Abstract
Glandular secreting trichomes of cultivated tomato (Solanum lycopersicum) produce a wide array of volatile and nonvolatile specialized metabolites. Many of these compounds contribute to the characteristic aroma of tomato foliage and constitute a key part of the language by which plants communicate with other organisms in natural environments. Here, we describe a novel recessive mutation called odorless-2 (od-2) that was identified on the basis of an altered leaf-aroma phenotype. od-2 plants exhibit pleiotrophic phenotypes, including alterations in the morphology, density, and chemical composition of glandular trichomes. Type VI glandular trichomes isolated from od-2 leaves accumulate only trace levels of monoterpenes, sesquiterpenes, and flavonoids. Other foliar defensive compounds, including acyl sugars, glycoalkaloids, and jasmonate-regulated proteinase inhibitors, are produced in od-2 leaves. Growth of od-2 plants under natural field conditions showed that the mutant is highly susceptible to attack by an indigenous flea beetle, Epitrix cucumeris, and the Colorado potato beetle, Leptinotarsa decemlineata. The increased susceptibility of od-2 plants to Colorado potato beetle larvae and to the solanaceous specialist Manduca sexta was verified in no-choice bioassays. These findings indicate that Od-2 is essential for the synthesis of diverse trichome-borne compounds and further suggest that these compounds influence host plant selection and herbivore community composition under natural conditions.
The plant epidermal surface provides a formidable protective barrier to invasion by pathogens and arthropod herbivores. Hair-like protuberances, called trichomes, are among the most conspicuous defense-related structures on the aerial epidermis of leaves, stems, and floral organs. Trichomes are typically classified morphologically as being either nonglandular or glandular. Nonglandular trichomes physically impede the movement of small arthropod herbivores on the plant surface. Molecular and ecological studies indicate that trichome density is both a highly adaptive and a functionally important trait for resistance to herbivory (Kennedy, 2003; Kivimaki et al., 2007). In-depth knowledge of the molecular mechanisms that control trichome development in Arabidopsis (Arabidopsis thaliana), which produces unicellular nonglandular trichomes, has provided significant insight into the genetic basis of variation in trichome habit (Marks, 1997; Karkkainen and Agren, 2002; Yoshida et al., 2009).
In contrast to our understanding of nonglandular trichomes, much less is known about the development and ecological function of glandular trichomes, many of which are multicellular. These epidermal structures synthesize a diverse array of specialized (i.e. secondary) metabolites that exert toxic or repellent effects on myriad phytophagous animals (Kennedy, 2003; Shepherd et al., 2005; Schilmiller et al., 2008). Rupture of the cuticle upon insect contact releases gland contents, which can rapidly oxidize to form a sticky exudate that physically entraps small insects. Among the major classes of compounds involved in trichome-mediated resistance are terpenoids, alkaloids, flavonoids, and defensive proteins (Shepherd and Wagner, 2007; Schilmiller et al., 2008). Large-scale sequencing of ESTs isolated from purified glands has provided unprecedented insight into the biochemical pathways that operate in glandular trichomes (Lange et al., 2000; Aziz et al., 2005; Wang et al., 2008, 2009; Xie et al., 2008; Schilmiller et al., 2009a; Dai et al., 2010). Many key biosynthetic genes in these pathways have been identified and characterized (Iijima et al., 2004; Falara et al., 2008; Slocombe et al., 2008; Ben-Israel et al., 2009; Marks et al., 2009; Schilmiller et al., 2009a).
Cultivated tomato (Solanum lycopersicum) and its wild relatives produce several different types of nonglandular and glandular trichomes on aerial tissues (Luckwill, 1943; Kang et al., 2010). The chemical composition of glandular trichomes varies significantly within and between tomato species (Antonious, 2001; Schilmiller et al., 2008; Besser et al., 2009). Acyl sugars secreted by Solanum pennellii type IV trichomes provide effective resistance to a wide range of insects (Goffreda et al., 1990; Rodriguez et al., 1993; Juvik et al., 1994). Methyl ketone and sesquiterpene derivatives produced in type VI glands of Solanum habrochaites also exert powerful toxic and repellent effects on numerous insect pests (Williams et al., 1980; Maluf et al., 2001; Antonious and Snyder, 2006). Recent studies indicate that trichomes are also an important component of induced anti-insect defenses that are regulated by the plant hormone jasmonate (JA). For example, the density of type VI trichomes on tomato leaves is regulated by the JA pathway (Li et al., 2004; Boughton et al., 2005; Peiffer et al., 2009). JA also plays a role in controlling the accumulation of defense-related terpenoids in type VI glands (Li et al., 2004; van Schie et al., 2007). Recent studies provide evidence that type VI trichomes accumulate JA and may function as sensors for detecting insect movement on the leaf surface (Peiffer et al., 2009). These collective observations highlight the importance of glandular trichomes in shaping plant-insect relations.
Our current understanding of the role of trichomes in mediating S. lycopersicum interaction with arthropod herbivores comes mainly from insect bioassays performed under controlled laboratory conditions (Kennedy, 2003; Li et al., 2004; Bleeker et al., 2009; Peiffer et al., 2009; Kang et al., 2010). Much less is known about the ecological relevance of trichomes in tomato plants grown under more natural conditions in the field. Here, we report the characterization of a tomato mutant, odorless-2 (od-2), that was identified on the basis of an altered leaf-aroma phenotype. This mutant exhibits defects in the development and density of glandular trichomes. Detailed chemical analysis of isolated type VI glands showed that od-2 disrupts the production of diverse specialized metabolites, including volatile terpenes and flavonoids. Consistent with important ecological roles for these compounds in host plant selection and defense, we show that od-2 plants are highly susceptible to natural populations of insect herbivores. Our results suggest that trichome-based chemical defenses play a major role in the resistance of cultivated tomato to opportunistic herbivores and also influence herbivore community composition under natural conditions.
RESULTS
Identification of a Tomato Mutant Affected in Leaf Aroma and Terpene Production
During an Agrobacterium tumefaciens-mediated transformation experiment to overexpress the hydroperoxide lyase (HPL) gene in tomato, we regenerated a primary (T0) line from tissue culture whose foliage lacked the distinct tomato leaf odor. The altered aroma phenotype of this line, which we called od-2, was heritable in the next (T1) generation. Genomic DNA-blot analysis and retesting of T1 seedlings for kanamycin resistance failed to provide evidence for transgenesis (see “Materials and Methods”). These observations and subsequent genetic analyses (see below) indicated that the mutation responsible for the od-2 phenotype likely occurred spontaneously or was generated as a result of the tissue culture procedure, which is known to be mutagenic (Phillips et al., 1994). In addition to the leaf-aroma phenotype, the overall growth stature and leaf size of od-2 plants were decreased in comparison with the wild-type parental line. Comparison of 3-week-old seedlings showed that od-2 leaf area and mass were 59% and 80%, respectively, of those of wild-type plants (Supplemental Fig. S1).
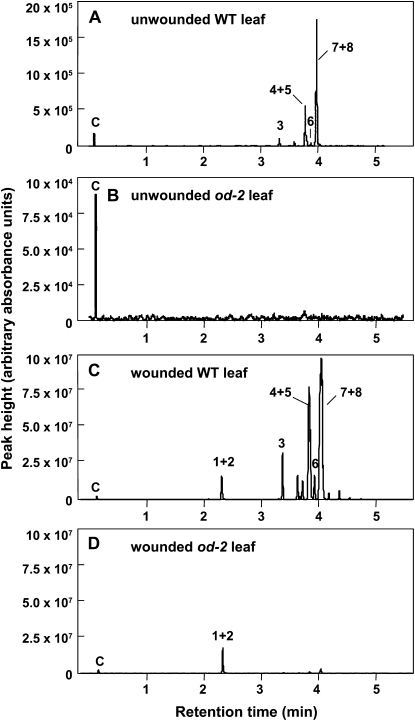
To investigate the biochemical basis of the altered aroma phenotype, we used a solid-phase microextraction (SPME) fiber and gas chromatography-mass spectrometry (GC-MS) to analyze volatile compounds emitted by wild-type and od-2 leaves. Under the GC conditions used, six monoterpenes (α-pinene, 2-carene, α-phellandrene, α-terpinene, limonene, and β-phellandrene; Fig. 1A) and lower levels of two sesquiterpenes (β-caryophyllene and α-humulene; data not shown) were identified in the head space collected from unwounded wild-type leaves. These compounds were not emitted from unwounded od-2 leaves (Fig. 1B). Mechanical wounding of the lamina of wild-type leaves prior to head space sampling resulted in a large increase (approximately 50-fold) in terpene emission as well as the production of the C6 green-leaf volatile cis-3-hexenal (Fig. 1C). Wounded od-2 leaves emitted wild-type levels of cis-3-hexenal but only trace amounts (less than 0.5% wild-type levels) of monoterpenes and sesquiterpenes (Fig. 1D). These findings indicate that od-2 impairs the production of volatile terpenes but does not affect the HPL pathway leading to the production of cis-3-hexenal.
