Abstract
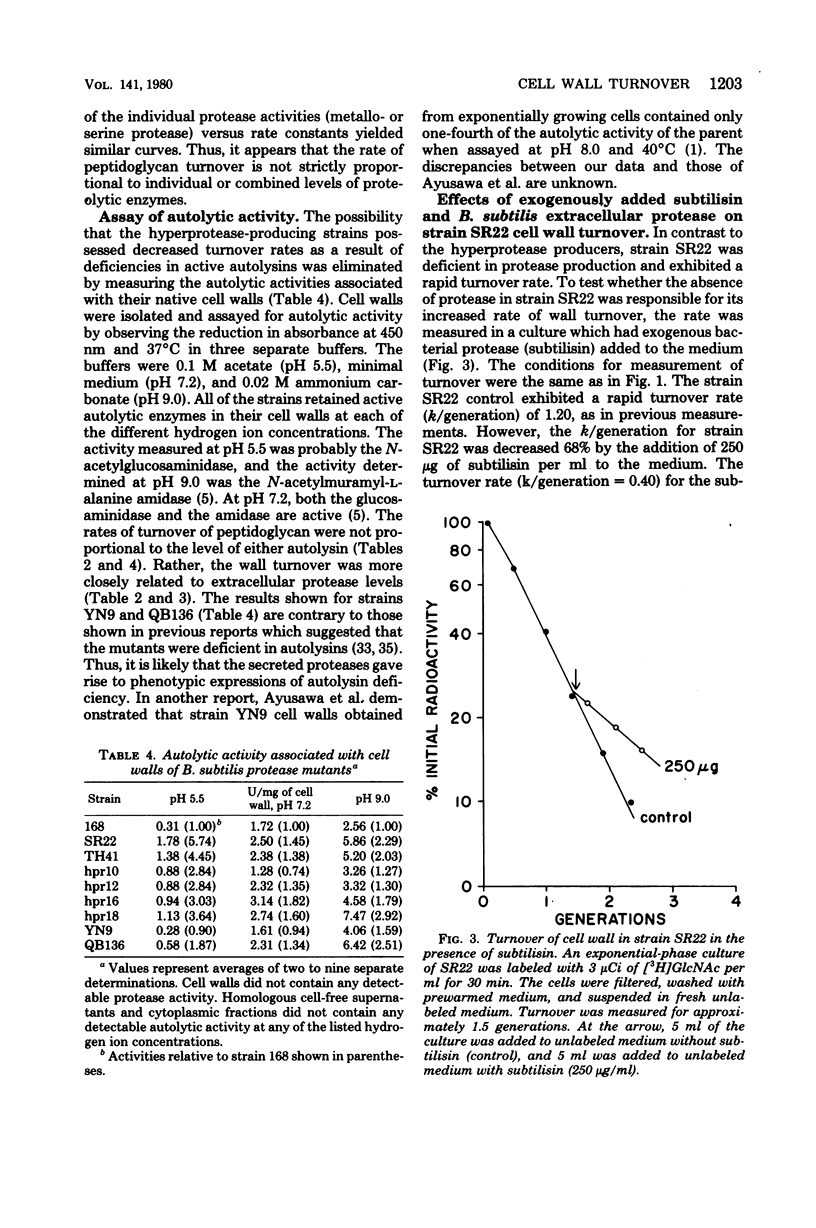
The rate of turnover of peptidoglycan in exponentially growing cultures of Bacillus subtilis was observed to be sensitive to extracellular protease. In protease-deficient mutants the rates of cell wall turnover were greater than that of wild-type strain 168, whereas hyperprotease-producing strains exhibited decreased rates of peptidoglycan turnover. The rate of peptidogylcan turnover in a protease-deficient strain was decreased when the mutant was grown in the presence of a hyperprotease-producing strain. The addition of phenylmethylsulfonyl fluoride, a serine protease inhibitor, to cultures of hyperprotease-producing strains increased their rates of cell wall turnover. Isolated cell walls of all protease mutants contained autolysin levels equal to or greater than that of wild-type strain 168. The presence of filaments, or cells with incomplete septa, was observed in hyperprotease-producing strains or when a protease-deficient strain was grown in the presence of subtilisin. The results suggest that the turnover of cell walls in B. subtilis may be regulated by extracellular proteases.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayusawa D., Yoneda Y., Yamane K., Maruo B. Pleiotropic phenomena in autolytic enzyme(s) content, flagellation, and simultaneous hyperproduction of extracellular alpha-amylase and protease in a Bacillus subtilis mutant. J Bacteriol. 1975 Oct;124(1):459–469. doi: 10.1128/jb.124.1.459-469.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. C. Rapid methods for extracting autolysins from Bacillus subtilis. Appl Microbiol. 1973 Feb;25(2):295–300. doi: 10.1128/am.25.2.295-300.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. C., Young F. E. Dynamic interactions between cell wall polymers, extracellular proteases and autolytic enzymes. Biochem Biophys Res Commun. 1970 Feb 20;38(4):564–568. doi: 10.1016/0006-291x(70)90618-2. [DOI] [PubMed] [Google Scholar]
- Cleveland R. F., Daneo-Moore L., Wicken A. J., Shockman G. D. Effect of lipoteichoic acid and lipids on lysis of intact cells of Streptococcus faecalis. J Bacteriol. 1976 Sep;127(3):1582–1584. doi: 10.1128/jb.127.3.1582-1584.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland R. F., Holtje J. V., Wicken A. J., Tomasz A., Daneo-Moore L., Shockman G. D. Inhibition of bacterial wall lysins by lipoteichoic acids and related compounds. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1128–1135. doi: 10.1016/0006-291x(75)90791-3. [DOI] [PubMed] [Google Scholar]
