Abstract
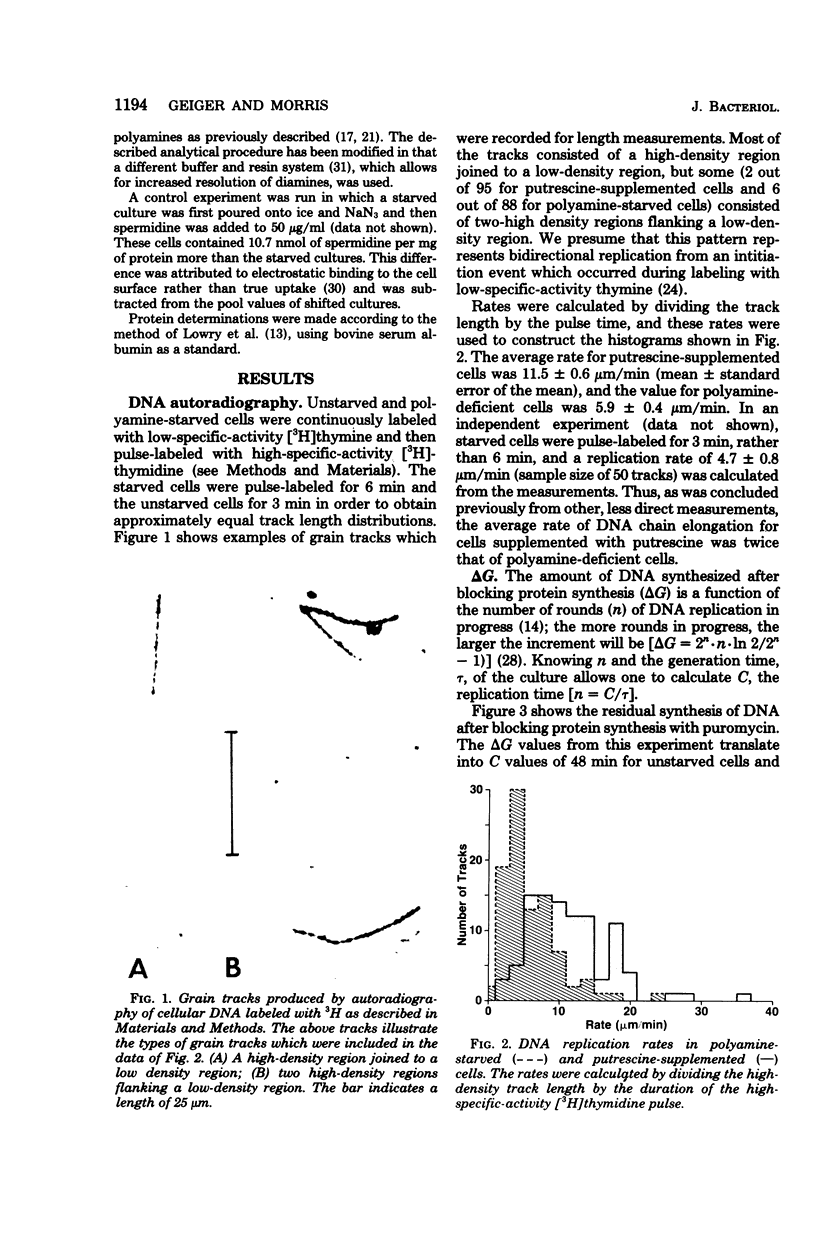
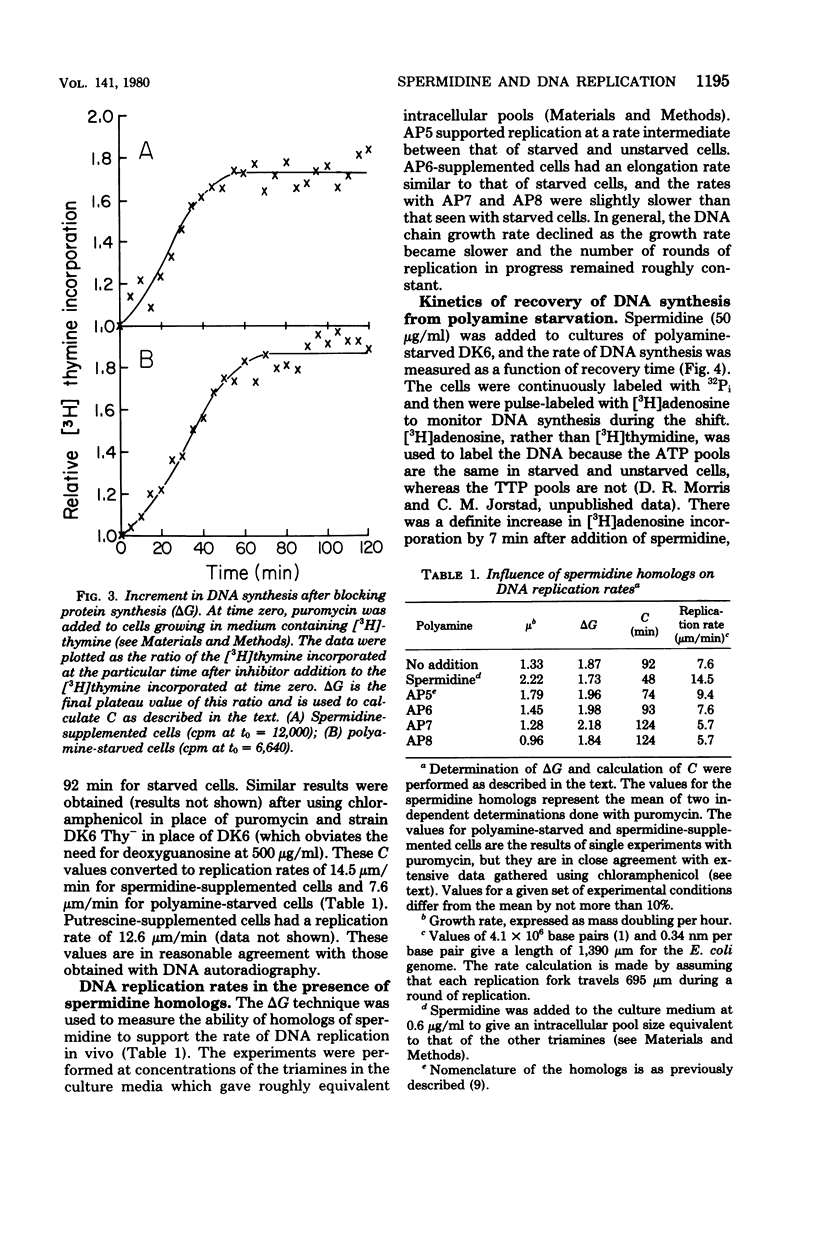
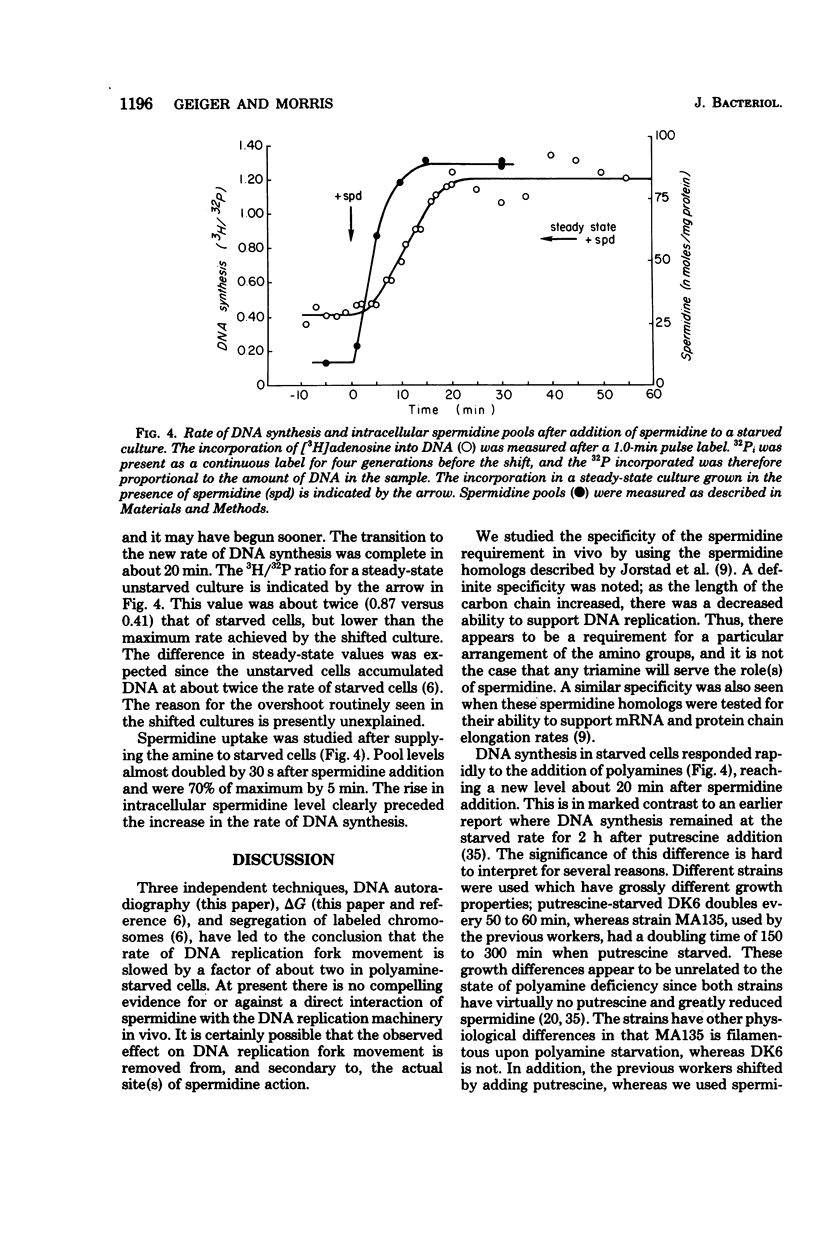
We examined the rate of deoxyribonucleic acid (DNA) replication fork movement in polyamine-deficient cells of Escherichia coli by two independent techniques. DNA autoradiography was used to directly visualize the length of DNA produced during a given time interval, and replication rates were calculated. The amount of DNA synthesized after blocking protein synthesis also allowed calculation of replication rates. We found that the DNA chain elongation rate in polyamine-deficient cells was about half that of putrescine- or spermidine-supplemented cells. We also found that spermidine homologs of increasing chain length, when present at equal intracellular concentrations, exhibited a decreasing ability to support growth and the rate of DNA replication fork movement. The kinetics of recovery of DNA synthesis from the polyamine-deficient state were also investigated. A new rate of DNA synthesis was reached about 20 min after addition of spermidine to polyamine-limited cells. The rise in the rate of DNA synthesis was preceded by a rise in the intracellular concentration of spermidine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen C., Baldwin R. L. Catalysis of DNA reassociation by the Escherichia coli DNA binding protein: A polyamine-dependent reaction. J Mol Biol. 1977 Sep 25;115(3):441–454. doi: 10.1016/0022-2836(77)90164-4. [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles S., Maas W. K. Isolation, characterization, and mapping of Escherichia coli mutants blocked in the synthesis of ornithine decarboxylase. J Bacteriol. 1975 Nov;124(2):791–799. doi: 10.1128/jb.124.2.791-799.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geider K., Kornberg A. Conversion of the M13 viral single strand to the double-stranded replicative forms by purified proteins. J Biol Chem. 1974 Jul 10;249(13):3999–4005. [PubMed] [Google Scholar]
