Abstract
Sarcopenia is the progressive generalized loss of skeletal muscle mass, strength, and function which occurs as a consequence of aging. With a growing older population, there has been great interest in developing approaches to counteract the effects of sarcopenia, and thereby reduce the age-related decline and disability. This paper reviews (1) the mechanisms of sarcopenia, (2) the diagnosis of sarcopenia, and (3) the potential interventions for sarcopenia. Multiple factors appear to be involved in the development of sarcopenia including the loss of muscle mass and muscle fibers, increased inflammation, altered hormonal levels, poor nutritional status, and altered renin–angiotensin system. The lack of diagnostic criteria to identify patients with sarcopenia hinders potential management options. To date, pharmacological interventions have shown limited efficacy in counteracting the effects of sarcopenia. Recent evidence has shown benefits with angiotensin-converting enzyme inhibitors; however, further randomized controlled trials are required. Resistance training remains the most effective intervention for sarcopenia; however, older people maybe unable or unwilling to embark on strenuous exercise training programs.
Keywords: aged, muscle function, sarcopenia
Background
Maintaining muscle function is vital to maintain functional independence. In our growing older population, muscle mass and force reach their peak between the second and fourth decades of life and thereafter show a steady decline with age.1 Sarcopenia is a syndrome characterized by progressive generalized loss of skeletal muscle mass and strength. It is usually accompanied by physical inactivity, decreased mobility, slow gait, and poor physical endurance which are also common features of the frailty syndrome.2 Rockwood et al3 described the concept of frailty as “a multidimensional syndrome which involves loss of reserves (energy, physical activity, cognition, and health) which gives rise to increased vulnerability”. Frailty involves a cumulative decline in multiple physiological systems including a decline in the neuromuscular system which is linked to the development of sarcopenia in later life. The loss of muscle mass during the ageing process is clinically important as it leads to reduced strength and exercise capacity, both of which are required to undertake normal daily living activities. Moreover, loss of muscle mass is a strong predictor of mortality in later life.4
It has been estimated that up to 15% of people older than 65 years and as many as 50% of people older than 80 years have sarcopenia.5 Sarcopenia has a major impact on public health and the cost in the united States alone was estimated at $18.5 billion in 2000.6 With the increasing number of older people worldwide, the cost is ever increasing. There has, therefore, been great interest in developing approaches to counteract the effects of sarcopenia and thereby help in reducing the age-related decline and disability.
What causes sarcopenia?
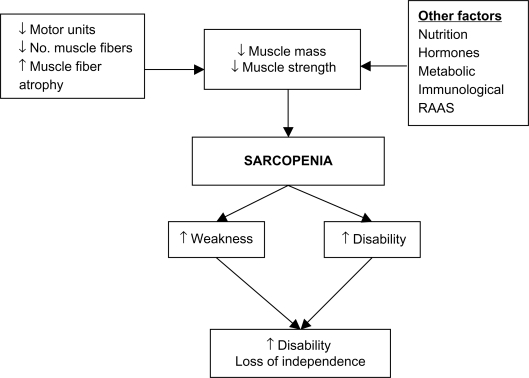
Understanding the mechanisms that have been implicated in the development of sarcopenia can help direct sarcopenia treatments. Research is still ongoing but as yet no primary cause of sarcopenia has been identified. Multiple factors appear to be involved in the development of sarcopenia (Figure 1). A reduction in muscle strength is primarily linked to a reduction in overall muscle mass.7 This reduction in muscle mass may occur due to a combination of the loss of muscle fibers as well as muscle fiber atrophy with a preferential atrophy of type 2 fast twitch fibers. Denervation of motor units which is then reinnervated with slow motor units can lead to increased muscle fatigability.8 Although the overall biological mechanism of sarcopenia is not fully understood, observational studies have shown that satellite cells which are involved in muscle regeneration are much lower in older people and, therefore, could play a role in sarcopenia.9
Figure 1.
Mechanism of sarcopenia.
Abbreviation: RAAS, renin–angiotensin–aldosterone system.
Other factors including hormonal changes including growth hormone (GH) and insulin-like growth factor (IGF-1) and androgens which help regulate growth and development of skeletal muscle appear to decrease in old age. It has been suggested that the renin–angiotensin system may play a role in modulating muscle function. Circulating angiotensin 2 is associated with muscle wasting, reduced IGF-1 levels, and insulin resistance and could, therefore, contribute to sarcopenia.10 Sarcopenia is also associated with chronic inflammation, and observational studies have shown increased levels of proinflammatory cytokines, tumor necrosis factor-α, and interleukin-6 in aging muscle.11 Studies looking at treating sarcopenia have attempted to address some of the factors implicated in the development of sarcopenia and we discuss the evidence surrounding this in our review.
Diagnosis of sarcopenia
The first step in the management of sarcopenia is to diagnose the condition. Unfortunately, at present there are no standardized diagnostic criteria for sarcopenia. Although sarcopenia is considered as a dynamic process incorporating both changes in muscle mass and function, many observational studies have concentrated on assessing changes in muscle mass. Table 1 summarizes the measurement techniques, what they measure, and their limitations. Although magnetic resonance imaging (MRI) is considered to be the most accurate measure of muscle mass, the currently preferred method is dual energy X-ray absorptiometry (DXA) as it measures both fat mass and bone mass and is useful for assessing appendicular muscle mass. DXA closely correlates with measurements achieved via MRI scanning.12 The main limitation with DXA is that it may underestimate the extent of sarcopenia as it can overestimate skeletal muscle mass by as much as 8% due to difficulty in distinguishing muscle from water retention and muscle fat infiltration.13 Bioelectric Impedence Analysis is a quick noninvasive method for measuring body composition via tissue conductivity.14 However, its reliability has been called into question as measurements can vary depending on an individual’s hydration status, ethnicity, physical fitness, and age.15
Table 1.
Measuring techniques for sarcopenia
| Measuring techniques | Measurements | Comments |
|---|---|---|
| Muscle size | ||
| CT Scan | Muscle cross-sectional area | Radiation exposure, expensive |
| MRI Scan | Muscle cross-sectional area | Expensive, availability of MRI |
| BIA | Tissue conductivity | ? reliability |
| Muscle circumferences | Mid arm and calf circumference | Measurements effected by subcutaneous fat |
| DXA scan | Total skeletal muscle mass | Reliable, low radiation exposure |
| Physical performance | ||
| SPPB | Lower extremity function | Validated tool for older people |
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; BIA, bioelectric impedence analysis; DXA, dual energy X-ray absorptiometry; SPPB, Short Physical Performance Battery.
Baumgartner5 in 1998 proposed a method for diagnosing sarcopenia. The degree of sarcopenia was measured by taking the muscle mass relative to a person’s height. Appendicular skeletal mass (ASM) was measured in all four limbs with DXA. Individuals with ASM/height2 (kg/m2) of two standard deviations (SDs) below the mean for gender specific healthy younger adults were more likely to have sarcopenia. Janssen et al16 measured skeletal muscle mass using bioimpedence and defined sarcopenia as a skeletal muscle index (skeletal muscle mass/whole body mass × 100) less than 1 SD below the mean for young adult values. These definitions fail to incorporate measures of disability and physical performance.16
Since then attempts have been made to refine the definition of sarcopenia. More recently, a joint effort has been made between the European Society on Clinical Nutrition and Metabolism and the Special Interest Needs group on geriatric nutrition on cachexia – anorexia and chronic wasting diseases.17 A diagnosis of sarcopenia was based on two of the following:
A low muscle mass, ie, a percentage of muscle mass >2 SDs below the mean measured in groups of young adults of the same sex and ethnic background.
Low gait speed, ie, walking speed below 0.8 m/s in the 4-meter walking test. However, this could be replaced with one well-established functional test utilized locally as part of a comprehensive geriatric assessment.
Although this definition includes measures of functional performance and helps to eliminate variance between ethnicity and sex, it fails to set age-specific populations. It has been suggested that a T-score system similar to that for osteoporosis is needed to include reference values for different populations. However, further refinement is required.