- Dancer B. N., Mandelstam J. Production and possible function of serine protease during sporulation of Bacillus subtilis. J Bacteriol. 1975 Feb;121(2):406–410. doi: 10.1128/jb.121.2.406-410.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein J. E., Rogers H. J. Autolytic enzyme-deficient mutants of Bacillus subtilis 168. J Bacteriol. 1976 Sep;127(3):1427–1442. doi: 10.1128/jb.127.3.1427-1442.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser L., Lindsay B. Relation between cell wall turnover and cell growth in Bacillus subtilis. J Bacteriol. 1977 May;130(2):610–619. doi: 10.1128/jb.130.2.610-619.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman J. H., Carlton B. C. An enzymatic and immunological comparison of two proteases from a transformable Bacillus subtilis with the "subtilisins". Arch Biochem Biophys. 1970 Jul;139(1):67–79. doi: 10.1016/0003-9861(70)90045-7. [DOI] [PubMed] [Google Scholar]
- Herbold D. R., Glaser L. Bacillus subtilis N-acetylmuramic acid L-alanine amidase. J Biol Chem. 1975 Mar 10;250(5):1676–1682. [PubMed] [Google Scholar]
- Herbold D. R., Glaser L. Interaction of N-acetylmuramic acid L-alanine amidase with cell wall polymers. J Biol Chem. 1975 Sep 25;250(18):7231–7238. [PubMed] [Google Scholar]
- Higerd T. B., Hoch J. A., Spizizen J. Hyperprotease-producing mutants of Bacillus subtilis. J Bacteriol. 1972 Nov;112(2):1026–1028. doi: 10.1128/jb.112.2.1026-1028.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A. Genetics of bacterial sporulation. Adv Genet. 1976;18:69–98. doi: 10.1016/s0065-2660(08)60437-x. [DOI] [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Specific recognition of choline residues in the cell wall teichoic acid by the N-acetylmuramyl-L-alanine amidase of Pneumococcus. J Biol Chem. 1975 Aug 10;250(15):6072–6076. [PubMed] [Google Scholar]
- Kawagishi S., Araki Y., Ito E. A novel glycosidase, an endo-glucosaminidase active on the cell wall peptidoglycan with N-unsubstituted glucosamine residues. FEBS Lett. 1979 Jan 1;97(1):20–22. doi: 10.1016/0014-5793(79)80042-3. [DOI] [PubMed] [Google Scholar]
- Kunst F., Pascal M., Lepesant-Kejzlarova J., Lepesant J. A., Billault A., Dedonder R. Pleiotropic mutations affecting sporulation conditions and the syntheses of extracellular enzymes in Bacillus subtilis 168. Biochimie. 1974;56(11-12):1481–1489. doi: 10.1016/s0300-9084(75)80270-7. [DOI] [PubMed] [Google Scholar]
- Mauck J., Chan L., Glaser L. Turnover of the cell wall of Gram-positive bacteria. J Biol Chem. 1971 Mar 25;246(6):1820–1827. [PubMed] [Google Scholar]
- Mauck J., Glaser L. On the mode of in vivo assembly of the cell wall of Bacillus subtilis. J Biol Chem. 1972 Feb 25;247(4):1180–1187. [PubMed] [Google Scholar]
- Monodane T., Matsushima Y., Hirachi Y., Kotani S. Cell wall autolysis in log phase cells of Micrococcus lysodeikticus (luteus). Microbiol Immunol. 1978;22(2):57–66. doi: 10.1111/j.1348-0421.1978.tb00349.x. [DOI] [PubMed] [Google Scholar]
- Monodane T., Matsushima Y., Kotani S. Demonstration of the physiological role of autolysis by a comparative study with a wild-type and its non-autolytic mutant of Micrococcus lysodeikticus (luteus) cultivated with externally added proteolytic enzymes. Microbiol Immunol. 1978;22(2):67–80. doi: 10.1111/j.1348-0421.1978.tb00350.x. [DOI] [PubMed] [Google Scholar]