- Geiger L. E., Morris D. R. Polyamine deficiency reduces the rate of DNA replication fork movement in Escherichia coli. Nature. 1978 Apr 20;272(5655):730–732. doi: 10.1038/272730a0. [DOI] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Nash H. A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., O'Dea M. H., Itoh T., Tomizawa J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4474–4478. doi: 10.1073/pnas.73.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorstad C. M., Harada J. J., Morris D. R. Structural specificity of the spermidine requirement of an Escherichia coli auxotroph. J Bacteriol. 1980 Feb;141(2):456–463. doi: 10.1128/jb.141.2.456-463.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorstad C. M., Morris D. R. Polyamine limitation of growth slows the rate of polypeptide chain elongation in Escherichia coli. J Bacteriol. 1974 Sep;119(3):857–860. doi: 10.1128/jb.119.3.857-860.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuempel P. L. Deoxyribonucleic acid-deoxyribonucleic acid hybridization assay for replication origin deoxyribonucleic acid of Escherichia coli. J Bacteriol. 1972 Jun;110(3):917–925. doi: 10.1128/jb.110.3.917-925.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lane H. E., Denhardt D. T. The rep mutation. IV. Slower movement of replication forks in Escherichia coli rep strains. J Mol Biol. 1975 Sep 5;97(1):99–112. doi: 10.1016/s0022-2836(75)80025-8. [DOI] [PubMed] [Google Scholar]