The main effect of loss of muscle mass is loss of strength. Low muscle strength is associated with increased mortality.18 Although complex measures of power and torque are available, hand-held dynamometers to measure hand grip and quadriceps strength have been commonly used with good reproducibility and validity and are simple to use and can be easily used in the clinical setting.19
Physical performance measures can complement measures of muscle mass and strength in the diagnosis of sarcopenia. The Short Physical Performance Battery assesses muscle function and strength using measures which are reproducible to activities of daily living. The assessment involves balance tests, a timed 4-meter walk, and timed chair rise which can be easily performed in the clinical setting. It can predict the risk of future disability and, therefore, may be useful in identifying people in the preclinical stage of sarcopenia who may benefit from interventions.20
Comorbidities and factors like pain from osteoarthritis which are unrelated to sarcopenia may limit performance and underestimate muscle strength. Fat mass may contribute to functional decline independent of muscle mass.21 Individuals with sarcopenic obesity (high fat mass and low muscle mass) are more susceptible to mobility problems and disability than those who are simply obese or sarcopenic.22 It is, therefore, imperative that sarcopenia is diagnosed under its paradigm as a dynamic process by assessing lean body mass and physical performance. As yet this may be more difficult to reproduce in a clinical setting.
The main difficulties in diagnosing sarcopenia are the lack of consensus in the definition of sarcopenia as well as the difficulty in measuring changes in muscle mass and function over time in older people. In clinical practice, the diagnosis is often missed as it is usually made in patients who appear to have “small muscle mass”. Simple measures of muscle strength and physical performance measures can supplement clinical diagnosis for the early recognition of people at risk of disability.
Potential interventions for sarcopenia
Exercise and physical activity
Physical activity refers to the body movement that is produced by skeletal muscle contractions and that increases energy expenditure.23 Evidence has shown that older adults who are less physically active are more likely to have lower skeletal muscle mass and strength and are at increased risk of developing sarcopenia.24,25
In aerobic exercise, the larger muscles in the body move in a rhythmic manner for a prolonged period of time, whereas resistance exercise involves muscles working hard against an applied force or weight such as in weight-lifting. Both aerobic and resistance-type exercise training have shown to improve the rate of decline in muscle mass and strength with age (Table 2).26
Table 2.
Summary of treatment options
| Intervention | Effect | Comments |
|---|---|---|
| Exercise | Increased cardiovascular fitness with increased endurance | Pros: overall beneficial effects of exercise to individual |
| Aerobic | Increases mitochondrial volume and activity | |
| Resistance | Increased muscle mass and strength | Cons: motivation to exercise remains low |
| Increased skeletal muscle protein synthesis and muscle fiber size | ||
| Improvement in physical performance | ||
| Nutritional supplement | Varying evidence of increased muscle mass and strength | Pros: ensures good protein intake |
| Cons: may reduce natural food intake | ||
| Hormone therapy | Varying evidence of increased muscle mass and strength | Cons: masculinization of women; increased risk of prostatic cancer in men |
| Testosterone | ||
| Estrogen | Poor evidence of increased muscle mass but not function | Cons: risk of breast cancer |
| Growth hormone | Some evidence for increased muscle mass. Varying evidence for increased muscle strength | Cons: side effects including fluid retention, orthostatic hypotension |
| Vitamin D | Variable evidence for increased muscle strength | Pros: fracture reduction; possible cardiovascular benefits |
| Reduced falls in nursing home residents | ||
| ACE inhibitors | Some evidence for increased exercise capacity | Pros: other cardiovascular benefits |
| Cons: renal function needs monitoring | ||
| Creatine | Variable evidence of increased muscle strength and endurance especially when combined with exercise | Cons: reports of nephritis |
| Potential new treatments | ||
| Myostatin antagonists | No trials in older people | |
| PPAR [δ] agonist | No human trials | |
| AICAR | No human trials |
Abbreviations: PPAR-δ, peroxisome-proliferator-activated receptor-δ; AICAR, 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside; ACE, angiotensin-converting enzyme.
Aerobic activity (swimming, running, and walking) has long been linked to improvements in cardiovascular fitness and endurance capacity. Although aerobic exercise is less likely to contribute to muscle hypertrophy, it can increase the cross-sectional area (CSA) of muscle fibers.27 Mitochondrial volume and enzyme activity increase after aerobic exercise demonstrate that muscle protein synthesis and muscle quality improve irrespective of age.28 Aerobic exercise can also reduce body fat including intramuscular fat which in turn improves the functional role of muscle relative to body weight.29
In contrast to aerobic exercise training, resistance exercise training appears to have a larger effect on augmenting muscle mass and strength and attenuates the development of sarcopenia.30,31 Improvements in muscle strength can be achieved with as little as one resistance exercise training session per week.32 Frontera et al33 demonstrated improvements in muscle CSA by 11% as well as improvement in muscle strength (>100%) after a 12-week period of high intensity resistance exercise training in older men. Similar improvements were seen in muscle strength even in the people aged >90 years with as little as 10–12 weeks of training.34
Muscle hypertrophy occurs when muscle protein synthesis outweighs protein breakdown. Older people performing resistance exercise show a marked increase in skeletal muscle protein synthesis without an increase in whole body muscle breakdown. Resistance training in older people increases both mixed-muscle protein synthesis and specific major histocompatibility complex synthesis to the same levels as younger adults.35 Evidence points to increases in size of both type 1 and type 2 muscle fibers which could explain the improvements in muscle strength and endurance.33,36 More recently, it has been reported that when using moderate levels of resistance exercise training, improvements in muscle strength and size in healthy older people were comparable to muscle strength seen in younger individuals. Roth et al37 demonstrated that 6 months of whole body resistance training in older people (65–75 years) produced gains in muscle CSA similar to those achieved in younger individuals aged 20–30 years.
Progressive resistance training (PRT) is the most commonly used resistance therapy in older people. A Cochrane review of 121 randomized controlled trials of PRT in older people showed that doing PRT 2–3 times per week improved physical function, gait speed, timed get-up and go, climbing stairs and balance, and more importantly had a significant effect on muscle strength especially in the high intensity training groups.38
The majority of studies have shown that resistance exercise training must be carried out at a high intensity in order to show substantial improvements in muscle strength.33,36,37 In contrast, Vincent et al39 performed a 26-week study in older healthy adults at both low and high intensity resistance exercise training programs and found only a modest improvement in thigh muscle strength in the high intensity resistance exercise training group.
Resistance exercise training appears to be relatively safe to perform even in participants with multiple comorbidities and can help in prevention of falls.40 Resistance exercise increases muscle CSA as well as type 2 (fast twitch) muscle fibers, which leads to overall improvement in muscle power and the ability to improve physical functioning. As a result, this can lead to enhanced ability to perform activities of daily living, preventing in functional decline and disability. Even in very old nursing home residents, resistance exercise training showed substantial improvements in muscle fiber CSA (3%–9%), muscle strength (>100%) as well as improvements in physical performance such as gait speed and stair climbing.36,38 However, participation in regular exercise training requires motivation by the individual which may be difficult for some older individuals; therefore, nonexercise interventions may offer a useful alternative.