Figure 1.
Volatile profiles of wild-type (WT) and od-2 leaves. A and B, GC traces of volatiles released from detached wild-type (A) and od-2 (B) leaflets. C and D, GC traces of volatiles released from detached wild-type (C) and od-2 (D) leaflets that were mechanically damaged prior to collection of head space-containing volatiles with a SPME fiber. Numbers and letters correspond to the following compounds: C, CO2; 1, hexanal; 2, cis-3-hexenal (coeluting with hexanal); 3, α-pinene; 4, 2-carene; 5, α-phellandrene; 6, α-terpinene; 7, limonene; 8, β-phellandrene. Sesquiterpenes (β-caryophyllene and α-humulene) eluted at later retention times (data not shown).
od-2 Is a Single Recessive Mutation on Chromosome 11
The terpene deficiency of od-2 leaves provided a robust phenotype with which to study the genetic basis of the mutation. F1 hybrid plants obtained from a cross between od-2 and its wild-type parent (S. lycopersicum ‘Castlemart’) showed normal terpene levels, indicating that the mutation is recessive. Analysis of an F2 population (186 plants) produced from self-pollination of an Od-2/od-2 heterozygote showed that the proportion of terpene-producing (terp+) to terpene-deficient (terp−) progeny was 140:46 (3.04:1). This result is in good agreement with the ratio predicted for a single recessive mutation (χ2 = 0.007; P = 0.933).
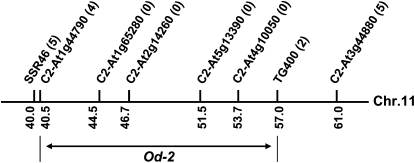
Genetic mapping of single-gene traits in S. lycopersicum is typically performed with F2 populations derived from crosses with S. pennellii or other suitable wild species. Our initial attempt to map Od-2 using such an F2 population was unsuccessful owing to the lack of discrete terp+ and terp− phenotypic classes among the F2 progeny (Supplemental Fig. S2). This phenomenon likely reflects large differences in the density, terpene composition, and distribution of trichome types between S. lycopersicum and S. pennellii (Schilmiller et al., 2009a). Mapping with a backcross population (BC1) can circumvent this problem because the genetic background of BC1 progeny is predominantly that of the recurring S. lycopersicum parent, thus favoring the appearance of discrete phenotypic traits (Li et al., 2003, 2005). We used S. pennellii to generate an interspecific BC1 population and then employed a quantitative GC-based “leaf-dip” assay (Kang et al., 2010) to measure terpene levels in single leaflets from 153 BC1 progeny. The ratio of terp+ to terp− plants in the population was 102:51. Although this value deviates from the expected ratio of 1:1 (χ2 = 17; P < 0.0001), segregation of unambiguous terp+ and terp− phenotypes indicated that the population was suitable for use in mapping experiments. collection of PCR-based markers dispersed among the 12 S. lycopersicum chromosomes (Frary et al., 2005) was used to genotype 98 BC1 individuals, including 63 terp−35 terp+ progeny. The resulting mapping data positioned Od-2 within a 6-centimorgan interval between markers C2_At1g44790 and TG400 on chromosome 11 (Fig. 2). No recombination events were observed between the target locus and four linked markers (C2_At1g65280, C2_At2g14260, C2_At5g13390, and C2_At4g10050) located in this interval (Fig. 2).
Figure 2.
Genetic map of Od-2. Od-2 was mapped in a BC1 population (98 plants) to a genetic interval between C2_At1g44790 and TG400 on chromosome 11. Molecular markers are indicated above the line. Numbers in parentheses indicate the number of recombination events identified between that marker and the target gene. Numbers under the line indicate genetic distances relative to the top (0 centimorgan) and bottom (100 centimorgan) of chromosome 11, according to the Tomato-EXPEN 2000 map (http://solgenomics.net/).
od-2 Affects Trichome Development and Density
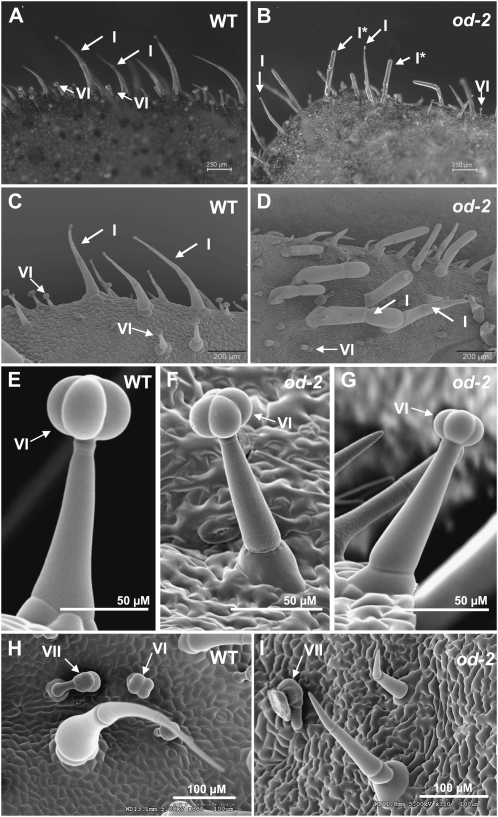
Light microscopy and scanning electron microscopy (SEM) showed that the most conspicuous trichome-related phenotype of od-2 leaves was the failure of type I trichomes to taper toward the tip, resulting in the appearance of swollen or rod-shaped structures (Fig. 3; Supplemental Fig. S3). Additional trichome-related defects were observed, including trichome clustering and a reduction in the size of type VI glandular heads (Fig. 3; Supplemental Fig. S3). The diameter of wild-type and od-2 type VI glands on the adaxial leaf surface was 68 ± 1 μm and 54 ± 1 μm, respectively (mean ± se; n = 17–24 type VI glands on each of four leaflets; unpaired t test, P < 0.001). The density of type VI trichomes on od-2 leaves (621 ± 187 cm−2) was also reduced in comparison with the wild-type (1,112 ± 180 cm−2; n = 6 per genotype; unpaired t test, P < 0.001), as was the density of type VI trichomes on od-2 stems (Supplemental Fig. S3, G and H). SEM analysis showed that epidermal pavement cells of od-2 leaves are more raised and irregularly shaped than pavement cells on wild-type leaves (Fig. 3, H and I).
Figure 3.
Trichome morphology on leaves in wild-type (WT) and od-2 plants. A and B, Light microscopic images of the adaxial surface of wild-type (A) and od-2 (B) leaves. C and D, Scanning electron micrographs of the adaxial surface of wild-type (C) and od-2 (D) leaves. E to I, Cryoscanning electron micrographs of the adaxial surface of wild-type (E and H) and od-2 (F, G, and I) leaves. All images were taken from plants at the seedling (approximately 3-week-old) stage. Type I, VI, and VII trichomes are indicated by arrows and uppercase characters. I* in B denotes abnormal rod-shaped type I trichomes.
