Abstract
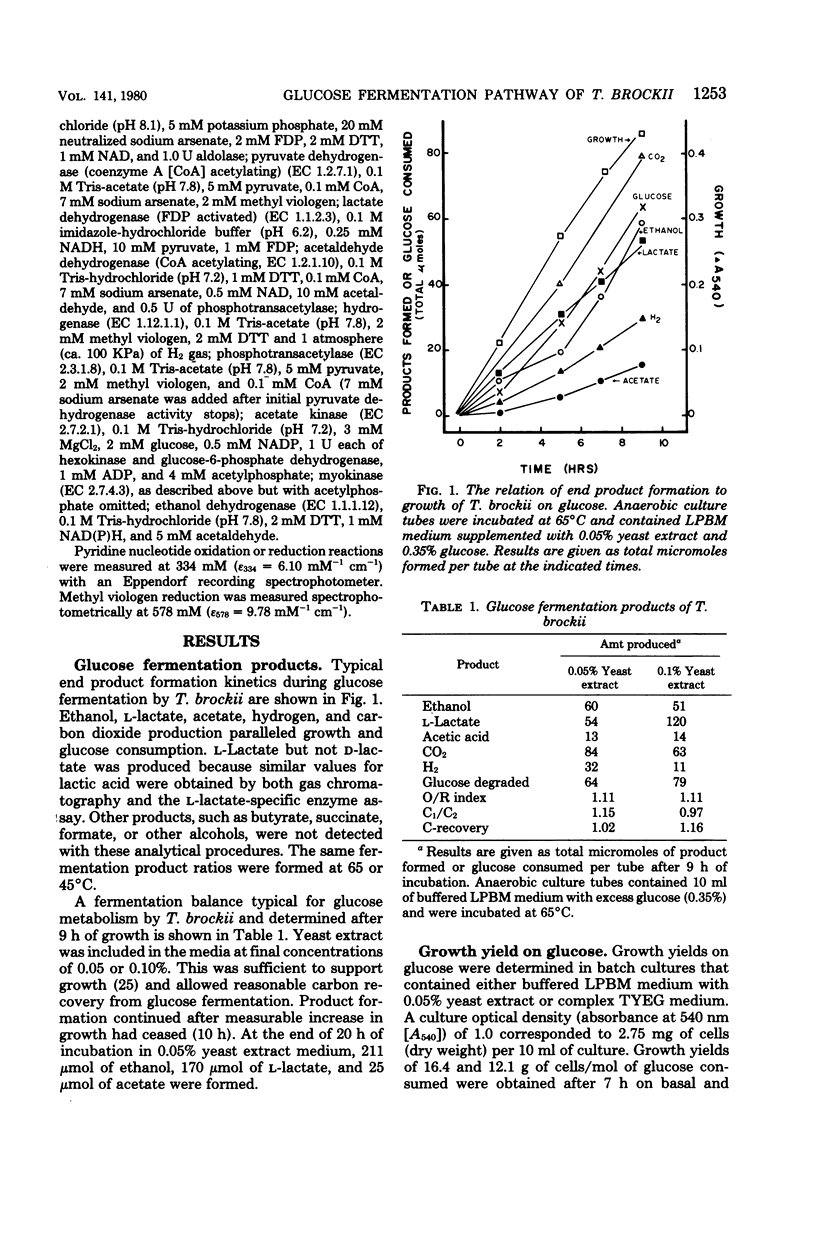
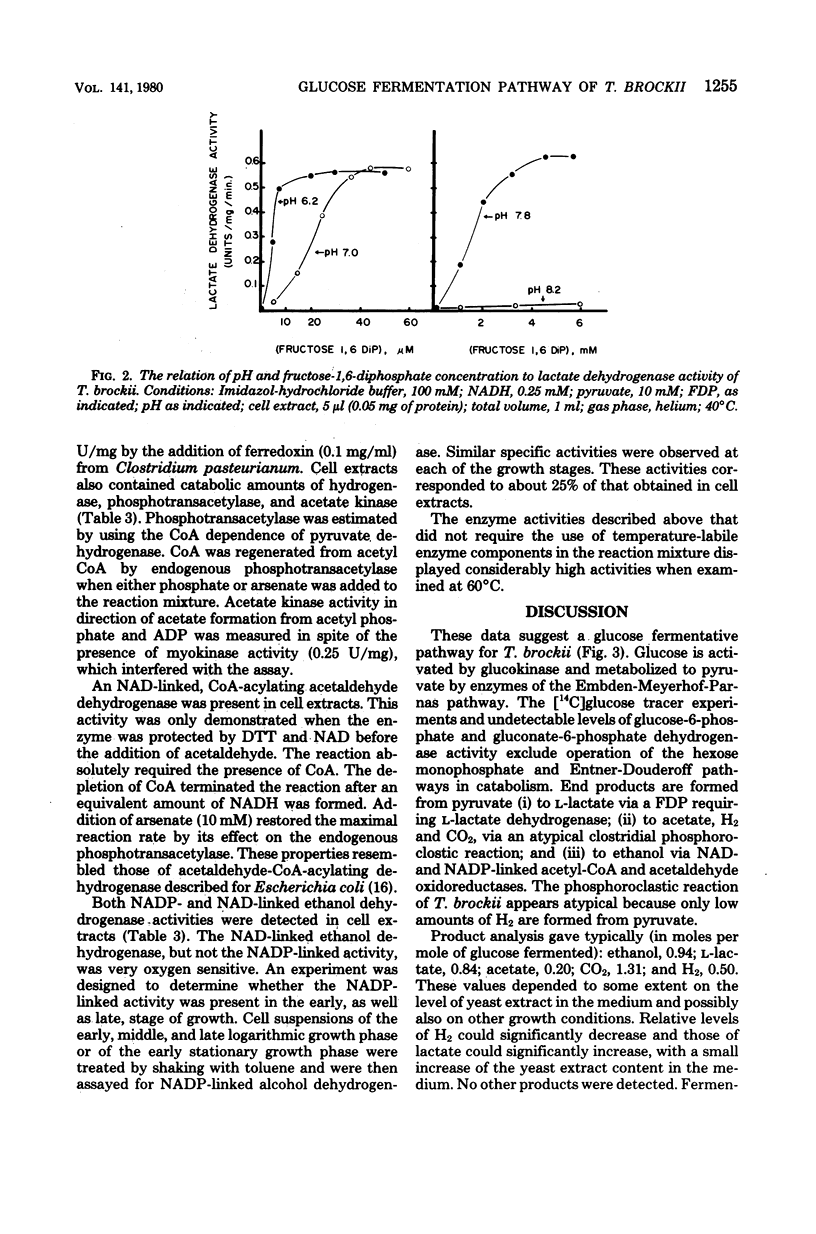
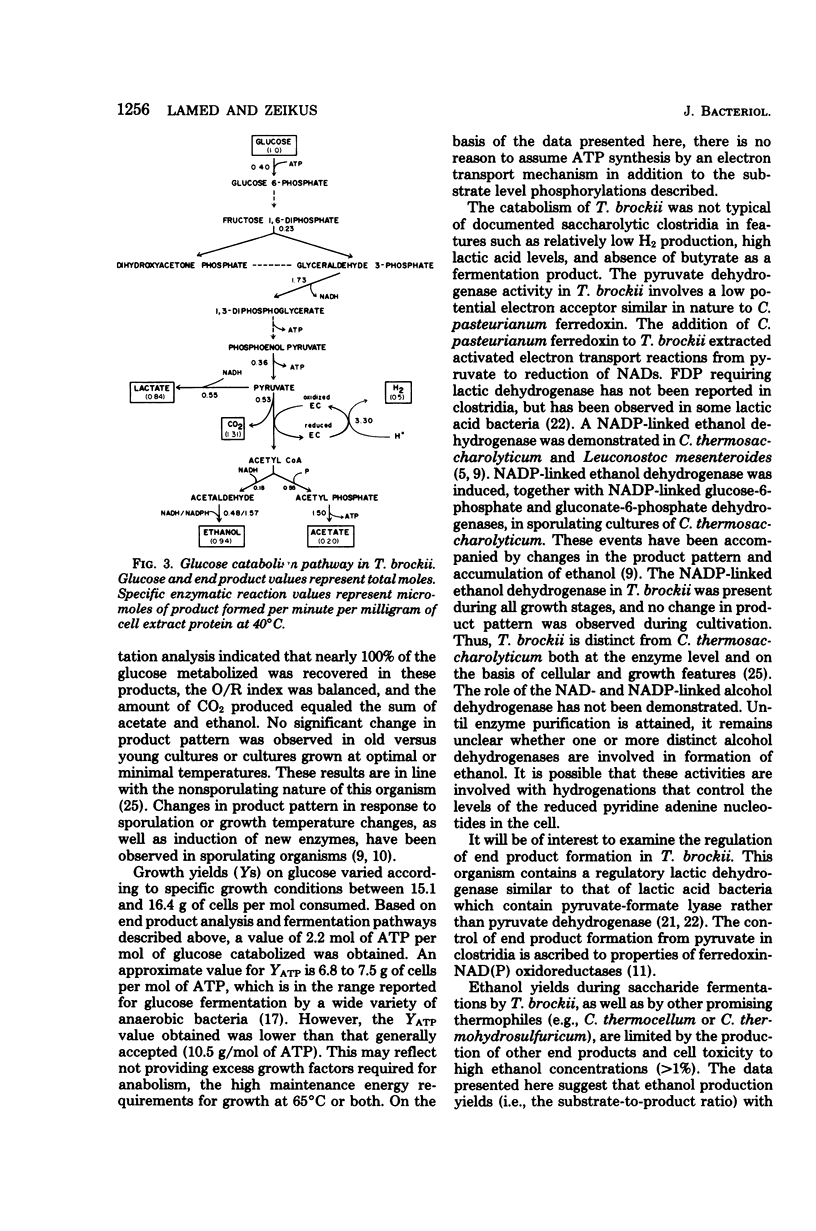
Thermoanaerobium brockii was shown to catabolize glucose via the Embden-Meyerhof-Parnas pathway into ethanol, acetic acid, H2-CO2, and lactic acid. Radioactive tracer studies, employing specifically labeled [14C]glucose, demonstrated significant fermentation of 14CO2 from C-3 and C-4 of the substrate exclusively. All extracts contained sufficient levels of activity (expressed in micromoles per minute per milligram of protein at 40°C) to assign a catabolic role for the following enzymes: glucokinase, 0.40; fructose-1,6-diphosphate aldolase, 0.23; glyceraldehyde-3-phosphate dehydrogenase, 1.73; pyruvate kinase, 0.36; lactate dehydrogenase (fructose-1,6-diphosphate activated), 0.55; pyruvate dehydrogenase (coenzyme A acetylating), 0.53; hydrogenase, 3.3; phosphotransacetylase, 0.55; acetaldehyde dehydrogenase (coenzyme A acetylating), 0.15; ethanol dehydrogenase, 1.57; and acetate kinase, 1.50. All pyridine nucleotide-linked oxidoreductases examined were specific for nicotinamide adenine dinucleotide, except ethanol dehydrogenase which displayed both nicotinamide adenine dinucleotide- and nicotinamide adenine dinucleotide phosphate-linked activities. Fermentation product balances and cell growth yields supported the glucose catabolic pathway described. Representative