scholar]
- Switzer R. L. The inactivation of microbial enzymes in vivo. Annu Rev Microbiol. 1977;31:135–157. doi: 10.1146/annurev.mi.31.100177.001031. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Lerner S. A., Lin E. C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967 Feb;93(2):642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. T., Stellwagen E. Binding of Cibacron blue F3GA to proteins containing the dinucleotide fold. Proc Natl Acad Sci U S A. 1976 Feb;73(2):361–365. doi: 10.1073/pnas.73.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbough C. L., Jr, Switzer R. L. Oxygen-dependent inactivation of glutamine phosphoribosylpyrophosphate amidotransferase in vitro inactivation. J Bacteriol. 1975 Jan;121(1):115–120. doi: 10.1128/jb.121.1.115-120.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waindle L. M., Switzer R. L. Inactivation of aspartic transcarbamylase in sporulating Bacillus subtilis: demonstration of a requirement for metabolic energy. J Bacteriol. 1973 May;114(2):517–527. doi: 10.1128/jb.114.2.517-527.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wellner D., Hayes M. B. Isoelectric focusing in polyacrylamide gels. Ann N Y Acad Sci. 1973 Jun 15;209:34–43. doi: 10.1111/j.1749-6632.1973.tb47517.x. [DOI] [PubMed] [Google Scholar]
- Wieland O. H., Hartmann U., Siess E. A. Neurospora crassa pyruvate dehydrogenase: interconversion by phosphorylation and dephosphorylation. FEBS Lett. 1972 Nov 1;27(2):240–244. doi: 10.1016/0014-5793(72)80630-6. [DOI] [PubMed] [Google Scholar]
- Williams L. S., Neidhardt F. C. Synthesis and inactivation of aminoacyl-transfer RNA synthetases during growth of Escherichia coli. J Mol Biol. 1969 Aug 14;43(3):529–550. doi: 10.1016/0022-2836(69)90357-x. [DOI] [PubMed] [Google Scholar]
- Wong J. Y., Meyer E., Switzer R. L. Glutamine phosphoribosylpyrophosphate amidotransferase from Bacillus subtilis. A novel iron-sulfur protein. J Biol Chem. 1977 Nov 10;252(21):7424–7426. [PubMed] [Google Scholar]
- Zwaig N., Diéguez E. A bactericidal product obtained from a mutant of Escherichia coli. Biochem Biophys Res Commun. 1970 Sep 30;40(6):1415–1422. doi: 10.1016/0006-291x(70)90025-2. [DOI] [PubMed] [Google Scholar]