Nutrition
Many older adults do not consume sufficient amounts of dietary protein which leads to a reduction in lean body mass and increased functional impairment.41 The current recommended dietary allowance (RDA) of protein is 0.8 g/kg/day, almost 40% of people >70 years do not meet this RDA.42 Taking a low protein diet below the RDA leads to a significant decline in muscle strength and muscle mass in older women.43 However, even older people who take the recommended RDA for protein continue to have a negative nitrogen balance and may require a diet containing a higher protein content than the RDA to maintain their skeletal muscle.44 Protein and energy supplementation may increase muscle strength even in very old people in the short term, but a Cochrane review has found no definite functional benefit of nutritional supplementation.45–47
Although older people who exercise have increased protein requirements, studies investigating whether nutritional supplementation in combination with resistance training can augment muscle strength gains in older people have yielded inconsistent results. One randomized controlled trial in nursing home residents undergoing resistance training over 10 weeks found that an additional 360 calories supplement increased leg muscle strength.36 Another study investigating the effect of dietary protein supplementation in combination with a 12-week resistance training period found that protein supplementation increased muscle mass but not muscle strength.48 Nutritional supplementation may also result in an overall decrease in voluntary food intake and adherence to the supplements can be a problem.47
Testosterone
Testosterone is secreted by the Leydig cells in men and ovarian thecal cells in women.49 Testosterone appears to increase muscle mass and increase muscle protein synthesis.50 It also increases the number of satellite cells in both animals and humans which are essential for muscle cell function.51
A substantial number of older men are hypogonadal. Hypogonadism has been defined as a total testosterone concentration of <9.26 nmol/L (2 SD below the mean for healthy young men). As a result, approximately 20% of men >60 years and 50% men >80 years are categorized as hypogonadal.52 Circulating testosterone is highly bound to sex hormone binding globulin (SHBG) and as SHBG increases with age, the total amount of bioavailable testosterone decreases. This phenomenon has been termed the “male menopause” or “andropause” in older men. Testosterone decreases gradually at a rate of 1% per year and bioavailable testosterone by 2% per year in males from the age of 30 years.53 The overall reduction of testosterone is associated with loss of muscle strength, muscle mass, a reduction in bone mineral density, and increased risk of fracture risk following falls.54,55
Evidence to support testosterone supplementation is variable. Gruenewald and Matsumoto56 analyzed 29 randomized controlled trials investigating the effects of testosterone replacement in older men. Some studies found an increase in lean body mass and hand grip strength but no effect on knee extension and flexion strength.56 Other studies have shown up to 25% increase in leg strength in as little as 4 weeks of therapy.57 Some studies have found no increase in muscle strength or function but an improvement in lean body mass.58 Testosterone supplementation has been shown to increase the size of the prostate gland in men.59 This could be detrimental to men older than 60 years in which the prevalence of early stage prostate cancer is already high.60 The Baltimore Longitudinal Study on Aging involving 781 men showed a positive correlation between prostate cancer and the blood concentration of free testosterone levels. The likelihood of acquiring a high risk prostate cancer in men >65 years doubled for every 0.1 unit increase in free testosterone.61 This along with other potential side effects of testosterone therapy like fluid retention, gynecomastia, polycythemia, and sleep apnea limit its usefulness as a treatment for sarcopenia.59,62,63
Estrogens
The menopause is linked to reduced concentrations of circulating estradiol in middle aged and older women. There appears to be impaired muscle performance during the postmenopausal period when ovarian hormone production has decreased.64 It is easy to hypothesize that estrogens may play a role in sarcopenia in older women.
The effect of hormone replacement therapy (HRT) in women is controversial. HRT may attenuate the loss of muscle mass which occurs in the perimenopausal period.65 Estrogen replacement therapy has only modest benefits on muscle composition and this may not translate to improved physical functioning.66 HRT combined with resistance training may have a role in improving lower extremity function; however, more evidence is needed.67 HRT has been implicated as a risk factor for breast cancer and is, therefore, not recommended for sarcopenia.68
Growth hormones
GH is required for maintenance of muscle and bone. GH exerts most of its anabolic actions through IGF-1 which is synthesized in the liver for systemic release. IGF-1 helps improve muscle function by increasing production of muscle satellite cells as well as stimulating production of muscle contractile proteins.69 Not only do GH and IGF-1 levels decline with age, the amplitude and frequency of pulsatile GH release is also significantly reduced.70
Despite a number of studies which have assessed the administration of GH supplementation, there is still an ongoing debate as to the use of GH supplementation on muscle mass, strength, and physical performance. The strongest evidence for the use of GH supplementation appears to be in states of reduced GH secretion. In younger GH deficient adults, GH supplementation for 3 years increased thigh muscle mass, strength, and improved exercise capacity.71 However, in healthy non-GH deficient older people results are more controversial. Some studies have shown an increase in muscle mass but no improvement in muscle strength, whereas others have shown an increase in both muscle mass and strength after administration of GH supplementation.72–74 The failure of exogenous GH to mimic the pulsatile pattern of normal GH secretion has been blamed for the negative results. Alternative potential hormonal interventions include the use of GH releasing hormone which was found to have only a small improvement in muscle strength in older men.75
It is well known that muscle strength increases as a result of resistance exercise training in older adults.30,33 It was hypothesized that the combination of GH replacement and exercise training may have a synergistic effect on muscle function in older people. However, results proved disappointing and the addition of GH supplementation does not augment the improvements in skeletal muscle brought about by exercise alone.76,77 Low GH levels alone, therefore, may not be responsible for the leveling off of muscle strength seen in older exercising people and that other pathways may be involved.
As it currently stands the evidence for the use of GH supplementation to counter the effects of sarcopenia in older people is weak. Moreover, the majority of trials involving GH replacement therapy in older people have reported a high incidence of side effects, including increased fluid retention, gynecomastia, orthostatic hypotension, and carpel tunnel syndrome.72,78
Vitamin D
Vitamin D levels decline with age and cutaneous vitamin D levels are up to four times lower in older compared with younger individuals.79 It is known that vitamin D plays an important role in bone and muscle metabolism. Several mechanisms have been suggested for the role of vitamin D in muscle function. Vitamin D binding to the vitamin D receptor found in skeletal muscle promotes muscle protein synthesis and enhances calcium uptake across the cell membrane.80 Low vitamin D levels result in atrophy predominantly of the type 2 (fast twitch) muscle fibers in common with sarcopenia.81 Low levels of vitamin D have been found to be associated with an increase in sarcopenia.82 A myopathy has been reported in severe vitamin D deficiency.83 In older people low vitamin D levels may produce functional problems including proximal muscle weakness, difficultly rising from a chair, difficulties in ascending stairs, and axial balance problems.84
The evidence for a benefit in physical performance with supplementation of vitamin D is controversial. Some studies have shown an improvement in muscle strength with intermittent dosing and others have shown small gains in lower extremity strength and less body sway with daily dosing.85 This improvement has been hypothesized as the mechanism behind a fall reduction of 23%–53% in older nursing or residential home residents given vitamin D in addition to a reduction in fractures.86–88
Conversely, other studies have found no benefits on physical function, falls risk or quality of life with vitamin D supplementation in vitamin D deficient people.89–91 The difference in findings between studies may in part be attributed to differences in the dose of vitamin D used with better outcomes seen when higher doses are used.92 It has also been suggested that there is a gender difference in outcomes with women standing to gain more from supplementation.92 Variations between study populations may also affect outcomes with the biggest improvements in muscle function and physical performance seen in institutionalized older people.
The prevalence of vitamin D insufficiency (25(OH)D levels <40 nmol/L) in older people is high between 50% and 75% especially in the northern latitudes and low levels have been found even in summer months.93–95 A European epidemiological study showed the prevalence of vitamin D deficiency in older adults aged 71–76 years was 36% in older men and 47% in older women.96 It is recommended that 25(OH)D levels <40 nmol/L requires supplementation and 25(OH)D levels of >75 nmol/L is the level for optimum bone and muscle health.97 The recommended daily intake of vitamin D is between 400 IU and 600 IU per day which may be inadequate to raise serum vitamin D levels to a desirable level >70 nmol/L.98,99 Studies have shown that in order to achieve optimal levels of 75–100 nmol/L of 25(OH)D doses between 700 and 1,000 IU would be needed.90 In the United States, fortification of food such as milk and orange juice is mandatory, whereas in the UK only margarine is fortified with vitamin D. The question of whether it should be mandatory for UK food products to be fortified with vitamin D remains controversial.
Although it is plausible to associate low levels of vitamin D with a reduction in muscle strength and physical function, the evidence for supplementation has been inconsistent. Safety issues surrounding vitamin D supplementation in older people include increased risk of nephrolithiasis and hypercalcemia.100,101 Further large randomized controlled trials are required with a longer follow-up period in order to assess the safety profile of vitamin D supplementation in older people before it is recommended as a treatment for sarcopenia in clinical practice.