Type VI Glandular Trichomes on the od-2 Mutant Do Not Accumulate Terpenoid and Flavonoid Compounds
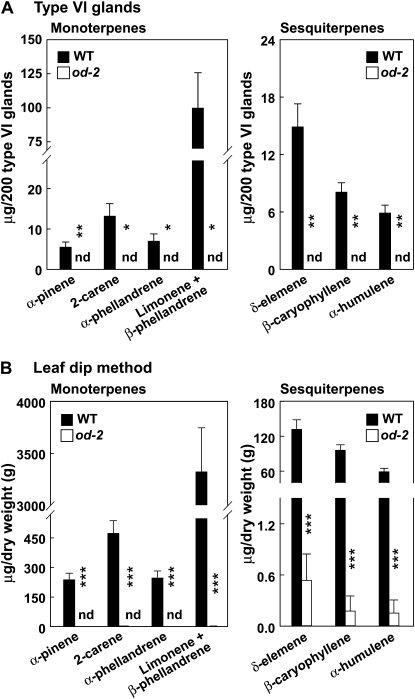
The reduced density and size of type VI glands on od-2 leaves cannot account for the severe terpene deficiency (less than 0.5% wild-type levels) of the mutant (Fig. 1). Therefore, we measured the terpene composition in isolated type VI glands. In one approach, a stretched-glass pipette was used to selectively collect individual glands into a solution containing methyl tert-butyl ether (MTBE) followed by GC-MS analysis. From 200 type VI glands collected from wild-type leaves, we identified four monoterpenes (α-pinene, 2-carene, α-phellandrene, and β-phellandrene) and three sesquiterpenes (δ-elemene, β-caryophyllene, and α-humulene; Fig. 4A). These compounds were not detected in extracts obtained from the same number of od-2 leaf trichomes. Analysis of leaf surface extracts obtained by brief immersion of detached leaflets in MTBE yielded very similar results; terpene levels in od-2 leaves were less than 0.5% of those from wild-type leaves (Fig. 4B). We also determined the terpene profile in type VI trichomes by applying a SPME fiber directly to the glandular head, followed by GC-MS analysis. This procedure was sufficiently sensitive to detect monoterpenes in a single wild-type type VI gland (Supplemental Fig. S4). Despite the high sensitivity of this method, monoterpenes and sesquiterpenes were not detected in od-2 trichomes. Cis-3-hexenal and other C6 green-leaf volatiles were not detected in the SPME-collected glands from either wild-type or od-2 leaves (Supplemental Fig. S4).
Figure 4.
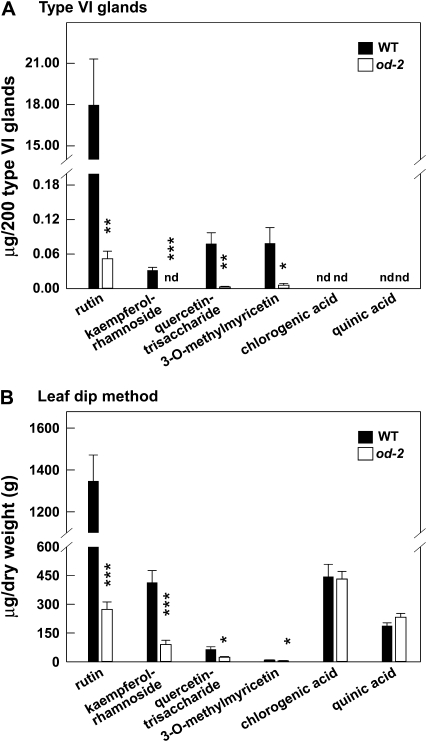
Comparison of terpene levels in isolated type VI glands from wild-type (WT) and od-2 leaves. A, The data show the amount of each of the indicated monoterpene (left panel) and sesquiterpene (right panel) compounds present in 200 type VI glands manually collected (into MTBE) from the adaxial leaf surface of 3-week-old plants. B, Measurement of the same compounds in leaf-dip extracts obtained by briefly immersing detached leaflets in MTBE. Under the GC conditions used, minor amounts of limonene coeluted with β-phellandrene. Each data point represents the mean + se of four biological replicates. Asterisks denote significant differences between the wild type and od-2 (unpaired t test: * P <0.05, ** P <0.01, *** P < 0.001). nd, Not detected.
The amount of rutin (a flavonol glycoside) in type VI glands collected from od-2 leaves was less than 1% of that in the wild-type (Fig. 5A). We also found that od-2 trichomes contain only trace amounts or undetectable levels of kaempferol-rhamnoside, quercetin-trisaccharide, and 3-O-methylmyricetin (Fig. 5A). Analysis of leaf surface extracts obtained by brief immersion of detached leaflets in an isopropanol/acetonitrile solvent system yielded similar results (Fig. 5B). Analysis of these extracts also showed that od-2 leaves contain normal levels of surface-extractable chlorogenic acid and quinic acid (Fig. 5B). The amounts of α-tomatine, dehydrotomatine, and acyl sugars in extracts from od-2 leaves were comparable to or slightly less (60%–77%) than those in extracts from wild-type leaves (Supplemental Fig. S5). These results support the hypothesis that od-2 affects metabolic pathways that operate mainly in type VI glands.
Figure 5.
Comparison of nonvolatile secondary metabolite levels in type VI glands from wild-type (WT) and od-2 leaves. A, The data show the amount of each of the indicated compounds present in 200 type VI glands collected from the adaxial leaf surface of 3-week-old plants. B, Measurement of the same compounds in leaf-dip extracts obtained by briefly immersing detached leaflets in a solution containing isopropanol-acetonitrile-water. Each data point represents the mean + se of five biological replicates. Asterisks represent significant differences between wild-type and od-2 plants (unpaired t test: * P < 0.05, ** P < 0.01, *** P < 0.001). nd, Not detected.
The od-2 Mutant Is Susceptible to Diverse Insect Herbivores
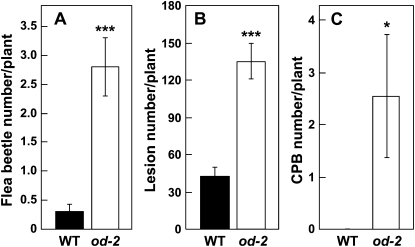
Wild-type and od-2 plants grown under natural field conditions revealed striking differences in the nature and prevalence of plant-herbivore interactions during the growing season. Epitrix cucumeris (potato flea beetle) was the most frequently observed insect on wild-type and mutant tomato plants (Supplemental Fig. S6A). The number of flea beetles observed on od-2 was approximately 8-fold higher than that on the wild type (Fig. 6A). As estimated from the number of lesions produced by flea beetle feeding, it was also apparent that these insects inflicted significantly more damage to od-2 foliage (Fig. 6B). Increased levels of flea beetle herbivory on the mutant were also observed in a second field trial performed at a different location (Supplemental Fig. S7).
Figure 6.
Field-grown od-2 plants are susceptible to natural populations of insect herbivores. A, Mean ± se number of flea beetles on wild-type (WT) and od-2 plants. B, Mean ± se number of flea beetle feeding sites (as measured by hole number) on each host genotype. Data in A and B were determined for 20 replicate plants per genotype 9 d after transplantation of seedlings to the field plot. C, Mean ± se number of CPB on each host genotype. Beetles were counted on 18 replicate wild-type and od-2 plants 40 d after plants were transplanted to the field plot. Asterisks represent significant differences between the wild type and od-2 (unpaired t test: * P < 0.05, *** P < 0.001).