- MAALOE O., HANAWALT P. C. Thymine deficiency and the normal DNA replication cycle. I. J Mol Biol. 1961 Apr;3:144–155. doi: 10.1016/s0022-2836(61)80041-7. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Hansen M. T. Influence of polyamine limitation on the chain growth rates of beta-galactosidase and of its messenger ribonucleic acid. J Bacteriol. 1973 Nov;116(2):588–592. doi: 10.1128/jb.116.2.588-592.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. R., Jorstad C. M. Growth and macromolecular composition of a mutant of Escherichia coli during polyamine limitation. J Bacteriol. 1973 Jan;113(1):271–277. doi: 10.1128/jb.113.1.271-277.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. R., Jorstad C. M. Isolation of conditionally putrescine-deficient mutants of Escherichia coli. J Bacteriol. 1970 Mar;101(3):731–737. doi: 10.1128/jb.101.3.731-737.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. R., Koffron K. L., Okstein C. J. An automated method for polyamine analysis. Anal Biochem. 1969 Sep;30(3):449–453. doi: 10.1016/0003-2697(69)90140-7. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott D. M., Kuempel P. L. Bidirectional replication of the chromosome in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2842–2845. doi: 10.1073/pnas.69.10.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STACEY K. A., SIMSON E. IMPROVED METHOD FOR THE ISOLATION OF THYMINE-REQUIRING MUTANTS OF ESCHERICHIA COLI. J Bacteriol. 1965 Aug;90:554–555. doi: 10.1128/jb.90.2.554-555.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman R., Weiner A., Kornberg A. Multienzyme systems of DNA replication. Science. 1974 Dec 13;186(4168):987–993. doi: 10.1126/science.186.4168.987. [DOI] [PubMed] [Google Scholar]