- PARK J. T., HANCOCK R. A fractionation procedure for studies of the synthesis of cell-wall mucopeptide and of other polymers in cells of Staphylococcus aureus. J Gen Microbiol. 1960 Feb;22:249–258. doi: 10.1099/00221287-22-1-249. [DOI] [PubMed] [Google Scholar]
- Pooley H. M. Layered distribution, according to age, within the cell wall of bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1139–1147. doi: 10.1128/jb.125.3.1139-1147.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M. Turnover and spreading of old wall during surface growth of Bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1127–1138. doi: 10.1128/jb.125.3.1127-1138.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., Pooley H. M., Thurman P. F., Taylor C. Wall and membrane growth in bacilli and their mutants. Ann Microbiol (Paris) 1974 Sep;125 B(2):135–147. [PubMed] [Google Scholar]
- Rogers H. J., Taylor C. Autolysins and shape change in rodA mutants of Bacillus subtilis. J Bacteriol. 1978 Sep;135(3):1032–1042. doi: 10.1128/jb.135.3.1032-1042.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Cheney M. C. Autolytic enzyme system of Streptococcus faecalis. V. Nature of the autolysin-cell wall complex and its relationship to properties of the autolytic enzyme of Streptococcus faecalis. J Bacteriol. 1969 Jun;98(3):1199–1207. doi: 10.1128/jb.98.3.1199-1207.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M., Kunst F., Dedonder R. Mapping of mutations affecting synthesis of exocellular enzymes in Bacillus subtilis. Identity of the sacUh, amyB and pap mutations. Mol Gen Genet. 1976 Nov 17;148(3):281–285. doi: 10.1007/BF00332902. [DOI] [PubMed] [Google Scholar]
- Tomasz A. Biological consequences of the replacement of choline by ethanolamine in the cell wall of Pneumococcus: chanin formation, loss of transformability, and loss of autolysis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):86–93. doi: 10.1073/pnas.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda Y., Maruo B. Mutation of Bacillus subtilis causing hyperproduction of alpha-amylase and protease, and its synergistic effect. J Bacteriol. 1975 Oct;124(1):48–54. doi: 10.1128/jb.124.1.48-54.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda Y., Yamane K., Maruo B. Membrane mutation related to the production of extracellular -amylase and protease in bacillus subtilis. Biochem Biophys Res Commun. 1973 Feb 5;50(3):765–770. doi: 10.1016/0006-291x(73)91310-7. [DOI] [PubMed] [Google Scholar]
- Young F. E. Requirement of glucosylated teichoic acid for adsorption of phage in Bacillus subtilis 168. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2377–2384. doi: 10.1073/pnas.58.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]