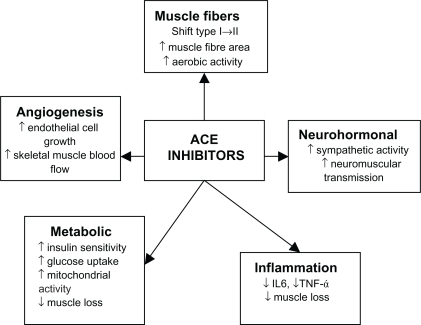
Angiotensin-converting enzyme inhibitors
Angiotensin-converting enzyme (ACE) inhibitors have long been used as a treatment in primary and secondary prevention in cardiovascular disease as well as secondary stroke prevention. It has now been suggested that ACE inhibitors may have a beneficial effect on skeletal muscle. ACE inhibitors may exert their beneficial effects on skeletal muscles through a number of different mechanisms (Figure 2). ACE inhibitors may improve muscle function through improvements in endothelial function, metabolic function anti-inflammatory effects, and angiogenesis thereby improving skeletal muscle blood flow. ACE inhibitors can increase mitochondrial numbers and IGF-I levels thereby helping to counter sarcopenia.102–107 People with the II genotype of the ACE gene who have low serum ACE levels show an increased response to physical endurance.108,109 Therefore, lowering serum ACE levels with ACE inhibitors may have a beneficial effect on physical function. Observational studies have shown that the long-term use of ACE inhibitors was associated with a lower decline in muscle strength and walking speed in older hypertensive people and a greater lower limb lean muscle mass when compared with users of other antihypertensive agents.110,111 Several studies have shown that ACE inhibitors improved exercise capacity in younger people with heart failure and this was also confirmed in older people with heart failure,110,112,113 no improvement in grip strength.114 Although this could be largely attributed to improvements in cardiac function, skeletal muscle atrophy is also associated with chronic heart failure so the evidence in muscle gains should not be discounted.
Figure 2.
Effects of ACE inhibitors on skeletal muscle.
Abbreviations: ACE, angiotensin-converting enzyme; IL-6, interleukin-6; TNF-α+ tumor necrosis factor-α.
Few interventional studies using ACE inhibitors for physical function have been undertaken. One study looking at functionally impaired older people without heart failure has shown that ACE inhibitors increase 6-minute walking distance to a degree comparable to that achieved after 6 months of exercise training.115 Another found that ACE inhibitors increased exercise time in older hypertensive men.116 However, a study comparing the effects of nifedipine with ACE inhibitors in older people found no difference between treatments in muscle strength, walking distance, or functional performance.117 It is possible that frailer subjects with slower walking speeds, who have a tendency to more cardiovascular problems, benefit more. This is reflected in the fact that adults with severe peripheral vascular disease significantly increase their walking time following treatment with ACE inhibitors.118
Further evidence is required before recommending ACE inhibitors to counter the effects of sarcopenia. However, ACE inhibitors are associated with cardiovascular benefits and as older people frequently have underlying cardiovascular problems these agents are already commonly prescribed.
Creatine
Creatine plays an important role in protein metabolism and cellular metabolism. It has been hypothesized that creatine increases the expression of myogenic transcription factors such as myogenin and myogenic regulatory factor-4, which increases muscle mass and strength.119 Creatine supplementation increases muscle phosphocreatine levels leading to a decrease in muscle relaxation time.120,121 This may increase the ability to perform high-intensity exercise as well as enhance muscle protein synthesis, lean skeletal muscle mass, and strength during periods of high intensity training.
To dates, several studies of creatine supplementation have shown increased muscle strength and power in younger men and women but few studies have looked at the effect of creatine supplementation in older people. Some studies have reported no effect of creatine supplementation on muscle strength or function.122,123 However, others have reported increments in muscle mass and increased muscle power without adverse effects.122,124 There is controversy over whether creatine supplementation increases the benefits of resistance training alone in older people. Some studies have found no added benefit of creatine supplementation to resistance exercise training and other studies have found a small increase in lean tissue mass with no residual benefit once resistance training was stopped.125–127
Creatine is a natural ingredient of food and the main source is from meat products with an average daily intake of 1 g/day. However, creatine supplementation may increase the risk of interstitial nephritis highlighting the need for particular caution about its use in older people.128 Creatine is not currently recommended for sarcopenia.
Myostatin
Myostatin is a natural inhibitor of growth factor. It was initially discovered when mutations of the myostatin gene was found to correlate with exaggerated muscle hypertrophy.129 The myostatin gene appears in skeletal muscle cells and functions as a negative regulator of muscle growth, antagonism of which increases satellite cell proliferation.130 In animal models, it appears that over expression of myostatin induces extensive muscle loss.131 Polymorphisms of the myostatin gene in humans correlated with measure of muscle mass, strength, and physical performance.132
Agents which target the myostatin pathway may be useful in increasing muscle mass and, therefore, play a vital role in muscle wasting disorders as well as sarcopenia of old age. Phase II trials have been carried out in muscular dystrophy and initial results have shown that MYO-29, a recombinant antibody to myostatin, had good safety and tolerability profile.133 Another potential therapeutic approach in development is a soluble activin type 2B receptor that binds to the myostatin and, therefore, reducing its availability. Initial results in mice have shown an increase in muscle weight larger than those achieved with myostatin inhibitors.134
Inhibition of myostatin with follistatin (myostatin antagonist) may have potential therapeutic benefits in the treatment of sarcopenia. Although myostatin deficiency increased muscle mass in mice it impaired, the structure and function of the muscle tendons thereby making the tendons smaller, stiffer, and more brittle.135,136 Older people who are already at increased risk of contraction induced injury may find it more difficult to sustain regular exercise. Further studies are required.
Other potential therapies
Following suggestions of a role for both the peroxisome-prolife rator-activated receptor-δ (PPAR-δ) and adenosine monophosphate (AMP)-activated protein kinase in regulating the metabolic and contractile characteristics of myofibers, studies looking at the effect of modulating these receptors have been carried out in mice. The PPAR-δ agonist GW1516 significantly increases exercise capacity when combined with exercise but not in sedentary mice. However, the activator of AMP-activated protein kinase called AICAR (5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside) increases exercise performance by 44% even in sedentary mice and may possible be the “exercise in a pill” for older people.137 It remains to be verified if these drugs are suitable for human beings especially older people.
Conclusion
Sarcopenia is an ever increasing global health concern that needs to be urgently addressed. Public awareness of the importance of physical activity need to be increased and exercise programs designed for older people should be developed. Although the primary aim would be to prevent the occurrence of the sarcopenia, like many other medical problems, we are ultimately left with having to deal with the condition and its consequences. As our review suggests, all is not lost by this stage and there is still scope for improvement even in the frail older person with a combination of measures.