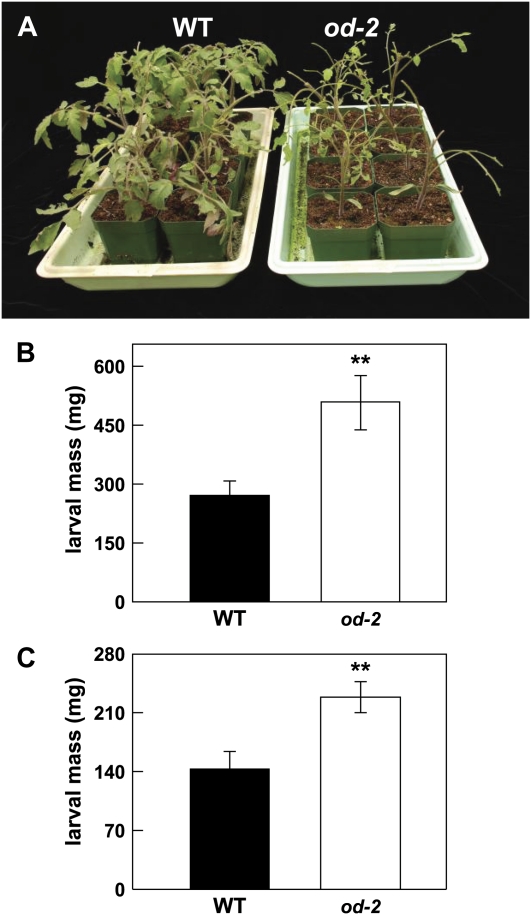
Many field-grown od-2 plants were heavily infested with Colorado potato beetle (CPB) larvae (Leptinotarsa decemlineata; Supplemental Fig. S6B). Remarkably, however, CPB larvae were not found on more than 90 wild-type (Od-2/Od-2) plants grown side by side with od-2 plants (Fig. 6C). No-choice feeding bioassays confirmed that od-2 is compromised in resistance to CPB larvae, as determined both by increased damage to od-2 foliage and an approximately 2.5-fold increase in the average weight of larvae reared on the mutant (Fig. 7; Supplemental Fig. S8). To determine whether od-2 affects host resistance to lepidopteran herbivores, wild-type and mutant plants were challenged with newly hatched larvae of the solanaceous specialist Manduca sexta. The results showed that M. sexta larvae grown on od-2 plants were significantly heavier than larvae reared on wild-type plants (Fig. 8).
Figure 7.
Effect of od-2 on host resistance to CPB larvae. No-choice bioassays were performed by placing newly hatched larvae on wild-type and od-2 mutant plants. A, Photograph of representative wild-type (left) and od-2 (right) plants taken 5 d after initiation of the feeding trial. B, CPB larvae recovered after 5 d of feeding on 12 replicate wild-type (left dish) and od-2 (right dish) plants.
Figure 8.
Effect of od-2 on host resistance to M. sexta larvae. No-choice bioassays were performed by placing first instar M. sexta larvae on wild-type (WT) and od-2 mutant plants. A, Photograph taken 12 d after initiation of the feeding trial. B, Mean ± se weight of M. sexta larvae (n = 16) reared for 12 d on either wild-type or od-2 plants. Each plant was challenged with two larvae. C, Results from an independent bioassay in which three M. sexta larvae were reared for 10 d on each of eight wild-type and od-2 plants. Data show the mean ± se weight of larvae (n = 24). Asterisks represent significant differences between wild-type and od-2 plants (unpaired t test: ** P < 0.01).
od-2 Does Not Impair the Accumulation of Wound-Inducible Proteinase Inhibitors
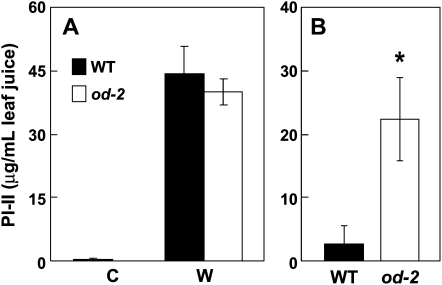
To test whether the increased performance of insect herbivores on od-2 plants results from reduced expression of JA-regulated defensive proteins, we measured the level of the wound-inducible Ser proteinase inhibitor (PI-II). In response to mechanical wounding, PI-II levels in od-2 leaves were comparable to those in wild-type leaves (Fig. 9A). PI-II levels in foliage of field-grown od-2 plants were much higher than in the wild type (Fig. 9B), which may reflect the increased level of herbivory on the mutant. These findings indicate that increased susceptibility of od-2 plants to herbivory is not caused by reduced expression of foliar proteinase inhibitors that are regulated by the JA pathway.
Figure 9.
od-2 plants are not defective in PI-II accumulation. A, Leaves of 15-d-old wild-type (WT) and od-2 plants (five replicates per genotype) were mechanically wounded (W) with a hemostat, and PI-II levels were measured 2 d after wounding. Control (C) plants were not wounded. Data show mean ± se PI-II levels in leaves from five replicate plants of each genotype. B, Mean ± se PI-II levels in leaf tissue from field-grown wild-type and od-2 plants. PI-II levels were measured 14 d after transplantation of plants to the field site. Asterisks represent significant differences between wild-type and od-2 plants (unpaired t test: * P < 0.05).
DISCUSSION
Volatile organic compounds are an integral part of the language with which plants communicate with other organisms (Pichersky and Gershenzon, 2002; Unsicker et al., 2009; Dicke and Baldwin, 2010). Identification of genes involved in the synthesis, storage, and emission of plant volatiles is an important goal of research aimed at deciphering this ancient form of communication. Here, we describe the characterization of a novel tomato mutant (od-2) that was identified on the basis of an altered leaf-aroma phenotype. Chemical profiling experiments revealed that od-2 leaves are severely deficient in both constitutive and damage-induced production of monoterpenoids and sesquiterpenoids. These compounds, together with C6 aldehyde derivatives, contribute to the aroma of tomato leaves (Buttery et al., 1987; Cañoles et al., 2006). The terpene-deficient phenotype of the mutant can be attributed to a defect in the metabolic function of type VI glandular trichomes, which are the major reservoir for terpenoids in tomato leaves. od-2 also impairs the production of the flavonoid compounds rutin, kaempferol- and quercetin-glycosides, and 3-O-methylmyricetin. Rutin is reported to be the major polyphenolic in type VI trichomes of cultivated tomato (Duffey and Isman, 1981). Based on these results, we conclude that Od-2 is required for the production of several chemical classes of compounds in type VI glandular trichomes.
The terpene deficiency in od-2 leaves provided a robust chemical phenotype with which to map Od-2. Mapping with a BC1 population allowed us to position Od-2 on chromosome 11 between markers C2_At1g44790 and TG400. This location distinguishes Od-2 from other previously described mutations affecting tomato leaf odor, including the Od locus on chromosome 3 (Mutschler et al., 1987) and the Spr-2 gene on chromosome 6, which encodes an ω-3 fatty acid desaturase (Li et al., 2003; Cañoles et al., 2006). Systematic characterization of these and other odor-related mutants promises to provide new insight into the biochemical pathways and ecological function of tomato leaf volatiles. Recent progress in sequencing the genome of cultivated and wild species of tomato (http://solgenomics.net) should facilitate these efforts.
The effect of od-2 on the accumulation of multiple classes of metabolites indicates that Od-2 is unlikely to encode an enzyme in the biosynthetic pathway for monoterpenes, sesquiterpenes, or flavonoids. A more plausible explanation is that Od-2 plays a role in the synthesis or transport of a primary metabolite, such as a substrate or intermediate in glycolysis, which supplies precursors for the synthesis of terpenoids and flavonoids. Radiotracer studies performed with isolated glands (McCaskill and Croteau, 1995) may be useful to address this hypothesis. It is also possible that Od-2 serves a regulatory function in coordinating the synthesis of specialized metabolites in glandular trichomes. A MYB-type transcription factor was shown to control the expression of genes involved in the production of benzenoid volatiles in petunia (Petunia hybrida; Verdonk et al., 2005). To our knowledge, there is no precedent for the existence of transcription factors that exert control over unrelated secondary metabolic pathways, such as those for the biosynthesis of terpenoids and flavonoids. Nevertheless, our results provide genetic evidence that terpenoid and flavonoid metabolism in type VI glands is coordinated.