balanced end product yields (in moles per mole of glucose fermented) were: ethanol, 0.94; l-lactate, 0.84; acetate, 0.20; CO2, 1.31; and H2, 0.50. Growth yields of 16.4 g of cells per mole of glucose were demonstrated. Both growth and end product yields varied significantly in accordance with the specific medium composition and incubation time.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- DeMOSS R. D., BARD R. C., GUNSALUS I. C. The mechanism of the heterolactic fermentation; a new route of ethanol formation. J Bacteriol. 1951 Oct;62(4):499–511. doi: 10.1128/jb.62.4.499-511.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollaus F., Sleytr U. On the taxonomy and fine structure of some hyperthermophilic saccharolytic Clostridia. Arch Mikrobiol. 1972;86(2):129–146. doi: 10.1007/BF00413368. [DOI] [PubMed] [Google Scholar]
- Hsu E. J., Ordal Z. J. Comparative metabolism of vegetative and sporulating cultures of Clostridium thermosaccharolyticum. J Bacteriol. 1970 May;102(2):369–376. doi: 10.1128/jb.102.2.369-376.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung L., Jost R., Stoll E., Zuber H. Metabolic differences in Bacillus stearothermophilus grown at 55 degrees C and 37 degrees C. Arch Mikrobiol. 1974 Feb 1;95(2):125–138. [PubMed] [Google Scholar]