The lack of diagnostic criteria to identify patients with sarcopenia hinders potential management options. Resistance exercise training remains the cornerstone of management for sarcopenia. As some older people are unable or unwilling to embark on exercise training program, alternative potential treatment options to counter the process of sarcopenia are being developed. Recent evidence has shown ACE inhibitors can improve muscle exercise capacity in functionally impaired older people; however, further randomized controlled trials are required. Other future prospects including the so called “exercise pill” have suggested potential methods to improve muscle performance in later life.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sayer AA, Syddall H, Martin H, Patel H, Baylis D, Cooper C. The developmental origins of sarcopenia. J Nutr Health Aging. 2008;12:427–432. doi: 10.1007/BF02982703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cesari M, Leeuwenburgh C, Lauretani F, et al. Frailty syndrome and skeletal muscle: results from the Invecchiare in Chianti study. Am J Clin Nutr. 2006;83:1142–1148. doi: 10.1093/ajcn/83.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. Gerontologist. 2005;45:386. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szulc P, Munoz F, Marchand F, Chapurlat R, Delmas PD. Rapid loss of appendicular skeletal muscle mass is associated with higher all-cause mortality in older men: the prospective MINOS study. Am J Clin Nutr. 2010;91:1227–1236. doi: 10.3945/ajcn.2009.28256. [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner RN. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755. doi: 10.1093/oxfordjournals.aje.a009520. Erratum in. Am J Epidemiol. 1999; 149:1161. [DOI] [PubMed] [Google Scholar]
- 6.Janssen I, Shepard D, Katzmarzyk PT, Roubenoff R. The health care costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 7.Akima H, Kano W, Enomoto Y, et al. Muscle function in 164 men and women aged 20–84 yr. Med Sci Sports Exerc. 2001;33:220–226. doi: 10.1097/00005768-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Erim Z, Beg MF, Burke DT, de Luca CJ. Effects of aging on motor-unit control properties. J Neurophysiol. 1999;82:2081–2091. doi: 10.1152/jn.1999.82.5.2081. [DOI] [PubMed] [Google Scholar]
- 9.Thornell LE, Lindstrom M, Renault V, Mouly V, Butler-Browne GS. Satellite cells and training in the elderly. Scand J Med Sci Sports. 2003;13:48–55. doi: 10.1034/j.1600-0838.2003.20285.x. [DOI] [PubMed] [Google Scholar]
- 10.Brink M, Wellen J, Delafontaine P. Angiotensin II causes weight loss and decreases circulating insulin-like growth factor I in rats through a pressor-independent mechanism. J Clin Invest. 1996;97:2509–2516. doi: 10.1172/JCI118698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaap LA, Pluijm SMF, Deeg DJH, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119:e9–e17. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Wang Z, Outwater E, et al. Is DXA a useful tool for assessing skeletal muscle mass in older women? J Bone Miner Res. 2005;20:S161. [Google Scholar]
- 13.Kim J, Wang ZM, Heymsfield SB, Baumgartner RN, Gallagher D. Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutr. 2002;76:378–383. doi: 10.1093/ajcn/76.2.378. [DOI] [PubMed] [Google Scholar]
- 14.Guo S, Roche AF, Houtkooper L. Fat-free mass in children and young-adults predicted from bioelectric impedance and anthropometric variables. Am J Clin Nutr. 1989;50:435–443. doi: 10.1093/ajcn/50.3.435. [DOI] [PubMed] [Google Scholar]
- 15.Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol. 2000;89:465–471. doi: 10.1152/jappl.2000.89.2.465. [DOI] [PubMed] [Google Scholar]
- 16.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 17.Muscaritoli M. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and nutrition in geriatrics. Clin Nutr. 2010;29:154–159. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Visser M, Harris TB, Langlois J, et al. Body fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham Heart Study. J Gerontol A Biol Sci Med Sci. 1998;53:M214–M221. doi: 10.1093/gerona/53a.3.m214. [DOI] [PubMed] [Google Scholar]
- 19.Martin HJ, Yule V, Syddall HE, Dennison EM, Cooper C, Sayer AA. Is hand-held dynamometry useful for the measurement of quadriceps strength in older people? A comparison with the gold standard biodex dynamometry. Gerontology. 2006;52:154–159. doi: 10.1159/000091824. [DOI] [PubMed] [Google Scholar]
- 20.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 22.Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904:437–48. doi: 10.1111/j.1749-6632.2000.tb06498.x. [DOI] [PubMed] [Google Scholar]
- 23.Chodzko-zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Salem GJ, Skinner JS. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exer. 2009;41:1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 24.Lee JSW, Auyeung TW, Kwok T, Lau EMC, Leung PC, Woo J. Associated factors and health impact of sarcopenia in older Chinese men and women: a cross-sectional study. Gerontology. 2007;53:404–410. doi: 10.1159/000107355. [DOI] [PubMed] [Google Scholar]
- 25.Rolland Y, Czerwinski S, van Kan GA, et al. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging. 2008;12:433–450. doi: 10.1007/BF02982704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frankel JE, Bean JF, Frontera WR. Exercise in the elderly: research and clinical practice. Clin Geriatr Med. 2006;22:256. doi: 10.1016/j.cger.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Coggan AR, Spina RJ, King DS, et al. Skeletal-muscle adaptations to endurance training in 60 year-old to 70 year-old men and women. J Appl Physiol. 1992;72:1780–1786. doi: 10.1152/jappl.1992.72.5.1780. [DOI] [PubMed] [Google Scholar]
- 28.Short KR, Vittone J, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metabo. 2004;286:E92–E101. doi: 10.1152/ajpendo.00366.2003. [DOI] [PubMed] [Google Scholar]
- 29.Misic MM, Rosengren KS, Woods JA, Evans EM. Muscle quality, aerobic fitness and fat mass predict lower-extremity physical function in community-dwelling older adults. Gerontology. 2007;53:260–266. doi: 10.1159/000101826. [DOI] [PubMed] [Google Scholar]
- 30.Sipila S, Suominen H. Effects of strength and endurance training on thigh and leg muscle mass and composition in elderly women. J Appl Physiol. 1995;78:334–340. doi: 10.1152/jappl.1995.78.1.334. [DOI] [PubMed] [Google Scholar]
- 31.Hughes VA, Roubenoff R, Wood M, Frontera WR, Evans WJ, Singh MAF. Anthropometric assessment of 10-y changes in body composition in the elderly. Am J Clin Nutr. 2004;80:475–482. doi: 10.1093/ajcn/80.2.475. [DOI] [PubMed] [Google Scholar]
- 32.Taaffe DR, Duret C, Wheeler S, Marcus R. Once-weekly resistance exercise improves muscle strength and neuromuscular performance in older adults. J Am Geriatr Soc. 1999;47:1214. doi: 10.1111/j.1532-5415.1999.tb05201.x. [DOI] [PubMed] [Google Scholar]
- 33.Frontera WR, Meredith CN, Oreilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men – skeletal-muscle hypertrophy and improved function. J Appl Physiol. 1988;64:1038–1044. doi: 10.1152/jappl.1988.64.3.1038. [DOI] [PubMed] [Google Scholar]