In addition to defects in metabolism, od-2 plants exhibit several developmental phenotypes, including reduced size, altered leaf shape, and aberrant trichome morphology. It is thus possible that Od-2 serves a primary role in a developmental process, the perturbation of which alters trichome- and defense-related traits. Recent studies have revealed links between the chemical composition and morphology of tomato trichomes (Ben-Israel et al., 2009; Kang et al., 2010). It is currently unclear whether the chemical deficiency in od-2 is an indirect consequence of a primary defect in a developmental process or whether a metabolic block created by od-2 results in pleiotrophic effects on growth and development. In support of the latter hypothesis, mutations affecting phenylpropanoid metabolism in Arabidopsis cause dwarfism, male sterility, and other developmental defects (Schilmiller et al., 2009b). Likewise, mutations affecting flavonol composition influence multiple aspects of development, including changes to the leaf epidermis (Ringli et al., 2008).
Interestingly, the od-2-mediated metabolic deficiency is accompanied by reduced density in type VI trichomes on leaves and stems. This observation implies the existence of a mechanism to coordinate the density and metabolic output of type VI glands. Tomato jai-1 mutants that are defective in the JA receptor also exhibit reduced trichome density and terpene-deficient phenotypes (Li et al., 2004; Katsir et al., 2008). A key role for JA in regulating trichome function is supported by studies showing that exogenous JA and wound-induced endogenous JA increase trichome density as well as the terpene content of type VI glands (Thaler et al., 2002; Ament et al., 2004; Boughton et al., 2005; van Schie et al., 2007; Peiffer et al., 2009; Yoshida et al., 2009). The hypersusceptibility of od-2 plants to insect attack also raised the possibility that the mutant might be defective in the JA pathway, which plays a central role in induced resistance of tomato to a broad spectrum of arthropod herbivores (Howe et al., 1996; Thaler, 1999; Li et al., 2002; Kant et al., 2004; Howe and Jander, 2008). However, this hypothesis is not supported by the ability of od-2 plants to accumulate the JA-regulated defensive protein PI-II in response to wounding. The od-2 mutant may provide a useful tool to disentangle the anti-insect role of trichomes from other aspects of JA-mediated defense in tomato foliage.
We conducted field studies as an unbiased approach to understand how od-2 affects the interaction of tomato with other organisms. These experiments revealed that od-2 plants are hypersusceptible to two coleopteran pests, namely the potato flea beetle and CPB. No-choice feeding assays confirmed that the performance of CPB and M. sexta larvae is significantly increased on od-2 plants in comparison with the wild type. CPB is the major insect pest of potato (Solanum tuberosum) and is responsible for significant economic losses worldwide (Hare, 1990). CPB is not considered a serious pest of tomato (Hare, 1990; Harding et al., 2002), which is consistent with the results of our field studies, in which CPB was observed on od-2 but not on wild-type plants. The od-2 mutant may be useful for future studies of CPB host specificity and the identification of compounds that confer resistance to this important pest.
Our results suggest that the increased susceptibility of od-2 to CPB and other insect herbivores results, at least in part, from a metabolic defect in type VI trichomes. Terpenes, which are produced at only trace levels in od-2 leaves, are well known for their role in mediating defenses that are directly toxic to insects as well as indirect defenses that serve to attract predators or parasitoids of the herbivore (De Moraes et al., 1998; Thaler, 1999; Kessler and Baldwin, 2001; Rasmann et al., 2005). The sesquiterpene zingiberene, which is produced in type VI trichomes of S. habrochaites, has been implicated as a factor for CPB resistance (Carter et al., 1989a, 1989b; Antonious and Kochhar, 2003). Zingiberene was not among the sesquiterpenes that we identified in the wild-type parent (cv Castlemart) of od-2. However, other sesquiterpenes in type VI trichomes of cultivated tomato, including δ-elemene, β-caryophyllene, and α-humulene, may also serve important roles in anti-insect defense (Eigenbrode et al., 1994; Antonious and Snyder, 2006). Reduced levels of rutin, 3-O-methylmyricetin, and conjugated forms of quercetin and kaempferol may also contribute to the increased susceptibility of the mutant, as these and related compounds exert growth-retarding effects on insects (Duffey and Isman, 1981; Elliger et al., 1981; Isman and Duffey, 1982; Koul, 2005). The ability of od-2 leaves to accumulate other defense-related metabolites, including chlorogenic acid, quinic acid, acyl sugars, and the glycoalkaloids α-tomatine and dehydrotomatine, indicates that these compounds are not likely responsible for the altered patterns of herbivory on the mutant.
Increased trichome density often correlates with the increased resistance of tomato to arthropod herbivores (Kennedy, 2003). The reduced density of type VI trichomes on od-2 leaves may thus contribute to the enhanced susceptibility of the mutant. Decreased numbers of type VI trichomes may not only reduce the total amount of insect toxins and repellents but may also impair the plant’s ability to properly sense and respond to insect movement on the leaf surface (Peiffer et al., 2009). Regardless of the precise mechanism involved, our results support the view that type VI glandular trichomes constitute a major component of cultivated tomato’s defense system against insect attack. Further characterization of the Od-2 locus should provide insight into the underlying mechanisms of metabolic control in glandular trichomes and may also have practical importance for breeding programs aimed at producing solanaceous crops with broad-spectrum resistance to insect pests.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Tomato (Solanum lycopersicum ‘Castlemart’ [LA2400]) was used as the wild type for all experiments. Seedlings were grown in Jiffy peat pots (Hummert International) in a growth chamber maintained under 17 h of light (265 mE m−2 s−1) at 27°C and 7 h of dark at 18°C and 60% humidity. Three- to 4-week-old plants were sampled for the analyses of trichome morphology, secondary metabolites, and for an herbivore feeding test. The od-2 mutant was identified serendipitously during an Agrobacterium tumefaciens-mediated transformation experiment. Tomato (cv Castlemart) cotyledon explants were infected with Agrobacterium (strain AGLO) containing the binary vector (pBI121) in which the LeHPL cDNA (Howe et al., 2000) was cloned behind the cauliflower mosaic virus 35S promoter. Primary (T0) transformants were selected for resistance to kanamycin and subsequently regenerated as described previously (Li and Howe, 2001). Leaves from one regenerated line (od-2) were noted to lack the distinct tomato leaf aroma. Genomic DNA-blot analysis performed with a 32P-labeled cDNA probe for HPL detected the endogenous HPL gene but not additional transgenic copies of the cDNA (data not shown). Consistent with this finding, germination and growth of od-2 seedlings (T1 generation) on Murashige and Skoog medium containing kanamycin (100 μg mL−1) showed that this line is fully sensitive to kanamycin. All experiments were performed with an od-2 mutant line that was backcrossed two times to its parent (cv Castlemart).
Genetic Analysis and Mapping of od-2
The od-2 mutant (T1 generation) was crossed to its wild-type parent (cv Castlemart [LA2400]), and the resulting F1 plant was self-pollinated to generate a segregating F2 population. F2 plants in this population were scored with a dissecting light microscope for the trichome morphology phenotype. In addition, a single leaflet from each F2 plant was used to prepare a leaf-dip extract for GC-based analysis of terpene levels. A genetic mapping population was constructed by crossing a homozygous od-2 plant with the wild tomato species Solanum pennellii (LA0716). A single F1 plant from this cross was backcrossed to the od-2 parental line to generate a BC1 mapping population. The terpene phenotype (terp+ or terp−) of 98 BC1 individuals was scored as described above. PCR-based anchor markers were used for mapping as described previously (Frary et al., 2005). Primer sequences used for mapping experiments, listed in Supplemental Table S1, are available from the Tomato-EXPEN 2000 map at the Solanaceae Genomics Network (http://www.solgenomics.net). Genomic DNA from parental lines and the individual BC1 plants was used as a template for PCR assays performed with a DNA Engine Dyad Thermal Cycler (Bio-Rad). Each 20-μL reaction contained 20 to 50 ng of template DNA, 10 pmol of each forward and reverse primer, and 10 μL of 2× Taq-Pro Red COMPLETE reaction mix (Denville Scientific). The amplification protocol included an initial 5-min denaturation step at 94°C, followed by 35 cycles in which the template was denatured for 45 s at 94°C, annealed for 30 s at 52°C, and extended for 1 min at 72°C, followed by a final incubation for 10 min at 72°C. Amplified products were separated on 2% to 3% agarose gels run at 4°C in 1× Tris-acetate-EDTA buffer at 100 V.