- Schekman R., Wickner W., Westergaard O., Brutlag D., Geider K., Bertsch L. L., Kornberg A. Initiation of DNA synthesis: synthesis of phiX174 replicative form requires RNA synthesis resistant to rifampicin. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2691–2695. doi: 10.1073/pnas.69.9.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N., Yoshikawa H. The chromosome of Bacillus subtilis. I. Theory of marker frequency analysis. Genetics. 1965 Oct;52(4):747–757. doi: 10.1093/genetics/52.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Dobbs L. G. Metabolism of 1,4-diaminobutane and spermidine in Escherichia coli: the effects of low temperature during storage and harvesting of cultures. J Biol Chem. 1970 Apr 25;245(8):2086–2091. [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H., Hafner E. W. Escherichia coli mutants completely deficient in adenosylmethionine decarboxylase and in spermidine biosynthesis. J Biol Chem. 1978 May 25;253(10):3671–3676. [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Transport systems for 1,4-diaminobutane, spermidine, and spermine in Escherichia coli. J Biol Chem. 1966 Aug 25;241(16):3714–3723. [PubMed] [Google Scholar]
- Tabor H., Tabor C. W., Irreverre F. Quantitative determination of aliphatic diamines and polyamines by an automated liquid chromatography procedure. Anal Biochem. 1973 Oct;55(2):457–467. doi: 10.1016/0003-2697(73)90136-x. [DOI] [PubMed] [Google Scholar]
- Weiner J. H., Bertsch L. L., Kornberg A. The deoxyribonucleic acid unwinding protein of Escherichia coli. Properties and functions in replication. J Biol Chem. 1975 Mar 25;250(6):1972–1980. [PubMed] [Google Scholar]
- Young D. V., Srinivasan P. R. Regulation of macromolecular synthesis by putrescine in a conditional Escherichia coli putrescine auxotroph. J Bacteriol. 1972 Oct;112(1):30–39. doi: 10.1128/jb.112.1.30-39.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