- 34.Brown AB, McCartney N, Sale DG. Positive adaptations to weight-lifting training in the elderly. J Appl Physiol. 1990;69:1725–1733. doi: 10.1152/jappl.1990.69.5.1725. [DOI] [PubMed] [Google Scholar]
- 35.Yarasheski KE. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am J Physiol. 1993;265:E210–E214. doi: 10.1152/ajpendo.1993.265.2.E210. [DOI] [PubMed] [Google Scholar]
- 36.Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 37.Roth SM, Martel GF, Ivey FM, et al. Skeletal muscle satellite cell characteristics in young and older men and women after heavy resistance strength training. J Gerontol A Biol Sci Med Sci. 2001;56:B240–B247. doi: 10.1093/gerona/56.6.b240. [DOI] [PubMed] [Google Scholar]
- 38.Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev. 2003:CD002759. doi: 10.1002/14651858.CD002759.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vincent KR, Braith RW, Feldman RA, et al. Resistance exercise and physical performance in adults aged 60 to 83. J Am Geriatr Soc. 2002;50:1100–1107. doi: 10.1046/j.1532-5415.2002.50267.x. [DOI] [PubMed] [Google Scholar]
- 40.Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009;3:CD007146. doi: 10.1002/14651858.CD007146.pub2. [DOI] [PubMed] [Google Scholar]
- 41.Bartali B, Frongillo EA, Bandinelli S, Lauretani F, Semba RD, Fried LP. Low nutrient intake is an essential component of frailty in older persons. J Gerontol A Biol Sci Med Sci. 2006;61:589–593. doi: 10.1093/gerona/61.6.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Houston DK, Nicklas BJ, Ding JZ, Harris TB, Tylavsky FA, Newman AB. Dietary intake is associated with lean mass change in older community-dwelling adults: the health aging and body composition (The Health ABC Study) study. Am J Clin Nutr. 2008;87:150–155. doi: 10.1093/ajcn/87.1.150. [DOI] [PubMed] [Google Scholar]
- 43.Castaneda C, Charnley JM, Evans WJ, Crim MC. Elderly women accommodate to a low-protein diet with losses of body cell mass, muscle function and immune-response. Am J Clin Nutr. 1995;62:30–39. doi: 10.1093/ajcn/62.1.30. [DOI] [PubMed] [Google Scholar]
- 44.Campbell WW, Trappe TA, Wolfe RR, Evans WJ. The recommended dietary allowance for protein may not be adequate for older people to maintain skeletal muscle. J Gerontol A Biol Sci Med Sci. 2001;56:M373–M380. doi: 10.1093/gerona/56.6.m373. [DOI] [PubMed] [Google Scholar]
- 45.Bonnefoy M, Cornu C, Normand S, et al. The effects of exercise and protein-energy supplements on body composition and muscle function in frail elderly individuals: a long-term controlled randomised study. Br J Nutr. 2003;89:731–738. doi: 10.1079/BJN2003836. [DOI] [PubMed] [Google Scholar]
- 46.Price R, Daly F, Pennington CR, Mcmurdo MET. Nutritional supplementation of very old people at hospital discharge increases muscle strength: a randomized controlled trial. Gerontology. 2005;51:185. doi: 10.1159/000083991. [DOI] [PubMed] [Google Scholar]
- 47.Milne AC, Potter J, Vivanti A, Avenell A. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Syst Rev. 2009;2:CD003288. doi: 10.1002/14651858.CD003288.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meridith CN, Frontera WR, O’Reilly KP, Evans WJ. Body-composition in elderly men – effect of dietary modification during strength training. J Am Geriatr Soc. 1992;40:162. doi: 10.1111/j.1532-5415.1992.tb01937.x. [DOI] [PubMed] [Google Scholar]
- 49.Brooks RV. Androgens. Clin Endocrinol Metab. 1975;4:503–520. doi: 10.1016/s0300-595x(75)80045-4. [DOI] [PubMed] [Google Scholar]
- 50.Griggs RC, Kingston W, Jozefowicz RF, Herr BE, Forbes G, Halliday D. Effect of testosterone on muscle mass and muscle protein synthesis. J Appl Physiol. 1989;66:489–503. doi: 10.1152/jappl.1989.66.1.498. [DOI] [PubMed] [Google Scholar]
- 51.Sinha-Hikim I, Taylor WE, Gonzalez-Cadavid NF, Zheng W, Bhasin S. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. Endocrinol Metab. 2004;89:5255. doi: 10.1210/jc.2004-0084. [DOI] [PubMed] [Google Scholar]
- 52.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 53.Morley JE, Kaiser FE, Perry HM, et al. Longitudinal changes in testosterone, luteinizing hormone and follicle stimulating hormone in healthy older men. Metab Clin Exp. 1997;46:410–413. doi: 10.1016/s0026-0495(97)90057-3. [DOI] [PubMed] [Google Scholar]
- 54.Mellstrom D, Johnell O, Ljunggren O, et al. Free testosterone is an independent predictor of BMD and prevalent fractures in elderly men: MrOS Sweden. J Bone Miner Res. 2006;21:529–535. doi: 10.1359/jbmr.060110. [DOI] [PubMed] [Google Scholar]
- 55.Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639–650. doi: 10.1210/jc.2009-1251. [DOI] [PubMed] [Google Scholar]
- 56.Gruenewald DA, Matsumoto AM. Testosterone supplementation therapy for older men: potential benefits and risks. J Am Geriatr Soc. 2003;51:101–115. doi: 10.1034/j.1601-5215.2002.51018.x. [DOI] [PubMed] [Google Scholar]
- 57.Urban RJ, Bodenburg YH, Gilkison C, et al. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol. 1995;269:E820–E826. doi: 10.1152/ajpendo.1995.269.5.E820. [DOI] [PubMed] [Google Scholar]
- 58.Emmelot-Vonk MH, Verhaar HJJ, Pour HRN, et al. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men – a randomized controlled trial. JAMA. 2008;299:39–52. doi: 10.1001/jama.2007.51. [DOI] [PubMed] [Google Scholar]
- 59.Snyder PJ, Peachey H, Berlin JA, et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85:2670–2677. doi: 10.1210/jcem.85.8.6731. [DOI] [PubMed] [Google Scholar]
- 60.Jemal A, Thomas A, Murray T, Thun M. Cancer statistics. CA Cancer J Clin. 2002;52:23–47. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- 61.Pierorazio PM, Ferrucci L, Kettermann AE, Metter EJ, Carter HB. Serum testosterone is associated with aggressive prostate cancer: results from the Baltimore longitudinal study of aging. J Urol. 2008;179:150. doi: 10.1111/j.1464-410X.2009.08853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schneider BK, Pickett CK, Zwillich CW, et al. Influence of testosterone on breathing during sleep. J Appl Physiol. 1986;61:618–623. doi: 10.1152/jappl.1986.61.2.618. [DOI] [PubMed] [Google Scholar]
- 63.Rhoden EL, Morgentaler A. Medical progress – risks of testosterone-replacement therapy and recommendations for monitoring. N Engl J Med. 2004;350:482–492. doi: 10.1056/NEJMra022251. [DOI] [PubMed] [Google Scholar]
- 64.Greeves JP, Cable NT, Reilly T, Kingsland C. Changes in muscle strength in women following the menopause: a longitudinal assessment of the efficacy of hormone replacement therapy. Clin Sci. 1999;97:79–84. [PubMed] [Google Scholar]
- 65.Dionne I. Sarcopenia and muscle function during menopause and hormone replacement therapy. J Nutr Aging Health. 2000;4:156–161. [PubMed] [Google Scholar]
- 66.Taaffe DR. Estrogen replacement, muscle composition and physical function: the health ABC study. Med Sci Sports Exerc. 2005;37:174–177. doi: 10.1249/01.mss.0000181678.28092.31. [DOI] [PubMed] [Google Scholar]
- 67.Sipila S. Effects of hormone replacement therapy and high impact physical exercise on skeletal muscle in post-menopausal women; a double randomized placebo controlled study. Clin Sci (London) 2001;101:147–151. [PubMed] [Google Scholar]