- Jungermann K., Thauer R. K., Leimenstoll G., Decker K. Function of reduced pyridine nucleotide-ferredoxin oxidoreductases in saccharolytic Clostridia. Biochim Biophys Acta. 1973 May 30;305(2):268–280. doi: 10.1016/0005-2728(73)90175-8. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Zeikus J. G. Rapid method for the radioisotopic analysis of gaseous end products of anaerobic metabolism. Appl Microbiol. 1974 Aug;28(2):258–261. doi: 10.1128/am.28.2.258-261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer P. J., Zeikus J. G. Fermentation of cellulose and cellobiose by Clostridium thermocellum in the absence of Methanobacterium thermoautotrophicum. Appl Environ Microbiol. 1977 Feb;33(2):289–297. doi: 10.1128/aem.33.2.289-297.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberger C. L., Angelo N. Purificationa and properties of a fructose-1,6-diphosphate-activated lactate dehydrogenase from Streptococcus faecalis. J Bacteriol. 1970 Mar;101(3):717–724. doi: 10.1128/jb.101.3.717-724.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Carlsson J. Regulation of lactate dehydrogenase and change of fermentation products in streptococci. J Bacteriol. 1975 Oct;124(1):55–61. doi: 10.1128/jb.124.1.55-61.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Fuchs G., Kenealy W., Thauer R. K. Oxidoreductases involved in cell carbon synthesis of Methanobacterium thermoautotrophicum. J Bacteriol. 1977 Nov;132(2):604–613. doi: 10.1128/jb.132.2.604-613.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