Analysis of Trichome Density and Morphology
A dissecting microscope (Leica MZ16) equipped with KL 2500 LCD light sources (Schott) and a Leica DFC 290 camera was used to document trichome morphology, size, and density. SEM and cryoSEM were performed as described previously (Kang et al., 2010). All measurements were performed on wild-type and od-2 plants grown side by side under the same growth conditions.
Volatile Analysis
For leaf volatile analysis, compounds in the head space were collected on a SPME fiber (65-μm PDMS-DVB; Supelco) following the procedure described by Song et al. (1997). One leaflet (approximately 40 mg) from each experimental unit was placed in a 25-mL glass vial and left intact or crushed five times with a rod (1 cm diameter) wrapped with Teflon. Vials were sealed with a cap housing a valved septum (Mininert; Supelco). A SPME fiber was held in the vial for 3 min to allow the absorption of volatile compounds. To analyze volatiles from glandular heads of type VI trichomes, we applied a SPME fiber directly to glandular heads for 10 s or less. Direct contact between the fiber and the glands ruptured the cuticle and allowed the released compounds to be absorbed by the SPME fiber. We desorbed the volatiles from the fiber coating by inserting the SPME fiber through a septumless injection port (Merlin Microseal; Supelco) and into a glass-lined injector port (200°C) of a GC instrument (HP-6890; Hewlett-Packard) interfaced to a time-of-flight mass spectrometer (Pegasus II; Leco). We separated volatiles using a capillary column (HP-5; 5 m × 0.1 mm i.d., 0.34-μm coating thickness) under conditions for GC separation and time-of-flight-MS analysis as described previously (Song et al., 1997), except that the GC was run in the split injection mode (split ratio = 2:1). Identification of volatile components was confirmed by comparison of collected mass spectra with those of authenticated chemicals and reference spectra in a mass spectrum library (NIST MS Search 1.5; National Institute for Standard Technology). Quantification of volatile compounds was performed by comparison with known concentrations of authenticated and high-purity compounds in an external standard mixture as described previously (Song et al., 1997). Standard mixtures were prepared with equal volumes of 1-hexanol, cis-3-hexen-1-ol, hexanal, cis-3-hexenal, trans-2-hexenal, α-pinene, 2-carene, α-phellandrene, α-terpinene, limonene, β-caryophyllene, and α-humulene. A 0.5-μL sample of the standard mixture was applied to a small paper filter disc at the bottom of a gas-tight 4.4-L glass volumetric flask, which was fitted with a tapered ground glass stopper and a gas-tight Mininert valve. The flask was sealed, and the liquid material was allowed to vaporize. Limonene was used as a standard to determine a response factor for monoterpenes. For trichome volatile analysis, a 0.1-μL sample of the standard mixture was applied directly to a SPME fiber, and GC was performed with a split ratio of 200:1. Quantification of terpene levels in leaf-dip extracts and isolated type VI glands (collected with a stretched Pasteur pipette) was performed as described previously (Kang et al., 2010).
Analysis of Flavonoid and Other Nonvolatile Compounds
Leaves from 4-week-old plants were used to prepare leaf-dip or type VI trichome exudates as described previously (Kang et al., 2010). Briefly, single leaflets were incubated in 1 mL of isopropanol:acetonitrile:water (3:3:2) for 5 min with gentle shaking. Alternatively, type VI glandular heads collected with a Pasteur pipette were dissolved in 100 μL of isopropanol:acetonitrile:water (3:3:2). We analyzed the resulting extracts (10 μL) by liquid chromatography-MS with a Waters LCT Premier mass spectrometer coupled to a Shimadzu LC-20AD HPLC ternary pump and SIL-5000 autosampler as described previously (Kang et al., 2010). Quantification of flavonoids, tomatines, and acyl sugars was performed as described by Kang et al. (2010).
Plant Interactions with Insect Herbivores
Field experiments were performed in the summer of 2007 and 2008 at two field sites in East Lansing, Michigan. Four- to 5-week-old plants grown in the greenhouse were transferred to a field plot on the Department of Plant Pathology Research Farm, Michigan State University, or at a second site located on the main Michigan State University campus. Wild-type and od-2 plants were grown in alternating rows, with 100-cm spacing between plants within and between rows. Plants were watered manually every other day for 1 week, after which they were allowed to grow under natural conditions. Plants were monitored twice a week for the presence of naturally occurring herbivores as well as for feeding damage caused by insect herbivores.
No-choice feeding bioassays were performed with 4-week-old wild-type and od-2 plants maintained in a growth chamber as described above. Tobacco hornworm (Manduca sexta) eggs and artificial diet were obtained from the Department of Entomology, North Carolina State University in Raleigh. Eggs were hatched at 26°C, as recommended by the supplier. Hatched larvae were reared on artificial diet for 4 d before transfer to tomato plants. CPB (Leptinotarsa decemlineata) eggs were obtained from the Phillip Alampi Beneficial Insect Laboratory of the New Jersey Department of Agriculture. Eggs were incubated at 26°C, and hatched neonate larvae were directly transferred to 4-week-old tomato plants.
Proteinase Inhibitor Assays
PI-II levels in tomato leaves were determined by a radial immunodiffusion assay as described previously (Li et al., 2003). A hemostat was used to make crushing-type wounds on all leaflets of the lower (oldest) leaf of 15-d-old tomato plants that contained two expanded leaves and a third emerging leaf. Wounded plants were incubated for 2 d under standard growth conditions, after which the wounded leaf was harvested for determination of PI-II protein levels. Leaflets from the youngest leaf of plants grown under natural conditions for at least 2 weeks were used to measure PI-II levels in field-grown plants.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Phenotypic appearance of 3-week-old wild-type and od-2 plants.
Supplemental Figure S2. β-Phellandrene levels in F2 plants from an interspecific mapping population derived from a cross between S. lycopersicum (od-2/od-2) and S. pennellii (Od-2/Od-2).
Supplemental Figure S3. Trichome morphology on leaves and stems in wild-type and od-2 plants.
Supplemental Figure S4. Volatile compounds in type VI glands from wild-type and od-2 leaves.
Supplemental Figure S5. Tomatine and acyl sugar levels in wild-type and od-2 leaves.
Supplemental Figure S6. Insect herbivores observed on field-grown od-2 tomato plants.
Supplemental Figure S7. Herbivory on wild-type and od-2 plants grown at a second field site in East Lansing, Michigan.
Supplemental Figure S8. Performance of CPB larvae on wild-type and od-2 plants.
Supplemental Table S1. Description of PCR-based anchor markers.
Supplementary Material
Acknowledgments
We thank former lab members Erin Beach, Afreen Syed, Matt Oney, Eric Czuprenski, and Cody Depew for technical assistance and Ray Hammerschmidt and Gary Zehr (Michigan State University) for providing field space. Ewa Danielewicz (Michigan State University) and David Marks (University of Minnesota) are acknowledged for SEM and cryoSEM images, respectively. We are grateful to members of the Solanum Trichome Project (http://www.trichome.msu.edu/) for helpful discussions during the course of this work. We acknowledge the C.M. Rick Tomato Genetics Resource Center (University of California at Davis) for kindly providing tomato seed stocks.