- 68.Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast, cancer and mammography in healthy post-menopausal women – the women’s health initiative randomized trial. JAMA. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 69.Chakravarthy MV, Davis BS, Booth FW. IGF-I restores satellite cell proliferative potential in immobilized old skeletal muscle. J Appl Physiol. 2000;89:1365–1379. doi: 10.1152/jappl.2000.89.4.1365. [DOI] [PubMed] [Google Scholar]
- 70.Goya RG, Brown OA, Bolognani F. The thymus-pituitary axis and its changes during aging. Neuroimmunomodulation. 1999;6:137–142. doi: 10.1159/000026373. [DOI] [PubMed] [Google Scholar]
- 71.Jorgensen JOL, Thuesen L, Muller J, Ovesen P, Shakkebaek NE, Christiansen JS. 3 years of growth-hormone treatment in growth-hormone deficient adults – near normalization of body composition and physical performance. Eur J Endocrinol. 1994;130:224–228. doi: 10.1530/eje.0.1300224. [DOI] [PubMed] [Google Scholar]
- 72.Papadakis MA, Grady D, Black D, et al. Growth hormone replacement in healthy older men improves body composition but not functional ability. Ann Intern Med. 1996;124:708–716. doi: 10.7326/0003-4819-124-8-199604150-00002. [DOI] [PubMed] [Google Scholar]
- 73.Thompson JL, Butterfield GE, Gylfadottir UK, et al. Effects of human growth hormone, insulin-like growth factor I and diet and exercise on body composition of obese postmenopausal women. J Clin Endocrinol Metab. 1998;83:1477–1484. doi: 10.1210/jcem.83.5.4826. [DOI] [PubMed] [Google Scholar]
- 74.Welle S, Thornton C, Statt M, McHenry B. Growth hormone increases muscle mass and strength but does not rejuvenate myofibrillar protein synthesis in healthy subjects over 60 years old. J Clin Endocrinol Metab. 1996;81:3239–3243. doi: 10.1210/jcem.81.9.8784075. [DOI] [PubMed] [Google Scholar]
- 75.Vittone J, Blackman MR, BusbyWhitehead J, et al. Effects of single nightly injections of growth hormone-releasing hormone (GHRH 1–29 in healthy elderly men. Metab Clin Exp. 1997;46:89–96. doi: 10.1016/s0026-0495(97)90174-8. [DOI] [PubMed] [Google Scholar]
- 76.Taaffe DR, Pruitt L, Reim J, et al. Effect of recombinant human growth-hormone on the muscle strength response to resistance exercise in elderly men. J Clin Endocrinol Metab. 1994;79:1361–1366. doi: 10.1210/jcem.79.5.7525633. [DOI] [PubMed] [Google Scholar]
- 77.Lange KHW, Andersen JL, Beyer N, et al. GH administration changes myosin heavy chain isoforms in skeletal muscle but does not augment muscle strength or hypertrophy, either alone or combined with resistance exercise training in healthy elderly men. J Clin Endocrinol Metab. 2002;87:513–523. doi: 10.1210/jcem.87.2.8206. [DOI] [PubMed] [Google Scholar]
- 78.Yarasheski KE, Zachwieja JJ. Growth-hormone therapy for the elderly – the fountain of youth proves toxic. JAMA. 1993;270:1694. [PubMed] [Google Scholar]
- 79.MacLaughlin JH. Ageing decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985;76:1536–1538. doi: 10.1172/JCI112134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bischoff HA, Borchers M, Gudat F, et al. In situ detection of 1,25-dihydroxyvitamin D-3 receptor in human skeletal muscle tissue. Histochem J. 2001;33:19–24. doi: 10.1023/a:1017535728844. [DOI] [PubMed] [Google Scholar]
- 81.Ziambaras K, DagogoJack S. Reversible muscle weakness in patients with vitamin D deficiency. West J Med. 1997;167:435–439. [PMC free article] [PubMed] [Google Scholar]
- 82.Visser M, Deeg DJH, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (Sarcopenia): the longitudinal aging study Amsterdam. J Clin Endocrinol Metab. 2003;88:5766–5772. doi: 10.1210/jc.2003-030604. [DOI] [PubMed] [Google Scholar]
- 83.Schott GD, Wills MR. Muscle weakness in osteomalacia. Lancet. 1976;1:626–629. doi: 10.1016/s0140-6736(76)90428-1. [DOI] [PubMed] [Google Scholar]
- 84.Mowe M, Haug E, Bohmer T. Low serum calcidiol concentration in older adults with reduced muscular function. J Am Geriatr Soc. 1999;47:220–226. doi: 10.1111/j.1532-5415.1999.tb04581.x. [DOI] [PubMed] [Google Scholar]
- 85.Moreira-Pfrimer LDF, Pedrosa MAC, Teixeira L, Lazaretti-Castro M. Treatment of vitamin D deficiency increases lower limb muscle strength in institutionalized older people independently of regular physical activity: a randomized double-blind controlled trial. Ann Nutr Metab. 2009;54:291–300. doi: 10.1159/000235874. [DOI] [PubMed] [Google Scholar]
- 86.Bischoff HA, Stahelin HB, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18:343–351. doi: 10.1359/jbmr.2003.18.2.343. [DOI] [PubMed] [Google Scholar]
- 87.Flicker L, MacInnis RJ, Stein MS, et al. Should older people in residential care receive vitamin D to prevent falls? Results of a randomized trial. J Am Geriatr Soc. 2005;53:1881–1888. doi: 10.1111/j.1532-5415.2005.00468.x. [DOI] [PubMed] [Google Scholar]
- 88.Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327:1637–1642. doi: 10.1056/NEJM199212033272305. [DOI] [PubMed] [Google Scholar]
- 89.Witham MD, Crighton LJ, Gillespie ND, Struthers AD, McMurdo ME. The effects of vitamin D supplementation on physical function and quality of life in older heart failure patients: a randomised controlled trial. Circ Heart Fail. 2010;3:195–201. doi: 10.1161/CIRCHEARTFAILURE.109.907899. [DOI] [PubMed] [Google Scholar]
- 90.Annweiler C, Beauchet O, Berrut G, et al. Is there an association between serum 25-hydroxyvitamin D concentration and muscle strength among older women? Results from baseline assessment of the EPIDOS study. J Nutr Health Aging. 2009;13:90–95. doi: 10.1007/s12603-009-0013-1. [DOI] [PubMed] [Google Scholar]
- 91.Brunner RL, Cochrane B, Jackson RD, et al. Calcium, vitamin D supplementation, and physical function in the women’s health initiative. J Am Diet Assoc. 2008;108:1472–1479. doi: 10.1016/j.jada.2008.06.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, et al. Fall prevention with a supplemental and active forms of vitamin D: a meta analysis of randomised controlled trials. BMJ. 2009;339:b3692. doi: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Napiorkowska L, Budlewski T, Jakubas-Kwiatkowska W, Hamzy V, Gozdowski D, Franek E. Prevalence of low serum vitamin D concentration in an urban population of elderly women in Poland. Pol Arch Intern Med. 2009;119:699–703. [PubMed] [Google Scholar]
- 94.Hirani V, Tull K, Ali A, Mindell J. Urgent action needed to improve vitamin D status among older people in England. Age Ageing. 2010;39:62–68. doi: 10.1093/ageing/afp195. [DOI] [PubMed] [Google Scholar]
- 95.Bischoff-Ferrari HA, Can U, Staehelin HB, et al. Severe vitamin D deficiency in Swiss hip fracture patients. Bone. 2008;42:597–602. doi: 10.1016/j.bone.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 96.Vanderwielen RPJ, Lowik MRH, Vandenberg H, et al. Serum vitamin-D concentrations among elderly people in Europe. Lancet. 1995;346:207–210. doi: 10.1016/s0140-6736(95)91266-5. [DOI] [PubMed] [Google Scholar]
- 97.Geneva World Health Organization and Food Agricultural Organization World Health Organization: Vitamin and Mineral Requirements in Human Nutritionss. 2nd ed. 2004.
- 98.Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B. Positive association between 25-hydroxy, vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med. 2004;116:634–639. doi: 10.1016/j.amjmed.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 99.Yetley EA, Brule D, Cheney MC, et al. Dietary reference intakes for vitamin D: justification for a review of the 1997 values. Am J Clin Nutr. 2009;89:719–27. doi: 10.3945/ajcn.2008.26903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barger-Lux MJ, Heaney RP, Dowell S, Chen TC, Holick MF. Vitamin D and its major metabolites: serum levels after graded oral dosing in healthy men. Osteoporos Int. 1998;8:222–230. doi: 10.1007/s001980050058. [DOI] [PubMed] [Google Scholar]
- 101.Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women (Nurses health study II) J Am Soc Nephrol. 2003;14:698A–699A. doi: 10.1001/archinte.164.8.885. [DOI] [PubMed] [Google Scholar]
- 102.Henriksen EJ, Jacob S. Modulation of metabolic control by angiotensin converting enzyme (ACE) inhibition. J Cell Physiol. 2003;196:171–179. doi: 10.1002/jcp.10294. [DOI] [PubMed] [Google Scholar]
- 103.Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fabre JE, Rivard A, Magner M, Silver M, Isner JM. Tissue inhibition of angiotensin-converting enzyme activity stimulates angiogenesis in vivo. Circulation. 1999;99:3043–3049. doi: 10.1161/01.cir.99.23.3043. [DOI] [PubMed] [Google Scholar]
- 105.Ferder L, Romano LA, Ercole LB, Stella I, Inserra F. Biomolecular changes in the aging myocardium – the effect of enalapril. Am J Hypertens. 1998;11:1297–1304. doi: 10.1016/s0895-7061(98)00152-6. [DOI] [PubMed] [Google Scholar]
- 106.de Cavanagh EMV, Piotrkowski B, Basso N, et al. Enalapril and losartan attenuate mitochondrial dysfunction in aged rats. FASEB J. 2003;17:1096–1098. doi: 10.1096/fj.02-0063fje. [DOI] [PubMed] [Google Scholar]
- 107.Maggio M, Ceda GP, Lauretani F, et al. Relation of angiotensin-converting enzyme inhibitor treatment to insulin-like growth factor-1 serum levels in subjects >65 years of age (the InCHIANTI study) Am J Cardiol. 2006;97:1525–1529. doi: 10.1016/j.amjcard.2005.11.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Williams AG, Rayson MP, Jubb M, et al. Physiology – the ACE gene and muscle performance. Nature. 2000;403:614. doi: 10.1038/35001141. [DOI] [PubMed] [Google Scholar]
- 109.Myerson S, Hemingway H, Budget R, Martin J, Humphries S, Montgomery H. Human angiotensin I-converting enzyme gene and endurance performance. J Appl Physiol. 1999;87:1313–1316. doi: 10.1152/jappl.1999.87.4.1313. [DOI] [PubMed] [Google Scholar]
- 110.Onder G, Penninx BWJH, Balkrishnan R, et al. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: an observational study. Lancet. 2002;359:926–930. doi: 10.1016/s0140-6736(02)08024-8. [DOI] [PubMed] [Google Scholar]
- 111.Di Bari M, Poll-Franse LV, Onder G, et al. Antihypertensive medications and differences in muscle mass in older persons: the health, aging and body composition study. J Am Geriatr Soc. 2004;52:961–966. doi: 10.1111/j.1532-5415.2004.52265.x. [DOI] [PubMed] [Google Scholar]
- 112.Dossegger L, Aldor E, Baird MG, et al. Influence of angiotensin-converting enzyme-inhibition on exercise performance and clinical symptoms in chronic heart-failure – a multicenter, double-blind, placebo-controlled trial. Eur Heart J. 1993;14:18–23. doi: 10.1093/eurheartj/14.suppl_c.18. [DOI] [PubMed] [Google Scholar]
- 113.Hutcheon SD, Gillespie ND, Crombie IK, Struthers AD, Mcmurdo MET. Perindopril improves six minute walking distance in older patients with left ventricular systolic dysfunction: a randomised double blind placebo controlled trial. Heart. 2002;88:373–377. doi: 10.1136/heart.88.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schellenbaum GD, Smith NL, Heckbert SR, et al. Weight loss, muscle strength, and angiotensin-converting enzyme inhibitors in older adults with congestive heart failure or hypertension. J Am Geriatr Soc. 2005;53:1996–2000. doi: 10.1111/j.1532-5415.2005.53568.x. [DOI] [PubMed] [Google Scholar]
- 115.Sumukadas D, Witham MD, Struthers AD, Mcmurdo MET. Effect of perindopril on physical function in elderly people with functional impairment: a randomized controlled trial. Can Med Assoc J. 2007;177:867–874. doi: 10.1503/cmaj.061339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Leonetti G, Mazzola C, Pasotti C, et al. Treatment of hypertension in the elderly – effects on blood-pressure, heart-rate, and physical-fitness. Am J Med. 1991;90:S12–S13. doi: 10.1016/0002-9343(91)90429-2. [DOI] [PubMed] [Google Scholar]
- 117.Bunout D, Barrera G, de la Maza MP, Leiva L, Backhouse C, Hirsch S. Effects of enalapril or nifedipine on muscle strength or functional capacity in elderly subjects. a double blind trial. J Renin Angiotensin Aldosterone Syst. 2009;10:77–84. doi: 10.1177/1470320309105338. [DOI] [PubMed] [Google Scholar]
- 118.Ahimastos AA, Lawler A, Reid CM, Blombery PA, Kingwell BA. Brief communication: Ramipril markedly improves walking ability in patients with peripheral arterial disease – a randomized trial. Ann Intern Med. 2006;144:660–664. doi: 10.7326/0003-4819-144-9-200605020-00009. [DOI] [PubMed] [Google Scholar]
- 119.Willoughby DS, Rosene JM. Effects of oral creatine and resistance training on myogenic regulatory factor expression. Med Sci Sports Exerc. 2003;35:923–929. doi: 10.1249/01.MSS.0000069746.05241.F0. [DOI] [PubMed] [Google Scholar]
- 120.Smith SA, Montain SJ, Matott RP, Zientara GP, Jolesz FA, Fielding RA. Creatine supplementation and age influence muscle metabolism during exercise. J Appl Physiol. 1998;85:1349–1356. doi: 10.1152/jappl.1998.85.4.1349. [DOI] [PubMed] [Google Scholar]
- 121.Van Leemputte M, Vandenberghe K, Hespel P. Shortening of muscle relaxation time after creatine loading. J Appl Physiol. 1999;86:840–844. doi: 10.1152/jappl.1999.86.3.840. [DOI] [PubMed] [Google Scholar]
- 122.Rawson ES. Acute creatine supplementation in older men. Int J Sports Med. 2000;21:71–75. doi: 10.1055/s-2000-8859. [DOI] [PubMed] [Google Scholar]
- 123.Rawson ES, Wehnert ML, Clarkson PM. Effects of 30 days of creatine ingestion in older men. Eur J Appl Physiol Occup Physiol. 1999;80:139–144. doi: 10.1007/s004210050570. [DOI] [PubMed] [Google Scholar]
- 124.Gotshalk LA, Kraemer WJ, Mendonca MAG, et al. Creatine supplementation improves musclular performance in older women. Eur J Appl Physiol Occup Physiol. 2008;102:223–231. doi: 10.1007/s00421-007-0580-y. [DOI] [PubMed] [Google Scholar]
- 125.Bermon S, Venembre P, Sachet C, Valour S, Dolisi C. Effects of creatine monohydrate ingestion in sedentary and weight-trained older adults. Acta Physiol Scand. 1998;164:147–155. doi: 10.1046/j.1365-201X.1998.00427.x. [DOI] [PubMed] [Google Scholar]
- 126.Crusch MJ. Creatine supplementation combined with resistance training in older men. Med Sci Sports Exerc. 2001;33:2111–2117. doi: 10.1097/00005768-200112000-00021. [DOI] [PubMed] [Google Scholar]
- 127.Candow DG, Chilibeck PD, Chad KE, Chrusch MJ, Davison KS, Burke DG. Effect of ceasing creatine supplementation while maintaining resistance training in older men. J Aging Phys Act. 2004;12:219–231. doi: 10.1123/japa.12.3.219. [DOI] [PubMed] [Google Scholar]
- 128.Koshy KM. Interstial nephritis in patient taking creatine. N Engl J Med. 1999;340:814–815. doi: 10.1056/NEJM199903113401017. [DOI] [PubMed] [Google Scholar]
- 129.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 130.Wagner KR, Liu XS, Chang XL, Allen RE. Muscle regeneration in the prolonged absence of myostatin. Proc Natl Acad Sci U S A. 2005;102:2519–2524. doi: 10.1073/pnas.0408729102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zimmer TA. Induction of cachexia in mice by systemically administered myostatin. Science. 2002;296:1486–1488. doi: 10.1126/science.1069525. [DOI] [PubMed] [Google Scholar]
- 132.Seibert MJ, Xue QL, Fried LP, Walstan JD. Polymorphic variation in human myostatin (GDF-8) gene and associations with strength measures in the Womens Health and Aging Study II cohort. J Am Geriatr Soc. 2001;49(8):1093–1096. doi: 10.1046/j.1532-5415.2001.49214.x. [DOI] [PubMed] [Google Scholar]
- 133.Wagner KR. Phase II trial of MYO-29 in adult subjects with muscular dystrophy. Ann Neurol. 2008;63:561–571. doi: 10.1002/ana.21338. [DOI] [PubMed] [Google Scholar]
- 134.Lee SJ. Regulation of muscle growth by multiple ligands signalling through activin type II receptors. PNAS. 2005;102:18117–18122. doi: 10.1073/pnas.0505996102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mendias CL, Bakhurin KI, Faulkner JA. Tendons of myostatin-deficient mice are small, brittle, and hypocellular. Proc Natl Acad Sci U S A. 2008;105:388–393. doi: 10.1073/pnas.0707069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kjaer M, Jespersen JG. The battle to keep or lose skeletal muscle with ageing. J Physiol (London) 2009;587:1–2. doi: 10.1113/jphysiol.2008.167049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Goodyear LJ. The exercise pill – too good to be true. N Engl J Med. 2008;359:1842–1844. doi: 10.1056/NEJMcibr0806723. [DOI] [PubMed] [Google Scholar]