References
- Ament K, Kant MR, Sabelis MW, Haring MA, Schuurink RC. (2004) Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiol 135: 2025–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonious GF. (2001) Production and quantification of methyl ketones in wild tomato accessions. J Environ Sci Health Part B Pestic Contam Agric Wastes 36: 835–848 [DOI] [PubMed] [Google Scholar]
- Antonious GF, Kochhar TS. (2003) Zingiberene and curcumene in wild tomato. J Environ Sci Health Part B Pestic Contam Agric Wastes 38: 489–500 [DOI] [PubMed] [Google Scholar]
- Antonious GF, Snyder JC. (2006) Natural products: repellency and toxicity of wild tomato leaf extracts to the two-spotted spider mite, Tetranychus urticae Koch. J Environ Sci Health Part B Pestic Contam Agric Wastes 41: 43–55 [DOI] [PubMed] [Google Scholar]
- Aziz N, Paiva NL, May GD, Dixon RA. (2005) Transcriptome analysis of alfalfa glandular trichomes. Planta 221: 28–38 [DOI] [PubMed] [Google Scholar]
- Ben-Israel I, Yu G, Austin MB, Bhuiyan N, Auldridge M, Nguyen T, Schauvinhold I, Noel JP, Pichersky E, Fridman E. (2009) Multiple biochemical and morphological factors underlie the production of methylketones in tomato trichomes. Plant Physiol 151: 1952–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser K, Harper A, Welsby N, Schauvinhold I, Slocombe S, Li Y, Dixon RA, Broun P. (2009) Divergent regulation of terpenoid metabolism in the trichomes of wild and cultivated tomato species. Plant Physiol 149: 499–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleeker PM, Diergaarde PJ, Ament K, Guerra J, Weidner M, Schutz S, de Both MTJ, Haring MA, Schuurink RC. (2009) The role of specific tomato volatiles in tomato-whitefly interaction. Plant Physiol 151: 925–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughton AJ, Hoover K, Felton GW. (2005) Methyl jasmonate application induces increased densities of glandular trichomes on tomato, Lycopersicon esculentum. J Chem Ecol 31: 2211–2216 [DOI] [PubMed] [Google Scholar]
- Buttery RG, Ling LC, Light DM. (1987) Tomato leaf volatile aroma components. J Agric Food Chem 35: 1039–1042 [Google Scholar]
- Cañoles MA, Beaudry RM, Li CY, Howe G. (2006) Deficiency of linolenic acid in Lefad7 mutant tomato changes the volatile profile and sensory perception of disrupted leaf and fruit tissue. J Am Soc Hortic Sci 131: 284–289 [Google Scholar]
- Carter CD, Gianfagna TJ, Sacalis JN. (1989a) Sesquiterpenes in glandular trichomes of a wild tomato species and toxicity to the Colorado potato beetle. J Agric Food Chem 37: 1425–1428 [Google Scholar]
- Carter CD, Sacalis JN, Gianfagna TJ. (1989b) Zingiberene and resistance to Colorado potato beetle in Lycopersicon hirsutum f. hirsutum. J Agric Food Chem 37: 206–210 [Google Scholar]
- Dai XB, Wang GD, Yang DS, Tang YH, Broun P, Marks MD, Sumner LW, Dixon RA, Zhao PX. (2010) TrichOME: a comparative omics database for plant trichomes. Plant Physiol 152: 44–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moraes CM, Lewis WJ, Pare PW, Alborn HT, Tumlinson JH. (1998) Herbivore-infested plants selectively attract parasitoids. Nature 393: 570–573 [Google Scholar]
- Dicke M, Baldwin IT. (2010) The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help.’ Trends Plant Sci 15: 167–175 [DOI] [PubMed] [Google Scholar]
- Duffey SS, Isman MB. (1981) Inhibition of insect larval growth by phenolics in glandular trichomes of tomato leaves. Experientia 37: 574–576 [Google Scholar]
- Eigenbrode SD, Trumble JT, Millar JG, White KK. (1994) Topical toxicity of tomato sesquiterpenes to the beet armyworm and the role of these compounds in resistance derived from an accession of Lycopersicon hirsutum f. typicum. J Agric Food Chem 42: 807–810 [Google Scholar]
- Elliger CA, Wong Y, Chan BG, Waiss AC. (1981) Growth-inhibitors in tomato (Lycopersicon) to tomato fruitworm (Heliothis zea). J Chem Ecol 7: 753–758 [DOI] [PubMed] [Google Scholar]
- Falara V, Fotopoulos V, Margaritis T, Anastasaki T, Pateraki I, Bosabalidis AM, Kafetzopoulos D, Demetzos C, Pichersky E, Kanellis AK. (2008) Transcriptome analysis approaches for the isolation of trichome-specific genes from the medicinal plant Cistus creticus subsp. creticus. Plant Mol Biol 68: 633–651 [DOI] [PubMed] [Google Scholar]
- Frary A, Xu YM, Liu JP, Mitchell S, Tedeschi E, Tanksley S. (2005) Development of a set of PCR-based anchor markers encompassing the tomato genome and evaluation of their usefulness for genetics and breeding experiments. Theor Appl Genet 111: 291–312 [DOI] [PubMed] [Google Scholar]
- Goffreda JC, Szymkowiak EJ, Sussex IM, Mutschler MA. (1990) Chimeric tomato plants show that aphid resistance and triacylglucose production are epidermal autonomous characters. Plant Cell 2: 643–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding CL, Fleischer SJ, Blom PE. (2002) Population dynamics of the Colorado potato beetle in an agroecosystem with tomatoes and potatoes with management implications to processing tomatoes. Environ Entomol 31: 1110–1118 [Google Scholar]
- Hare JD. (1990) Ecology and management of the Colorado potato beetle. Annu Rev Entomol 35: 81–100 [Google Scholar]
- Howe GA, Jander G. (2008) Plant immunity to insect herbivores. Annu Rev Plant Biol 59: 41–66 [DOI] [PubMed] [Google Scholar]
- Howe GA, Lee GI, Itoh A, Li L, DeRocher AE. (2000) Cytochrome P450-dependent metabolism of oxylipins in tomato: cloning and expression of allene oxide synthase and fatty acid hydroperoxide lyase. Plant Physiol 123: 711–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe GA, Lightner J, Browse J, Ryan CA. (1996) An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. Plant Cell 8: 2067–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima Y, Davidovich-Rikanati R, Fridman E, Gang DR, Bar E, Lewinsohn E, Pichersky E. (2004) The biochemical and molecular basis for the divergent patterns in the biosynthesis of terpenes and phenylpropenes in the peltate glands of three cultivars of basil. Plant Physiol 136: 3724–3736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isman MB, Duffey SS. (1982) Toxicity of tomato phenolic compounds to the fruitworm, Heliothis zea. Entomol Exp Appl 31: 370–376 [Google Scholar]
- Juvik JA, Shapiro JA, Young TE, Mutschler MA. (1994) Acylglucoses from wild tomatoes alter behavior and reduce growth and survival of Helicoverpa zea and Spodoptera exigua (Lepidoptera, Noctuidae). J Econ Entomol 87: 482–492 [Google Scholar]
- Kang JH, Shi F, Jones AD, Marks MD, Howe GA. (2010) Distortion of trichome morphology by the hairless mutation of tomato affects leaf surface chemistry. J Exp Bot 61: 1053–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant MR, Ament K, Sabelis MW, Haring MA, Schuurink RC. (2004) Differential timing of spider mite-induced direct and indirect defenses in tomato plants. Plant Physiol 135: 483–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkkainen K, Agren J. (2002) Genetic basis of trichome production in Arabidopsis lyrata. Hereditas 136: 219–226 [DOI] [PubMed] [Google Scholar]
- Katsir L, Schilmiller AL, Staswick PE, He SY, Howe GA. (2008) COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc Natl Acad Sci USA 105: 7100–7105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy GG. (2003) Tomato, pests, parasitoids, and predators: tritrophic interactions involving the genus Lycopersicon. Annu Rev Entomol 48: 51–72 [DOI] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT. (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291: 2141–2144 [DOI] [PubMed] [Google Scholar]
- Kivimaki M, Karkkainen K, Gaudeul M, Loe G, Agren J. (2007) Gene, phenotype and function: GLABROUS1 and resistance to herbivory in natural populations of Arabidopsis lyrata. Mol Ecol 16: 453–462 [DOI] [PubMed] [Google Scholar]
- Koul O. (2005) Insect Antifeedants. CRC Press, Boca Raton, FL [Google Scholar]
- Lange BM, Wildung MR, Stauber EJ, Sanchez C, Pouchnik D, Croteau R. (2000) Probing essential oil biosynthesis and secretion by functional evaluation of expressed sequence tags from mint glandular trichomes. Proc Natl Acad Sci USA 97: 2934–2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Schilmiller AL, Liu G, Lee GI, Jayanty S, Sageman C, Vrebalov J, Giovannoni JJ, Yagi K, Kobayashi Y, et al. (2005) Role of β-oxidation in jasmonate biosynthesis and systemic wound signaling in tomato. Plant Cell 17: 971–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Liu GH, Xu CC, Lee GI, Bauer P, Ling HQ, Ganal MW, Howe GA. (2003) The tomato Suppressor of prosystemin-mediated responses2 gene encodes a fatty acid desaturase required for the biosynthesis of jasmonic acid and the production of a systemic wound signal for defense gene expression. Plant Cell 15: 1646–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Williams MM, Loh YT, Lee GI, Howe GA. (2002) Resistance of cultivated tomato to cell content-feeding herbivores is regulated by the octadecanoid-signaling pathway. Plant Physiol 130: 494–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Howe GA. (2001) Alternative splicing of prosystemin pre-mRNA produces two isoforms that are active as signals in the wound response pathway. Plant Mol Biol 46: 409–419 [DOI] [PubMed] [Google Scholar]
- Li L, Zhao YF, McCaig BC, Wingerd BA, Wang JH, Whalon ME, Pichersky E, Howe GA. (2004) The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell 16: 126–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckwill LC. (1943) The Genus Lycopersicon: A Historical, Biological and Taxonomic Survey of the Wild and Cultivated Tomatoes. Aberdeen University Press, Aberdeen, UK [Google Scholar]
- Maluf WR, Campos GA, Cardoso MD. (2001) Relationships between trichome types and spider mite (Tetranychus evansi) repellence in tomatoes with respect to foliar zingiberene contents. Euphytica 121: 73–80 [Google Scholar]
- Marks MD. (1997) Molecular genetic analysis of trichome development in Arabidopsis. Annu Rev Plant Physiol Plant Mol Biol 48: 137–163 [DOI] [PubMed] [Google Scholar]
- Marks MD, Tian L, Wenger JP, Omburo SN, Soto-Fuentes W, He J, Gang DR, Weiblen GD, Dixon RA. (2009) Identification of candidate genes affecting Δ9-tetrahydrocannabinol biosynthesis in Cannabis sativa. J Exp Bot 60: 3715–3726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaskill D, Croteau R. (1995) Monoterpene and sesquiterpene biosynthesis in glandular trichomes of peppermint (Mentha × piperita) rely exclusively on plastid-derived isopentenyl diphosphate. Planta 197: 49–56 [Google Scholar]
- Mutschler MA, Tanksley SD, Rick CM. (1987) Linkage maps of the tomato (Lycopersicon esculentum). Report of the Tomato Genetics Cooperative 37: 5–34 [Google Scholar]
- Peiffer M, Tooker JF, Luthe DS, Felton GW. (2009) Plants on early alert: glandular trichomes as sensors for insect herbivores. New Phytol 184: 644–656 [DOI] [PubMed] [Google Scholar]
- Phillips RL, Kaeppler SM, Olhoft P. (1994) Genetic instability of plant tissue cultures: breakdown of normal controls. Proc Natl Acad Sci USA 91: 5222–5226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichersky E, Gershenzon J. (2002) The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol 5: 237–243 [DOI] [PubMed] [Google Scholar]
- Rasmann S, Kollner TG, Degenhardt J, Hiltpold I, Toepfer S, Kuhlmann U, Gershenzon J, Turlings TC. (2005) Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 434: 732–737 [DOI] [PubMed] [Google Scholar]
- Ringli C, Bigler L, Kuhn BM, Leiber RM, Diet A, Santelia D, Frey B, Pollmann S, Klein M. (2008) The modified flavonol glycosylation profile in the Arabidopsis rol1 mutants results in alterations in plant growth and cell shape formation. Plant Cell 20: 1470–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AE, Tingey WM, Mutschler MA. (1993) Acylsugars of Lycopersicon pennellii deter settling and feeding of the green peach aphid (Homoptera, Aphididae). J Econ Entomol 86: 34–39 [Google Scholar]
- Schilmiller AL, Last RL, Pichersky E. (2008) Harnessing plant trichome biochemistry for the production of useful compounds. Plant J 54: 702–711 [DOI] [PubMed] [Google Scholar]
- Schilmiller AL, Schauvinhold I, Larson M, Xu R, Charbonneau AL, Schmidt A, Wilkerson C, Last RL, Pichersky E. (2009a) Monoterpenes in the glandular trichomes of tomato are synthesized from a neryl diphosphate precursor rather than geranyl diphosphate. Proc Natl Acad Sci USA 106: 10865–10870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilmiller AL, Stout J, Weng JK, Humphreys J, Ruegger MO, Chapple C. (2009b) Mutations in the cinnamate 4-hydroxylase gene impact metabolism, growth and development in Arabidopsis. Plant J 60: 771–782 [DOI] [PubMed] [Google Scholar]
- Shepherd RW, Bass WT, Houtz RL, Wagner GJ. (2005) Phylloplanins of tobacco are defensive proteins deployed on aerial surfaces by short glandular trichomes. Plant Cell 17: 1851–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RW, Wagner GJ. (2007) Phylloplane proteins: emerging defenses at the aerial frontline? Trends Plant Sci 12: 51–56 [DOI] [PubMed] [Google Scholar]
- Slocombe SP, Schauvinhold I, McQuinn RP, Besser K, Welsby NA, Harper A, Aziz N, Li Y, Larson TR, Giovannoni J, et al. (2008) Transcriptomic and reverse genetic analyses of branched-chain fatty acid and acyl sugar production in Solanum pennellii and Nicotiana benthamiana. Plant Physiol 148: 1830–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Gardner BD, Holland JF, Beaudry RM. (1997) Rapid analysis of volatile flavor compounds in apple fruit using SPME and GC/time-of-flight mass spectrometry. J Agric Food Chem 45: 1801–1807 [Google Scholar]
- Thaler JS. (1999) Jasmonate-inducible plant defences cause increased parasitism of herbivores. Nature 399: 686–688 [Google Scholar]
- Thaler JS, Farag MA, Pare PW, Dicke M. (2002) Jasmonate-deficient plants have reduced direct and indirect defences against herbivores. Ecol Lett 5: 764–774 [Google Scholar]
- Unsicker SB, Kunert G, Gershenzon J. (2009) Protective perfumes: the role of vegetative volatiles in plant defense against herbivores. Curr Opin Plant Biol 12: 479–485 [DOI] [PubMed] [Google Scholar]
- van Schie CCN, Haring MA, Schuurink RC. (2007) Tomato linalool synthase is induced in trichomes by jasmonic acid. Plant Mol Biol 64: 251–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdonk JC, Haring MA, van Tunen AJ, Schuurink RC. (2005) ODORANT1 regulates fragrance biosynthesis in petunia flowers. Plant Cell 17: 1612–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GD, Tian L, Aziz N, Broun P, Dai XB, He J, King A, Zhao PX, Dixon RA. (2008) Terpene biosynthesis in glandular trichomes of hop. Plant Physiol 148: 1254–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Wang Y, Zhang Q, Qi Y, Guo D. (2009) Global characterization of Artemisia annua glandular trichome transcriptome using 454 pyrosequencing. BMC Genomics 10: 465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams WG, Kennedy GG, Yamamoto RT, Thacker JD, Bordner J. (1980) 2-Tridecanone: a naturally occurring insecticide from the wild tomato Lycopersicon hirsutum f. glabratum. Science 207: 888–889 [DOI] [PubMed] [Google Scholar]
- Xie Z, Kapteyn J, Gang DR. (2008) A systems biology investigation of the MEP/terpenoid and shikimate/phenylpropanoid pathways points to multiple levels of metabolic control in sweet basil glandular trichomes. Plant J 54: 349–361 [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Sano R, Wada T, Takabayashi J, Okada K. (2009) Jasmonic acid control of GLABRA3 links inducible defense and trichome patterning in Arabidopsis. Development 136: 1039–1048 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.